Introduction
The island of New Britain in the Bismarck Archipelago of Papua New Guinea is characterised by high avian endemism (BirdLife International 2016). Together with New Ireland and smaller satellite islands, New Britain forms an ‘Endemic Bird Area’ supporting 38 ‘restricted-range’ species (Stattersfield et al. Reference Stattersfield, Crosby, Long and Wege1998). These species are also under considerable threat from habitat loss – between 1989 and 2000 an estimated 20% of all forest below 100 m altitude was lost (Buchanan et al. Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008). Habitat loss primarily resulted from forest clearance for the production of palm oil. Oil palm is the world’s highest yielding and least expensive vegetable oil. Oil palm plantations are known to present a significant challenge to biodiversity and support far fewer species than forest (Fitzherbert et al. Reference Fitzherbert, Struebig, Morel, Danielsen, Bruhl, Donald and Phalan2008). Papua New Guinea saw a 72.3% increase in the planted area of oil palm from 1989 to 2013 of which 25% came from deforestation (Vijay et al. Reference Vijay, Pimm, Jenkins and Smith2016). Despite the conservation commitments of the largest palm oil company, New Britain Palm Oil Ltd (e.g. “no new plantings in High Carbon Stock take place in forest areas identified for conservation”; http://poig.org/poig-verification-indicators/), forest on New Britain continues to be cleared for oil palm plantations (Nelson et al. Reference Nelson, Gabriel, Filer, Banabas, Sayer, Curry, Koczberski and Venter2014). Industrial logging also remains a major threat on New Britain, and frequent and high-intensity logging of previously logged forests continues (Bryan and Shearman Reference Bryan and Shearman2015). Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008) analysed forest loss in altitudinal bands between 1989 and 2000 and found an overall rate of loss of 12% and 20% of lowland forest lost. A subsequent analysis between 2002 and 2014 suggested that forest loss had continued, albeit at a slower rate of 2.2% with 5.2% of forest logged across all altitudes (Bryan and Shearman Reference Bryan and Shearman2015). About 70% of New Britain (26,000 km2) was mapped as rainforest in 2014, including logged and unlogged forests at all altitudes (Bryan and Shearman Reference Bryan and Shearman2015).
Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008) assessed the IUCN Red List status of all 38 restricted-range bird species that occur on New Britain. The 23 species assessed as highly forest-dependent were assumed to have declined at a rate similar to that of forest loss (12% over 11 years) within their altitudinal range, or faster if also subject to high rates of hunting or trapping. To assess extinction risk, species that also occur on adjacent islands, including the large islands of New Ireland and Manus, were assumed to be declining at the same rate as New Britain. This is a very conservative approach, as there is much less oil palm on these islands, and New Ireland lost most of its lowland forest before 1989. Based on published and unpublished data, Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008) assessed 13 species as having small total population sizes. Based on Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008), the number of restricted-range species on New Britain that qualified as ‘elevated conservation concern’ (i.e. categorised as Critically Endangered, Endangered, Vulnerable or Near Threatened) increased from 12 to 21 (BirdLife International 2016).
Several of New Britain’s endemic birds remain very poorly known and consequently the estimates of population size and tolerance of forest degradation used in Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008) could be better refined with additional data. No further quantitative bird surveys have been published since Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008). The objective of our study was to use our own extensive bird survey dataset to review and where necessary, redefine the conservation status of New Britain’s restricted-range forest birds. We specifically aimed to assess which bird species were intolerant of degraded forest and to assess and revise population estimates for each of these species.
Methods
Taxonomy
This paper considers forest bird species listed as ‘restricted-range’ by Stattersfield et al. (Reference Stattersfield, Crosby, Long and Wege1998) and/or of elevated conservation concern by BirdLife International (2016). Taxonomy and nomenclature follow BirdLife International (2016). This includes three taxa endemic to the Bismarcks now recognised as species which we consider to be threatened, as they are tolerant of, and indeed more common in, degraded forest habitats: Black-capped Paradise-kingfisher Tanysiptera nigriceps, Ashy Myzomela Myzomela cineracea and Bismarck Crow Corvus insularis. An undescribed species of Microeca flycatcher is still only known from a handful of records and locations - although regularly seen on New Ireland, it has not been seen on New Britain for perhaps decades - and is likely to be threatened (Dutson Reference Dutson2011). BirdLife International (2016) assess the New Britain Flyrobin Monachella coultasi as ‘Near Threatened’. We recorded this non-forest species only on the Sulu River outside our forest surveys.
Source data and site descriptions
Two sources of bird survey data are utilised to complement the historical published and unpublished data used by Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008). The first derives from a study on the impacts of oil palm plantations on biodiversity, in which bird surveys were undertaken in the four largest accessible lowland forests within 50 km of Walindi in West New Britain (Davis et al. unpubl. data). Between 12 January and 12 February 2010, these were undertaken by walking non-overlapping 1-km, 30-minute transects along forest roads and other tracks. All birds seen and heard were recorded, creating a known bias towards species with loud and frequent vocalisations. Surveys were completed by three teams of two surveyors, one person recording and the other surveying.
The second data source derives from less standardised surveys conducted by GD between 8 and 29 August 1997 and between 6 December 1997 and 8 January 1998. These surveys were similar to the 2010 fieldwork, but were undertaken by one person, were of variable length and time, mostly along narrow forest trails, and conducted at multiple locations throughout New Britain. GD was tape-recording and investigating the source of any unknown calls, and estimated that the accuracy of his identification of every call came close to 99% with some known exceptions (e.g. the calls of hawks Accipiter spp.). Additional non-quantitative surveys by GD in 1997–2007 were used to inform the discussions about species status.
All surveys were diurnal and survey locations are shown in Figure 1. At some lowland sites, surveys occurred along roads and tracks that were utilised by villagers on foot and traffic. Although this introduces a potential bias, trial surveys along newly-cut tracks and off-track generally resulted in fewer bird observations because of poor visibility, difficulty of access and noisy walking. Observed biases included lower encounter rates of terrestrial and understory thicket species, and higher encounter rates of some forest-edge species such as cuckoo-doves Macropygia spp.
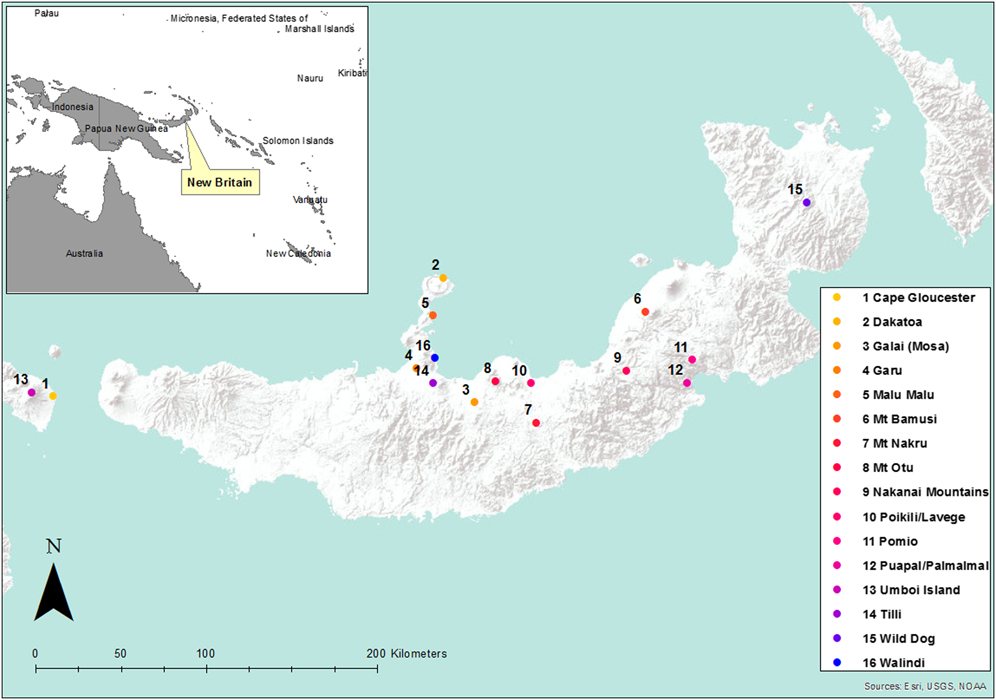
Figure 1. Location of New Britain, Papua New Guinea (inset) showing location of bird survey sites.
Surveys were conducted in seven broad habitat types defined by their elevation and vegetation status as listed below (detailed site descriptions are in Appendix S1 in the online supplementary materials):
Old-growth lowland forest, 0–300 m (79 hours at six sites)
Degraded lowland forest, 0–300 m (85 hours at five sites)
Oil palm plantations, 0–300 m (41 hours at one site)
Old-growth hill forest, 300–950 m (43 hours at one site)
Degraded hill forest, 300–950 m (19 hours at one site)
Old-growth montane forest, 700–1,400 m (28 hours at four sites)
Degraded montane forest, 700–1,400 m (15 hours at one site)
Degraded forest had been logged, had many subsistence gardens and regrowth, or had been significantly damaged by cyclones. Degraded forest retained large trees and patches of shaded closed-canopy – we did not survey more heavily degraded forests from which nearly all large trees had been extracted by multiple logging cycles or ongoing logging by landowners. Oil palm plantations were a monoculture of oil palms Elaeis guineensis. The divisions between the altitudinal categories were not clear-cut, but hill forest was dominated by steep ridges with lower trees, and montane forest had exposed mountain slopes with stunted vegetation.
Data analysis
All records from a particular habitat type were converted to an encounter rate per hour as follows:
Encounter rate = sum[total no. birds recorded in all surveys]/sum[total no. hours]
The total numbers of individual birds recorded visually or aurally were combined, and uncertain records were discarded.
Two parameters of particular relevance to the IUCN Red List status of these species were estimated. Species that were entirely or largely recorded in old-growth forest, and seldom or never recorded in degraded habitat were designated as ‘highly’ dependent on old-growth forest, species largely found in primary forest but regularly recorded, and may breed, in degraded habitat were designated as ‘moderate’ and species that may occur in primary forest, but were more abundant and breed in degraded habitat were designated as ‘low’, following Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008). It is emphasised that ‘degraded’ habitat is only lightly or moderately degraded (see above) and these ‘moderately’ tolerant species do not necessarily use heavily degraded habitat, such as forest that has been heavily re-logged.
Population size was estimated to fit into the intervals relevant to the IUCN Red List categories and criteria, based on a range of factors including encounter rate, estimated detectability, other published and unpublished records (notably as summarised in BirdLife International 2016), Extent of Occurrence, Area of Occupancy and population estimates for similar species given in BirdLife International (2016). We then compared our results to the estimates in Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008), explaining any deviations.
Results
A total of 415 hours of survey effort were available for analysis. A total of 17 species listed as being of elevated conservation concern by BirdLife International (2016) were recorded by us (Table 1), representing all species of elevated conservation concern, except for three species listed as ‘Vulnerable’: New Britain Bronzewing Henicophaps foersteri, Golden Masked-owl Tyto aurantia and Bismarck Thicketbird Megalurulus grosvenori.
Table 1. Encounter rates (records/100 hours and rounded to the nearest whole number) of bird species of elevated conservation concern on New Britain in each habitat type; and dependence on old growth forest population estimates compared with Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008). Full species names are listed in the species discussion below. Deviations from Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008) are in bold.
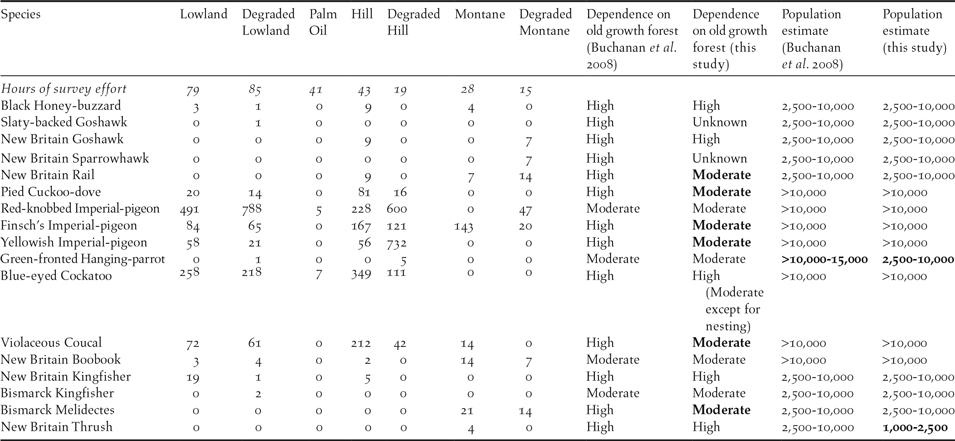
Among the most rarely recorded species in our surveys were the endemic birds of prey, including three ‘Vulnerable’ raptors: Slaty-backed Goshawk Accipiter luteoschistaceus, New Britain Goshawk Accipiter princeps and New Britain Sparrowhawk Accipiter brachyurus (Table 1). The ‘Near Threatened’ Bismarck Hanging-parrot Loriculus tener, ‘Vulnerable’ Bismarck Kingfisher Ceyx websteri and ‘Near Threatened’ New Britain Thrush Zoothera talaseae were also rarely encountered (Table 1). Three ‘Near Threatened’ species: Red-knobbed Imperial-pigeon Ducula rubricera, Finsch’s Imperial-pigeon Ducula finschii and Yellowish Imperial-pigeon Ducula subflavescens, as well as the ‘Vulnerable’ Blue-eyed Cockatoo Cacatua ophthalmica and ‘Near Threatened’ Violaceous Coucal Centropus violaceus were the most frequently encountered restricted-range, or elevated conservation concern species (Table 1).
Based on our observations, we considered all species of elevated conservation concern, with the possible exception of the Red-knobbed Imperial-pigeon and Yellowish Imperial-pigeon, to have a moderate to high dependence on old growth forest habitats (Table 1).
Individual species discussion
The current IUCN Red List category and criteria are given for each species based on BirdLife International (2016), along with our suggested revisions. We note that some categories were updated by BirdLife International (2017), based on a draft of this paper. We note that rates of species decline used for criterion C1 must be estimated from census data or an index of abundance, and cannot be inferred from indirect evidence such as rates of habitat loss (IUCN 2016). Criterion C1 had previously been used where the rates of habitat loss greatly exceed the thresholds, but current lower rates of forest loss no longer support this use.
Black Honey-buzzard
Henicopernis infuscatus – Vulnerable A2cd+3cd+4cd; C1+2a(ii); suggested: Vulnerable A2c; C2a(ii)
This species was probably under-recorded, especially when perched, as all records except one were of birds in flight above the canopy. A number of authors have commented on its rarity, but at least 30 records were noted in BirdLife International (2016), which together with our records support a (precautionarily) estimated population size of 2,500–10,000 mature individuals and the current IUCN Red List categorisation. However, we found no evidence of direct human interactions with this species, and suggest revising the categorisation to exclude the ‘d’ criterion for human exploitation. Further, given that the rate of habitat loss measured by Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008) has slowed significantly (Bryan and Shearman Reference Bryan and Shearman2015), we suggest that the species now only qualifies under criterion A2c for past declines in habitat extent and quality.
Slaty-backed Goshawk
Accipiter luteoschistaceus – Vulnerable C2a(ii); suggested: Vulnerable C2a(ii)
We reassess this species to have a single subpopulation (sensu IUCN 2016), as individuals on Umboi (Diamond Reference Diamond1971) are considered here to be able to cross the 25-km deep-water barrier to New Britain. This is based on the lack of subspecific variation, the ability of the likely allospecies A. albogularis to cross water (Mayr and Diamond Reference Mayr and Diamond2001) and the distribution of most New Guinea congeners on multiple smaller islands (Beehler and Pratt Reference Beehler and Pratt2016). Although we are not presenting new evidence on the species’ population structure, we are making a more precautionary interpretation of the limited evidence. We observed one individual and note the difficulty of estimating the population size of this very poorly known species. The fact that its vocalisations are unknown (Dutson Reference Dutson2011) and the relatively high rate of captures in mist-nets (two from only a handful of recent records; Richards and Gamui Reference Richards and Gamui2011, BirdLife International 2016) suggest that it may be more common than suspected, but unobtrusive and overlooked. Although the population might be < 2,500 mature individuals, which would qualify the species as ‘Endangered’ C2a(ii), we think it is more likely that the population is 2,500–10,000 and hence that the species is ‘Vulnerable’ C2a(ii).
New Britain Goshawk
Accipiter princeps – Vulnerable C2a(ii)
This species was recorded five times (Table 1), more often than the single records of A. luteoschistaceus and A. brachyurus, and without further data, we retain Buchanan et al.’s (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008) estimate of 2,500–10,000 mature individuals. Although we recorded one in degraded montane forest, we retain Buchanan et al.’s (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008) assessment of the species as highly dependent on old-growth forest.
New Britain Sparrowhawk
Accipiter brachyurus – Vulnerable C2a(i); suggested: Vulnerable C2a(ii).
As with A. luteoschistaceus, we reassess this species to have a single subpopulation (sensu IUCN 2016), as individuals on New Ireland (Beehler and Alonso Reference Beehler and Alonso2001) are considered here to be able to cross the 25-km deep-water barrier to New Britain. This is based on the limited evidence of other congeners crossing between islands (Mayr and Diamond Reference Mayr and Diamond2001). Although we are not presenting new evidence on the species’ population structure, we are making a more precautionary interpretation of the limited evidence. This species is also very rarely recorded, with one record from our observations, and the few additional records presented without evidence or discussion may be misidentifications (e.g. Richards and Gamui Reference Richards and Gamui2011). However, it appears to be more common on New Ireland where it was recorded on four out of 13 days in montane forest (Beehler and Alonso Reference Beehler and Alonso2001). Although the population might be < 2,500 mature individuals, categorising the species as ‘Endangered’ C2a(ii), without further evidence, we retain the population estimate of 2,500–10,000 and hence that the species is ‘Vulnerable’ C2a(ii).
New Britain Rail
Hypotaenidia insignis – Near Threatened C1; suggested: Near Threatened C2a(ii)
Despite the published literature suggesting that this is a rare species of lowland habitats (Bishop Reference Bishop1983, Bishop and Jones Reference Bishop and Jones2001), we recorded none ourselves in lowland forest (however, it was reported from this habitat by local people). On the other hand, we recorded several individuals in hill and montane forest. Based on our two sightings in logged forest, one in old-growth forest, and five in grassy roadsides in old-growth forest, the New Britain Rail appears to be tolerant of degraded forest. Any decline in the lowlands needs further investigation, especially as other flightless rails in the Solomon Islands co-exist with cats, dogs and hunters (GD pers. obs.). We suggest that the lowland records indicate a single subpopulation, and that it should be recategorized as ‘Near Threatened’ C2a(ii).
Yellow-legged Pigeon
Columba pallidiceps – Vulnerable C1
Although we failed to record this species on our quantitative surveys, we recorded one near sea-level at Poikili and there have been a handful of other recent records from New Britain (BirdLife International 2016). Given the similar paucity of records from across its range (BirdLife International 2016), with the exception of multiple records from Makira (Solomon Islands), this is consistent with the BirdLife International (2016) estimation of 2,500–10,000 mature individuals. (To review its threat status, more information is needed on the species’ tolerance of logged forest and rates of logging on Makira.)
Pied Cuckoo-dove
Reinwardtoena browni – Near Threatened A2c+3c+4c; suggested: Least Concern
Our encounter rates of 0.14 birds/hour in degraded lowland forest compared to 0.20 birds/hour in old-growth, and 0.16 birds/hour in degraded hill forest compared to 0.81 birds/hour in old-growth (Table 1) suggest that it is more tolerant of degraded forest than implied in Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008). However, our observations suggest that it is intolerant of heavily degraded forest with limited canopy cover. Given the current rates of about 2.7 % habitat loss plus about 6.5% logged in three generations (Bryan and Shearman Reference Bryan and Shearman2015), we suggest that it is categorised as ‘Least Concern’ (LC).
New Britain Bronzewing
Henicophaps foersteri – Vulnerable C1+2a(i); suggested: Vulnerable C2a(ii)
We reassess this species to have a single sub-population (sensu IUCN 2016) as individuals on Umboi (Diamond Reference Diamond1971) are considered here to be able to cross the 25-km deep water barrier to New Britain. This is based on an old record from the offshore island of Lolobau, the lack of any subspecific variation, and the ability of other similar ground-pigeons to cross water (Mayr and Diamond Reference Mayr and Diamond2001). Although we are not presenting new evidence on the species’ population structure, we are making a more precautionary interpretation of the limited evidence. We recorded one bird outside the quantitative surveys at 75 m in old-growth forest; although its call is known (Dutson Reference Dutson2011), this is one of only five records since the 1980s listed by BirdLife International (2016). Although the population might be < 2,500 mature individuals, categorizing the species as ‘Endangered’ C2a(ii), we think it is more likely that the population is 2,500–10,000 and hence that the species qualifies as ‘Vulnerable’ C2a(ii).
Red-knobbed Imperial-pigeon
Ducula rubricera – Near Threatened A2c+3c+4c; suggested Least Concern.
This vocal species had the highest encounter rate of any bird in this study and encounter rates were about twice as high in degraded forest as old-growth forest, suggesting that it is more tolerant of degraded forest than implied in Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008). This is supported by our observations elsewhere in the species range, suggesting that it may be better categorised as ‘Least Concern’ (LC), especially given the slower rate of forest loss across New Britain and New Ireland between 2002 and 2014 (Bryan and Shearman Reference Bryan and Shearman2015) and elsewhere in its range.
Finsch’s Imperial-pigeon
Ducula finschii – Near Threatened A2c+3c+4c; suggested Least Concern
With encounter rates in degraded lowland and hill forest more than half the rates in old-growth forest (Table 1), our surveys suggest that it is more tolerant of degraded forest than implied in Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008), and it may be better categorised as ‘Least Concern’ (LC), especially given the slower rate of forest loss across New Britain and New Ireland between 2002 and 2014 (Bryan and Shearman Reference Bryan and Shearman2015).
Yellowish Imperial-pigeon
Ducula subflavescens – Near Threatened A2c+3c+4c; suggested Least Concern
This species’ habit of flying large distances might result in higher detectability in open degraded forests and in hills, and might obscure a dependency on coastal and riparian habitats. Otherwise, it may be better categorised as ‘Least Concern’ (LC) given the slower rate of forest loss across New Britain, New Ireland and Manus between 2002 and 2014 (Bryan and Shearman Reference Bryan and Shearman2015).
Green-fronted Hanging-parrot
Loriculus tener - Near Threatened C1; suggested Least Concern.
This unobtrusive species with an unremarkable call is likely to have been overlooked and we recorded few individuals, all in the lowlands (our survey records are from 0 to 500 m, and additional opportunistic records are from 0 to 100 m). We suggest that its population is precautionarily re-estimated as 2,500–10,000 mature individuals across its range (New Britain, New Ireland, Lavongai = New Hanover and Duke of York). Given the slowed rate of forest loss across New Britain and New Ireland (Bryan and Shearman Reference Bryan and Shearman2015) and that the species does not qualify for listing under C1, we suggest that it may be better categorised as ‘Least Concern’ (LC), but highlighted as a distinctly uncommon species.
Blue-eyed Cockatoo
Cacatua ophthalmica – Vulnerable A2cd+3cd+4cd; suggested Near Threatened A2c+3c+4c
Although it has a long generation time (about 10 years) and is likely to be dependent on large hollow-bearing trees (Marsden and Pilgrim Reference Marsden and Pilgrim2003), it was commonly detected in all lowland and hill habitats, the rate of forest loss slowed to about 2.2% loss plus 5.2% logged between 2002 and 2014 (Bryan and Shearman Reference Bryan and Shearman2015), and we saw no evidence for any trapping for trade. This supports re-categorisation as ‘Near Threatened’ A2c+3c+4c.
Violaceous Coucal
Centropus violaceus - Near Threatened A2c+3c+4c; suggested Least Concern.
Our surveys suggest that it is more tolerant of degraded forest than implied in Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008), with nearly equal encounter rates in primary and degraded lowland forest, although it was much more common in primary hill forest than degraded (Table 1). Together with the slowing rate of forest loss, these data suggest that it may be better categorised as ‘Least Concern’.
Golden Masked-owl
Tyto aurantia – Vulnerable C1+2a(ii); suggested Vulnerable C2a(ii)
Despite asking our local guides and other informants, we were never able to confirm the presence of this species. However, there have been subsequent records in and around oil palm plantations at Walindi. The current population estimate of 2,500–10,000 mature individuals is consistent with that for other unobtrusive forest Tyto owls, but the indication that it might regularly feed in oil palm plantations needs further investigation.
New Britain Boobook
Ninox odiosa – Vulnerable A2c+3c+4c; suggested Near Threatened A2c+3c+4c.
Our opportunistic nocturnal records suggest that it is relatively tolerant of degraded forest, as implied in Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008), including almost nightly calling individuals at Walindi. Given the slowing rate of forest loss, it may be better categorised as ‘Near Threatened’ A2c+3c+4c.
New Britain Kingfisher
Todiramphus albonotatus – Near Threatened C1; suggested Vulnerable C2a(ii).
Although the calls of this endemic kingfisher are not particularly distinctive, the small number of records (25; all except one from old-growth forest and all except three from < 300 m altitude) is consistent with the precautionary population estimate of 2,500–10,000 mature individuals in Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008). Given the slowed rate of forest loss (Bryan and Shearman Reference Bryan and Shearman2015), we suggest that it is categorised as ‘Vulnerable’ C2a(ii).
Bismarck Kingfisher
Ceyx websteri - Vulnerable A2c+3c+4c; C1+2a(i); suggested Vulnerable A2c; C2a(i)
Although more of our opportunistic records were from degraded forest, this may have been an artefact of easier access to suitable small rivers in degraded habitats. However, given this apparent tolerance of degraded habitats, and the slowed rate of forest loss (Bryan and Shearman Reference Bryan and Shearman2015), we suggest that the species no longer meets the criteria for ‘Vulnerable’ A3c+4c or C1.
Bismarck Thicketbird
Megalurulus grosvenori – Vulnerable D1
We have no further information to add as there have been no further records of the Bismarck Thicketbird, which is known only from two specimens collected in karst forest in the Whiteman Mountains at about 1,575 m (Gilliard Reference Gilliard1960). Its habitat requirements are poorly known. It is notable that we recorded Rusty Thicketbird Megalurulus rubiginosus (LC) at 1,400 m, the highest altitude where we accessed (non-karst) forest, as did Richards and Gamui (Reference Richards and Gamui2011) in karst forest at 1,600 m.
Bismarck Melidectes
Melidectes whitemanensis –Near Threatened C1; suggested Near Threatened C2a(ii).
Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008) estimated the rate of habitat loss for this species at 6% over three generations based on an altitudinal range of 1,200–1,800 m, but we recorded this species as low as 900 m. We believe it may have a single subpopulation, and suggest that it is categorised as ‘Near Threatened’ C2a(ii).
New Britain Thrush
Zoothera talaseae – Near Threatened C1+C2a(i); suggested Least Concern
Although we recorded it in small numbers, it is known from multiple sites across New Britain and also Umboi, and is unlikely to meet criterion D. Buchanan et al. (Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008) estimated the rate of habitat loss for the species based on an altitudinal range of 600–1,500 m, but it is generally recorded > 1000 m (Dutson Reference Dutson2011). We have seen no evidence of logging in its habitat, or any other anthropogenic threats (congeners appear to co-exist with introduced cats and rats), and suggest that it is categorised as ‘Least Concern’.
General discussion and recommendations
Based on new data presented here, we recommend downward revisions (worsened status) for only one species, the New Britain Kingfisher (Table 1). We recommend upward revisions (improved status) for seven species: Pied Cuckoo-dove, Yellowish Imperial Pigeon, Green-fronted Hanging Parrot, Blue-eyed Cockatoo, Violaceous Coucal, New Britain Boobook and New Britain Thrush. (Table 1). Despite our widespread and comprehensive surveys, the Slaty-backed Goshawk, New Britain Sparrowhawk, New Britain Bronzewing, Golden Masked-owl, New Britain Flyrobin and New Britain Thrush remained very rarely recorded. The New Britain Sparrowhawk and New Britain Thrush were recorded in montane forest and due to the current low anthropogenic impacts on this habitat, may be under less threat than lowland forest-dependent species (e.g. Slaty-backed Goshawk and New Britain Bronzewing were each only recorded once, in lowland forest). However, the ecology of most species and their dependence on lowland forest is poorly known and requires further investigation. We stress that the lack of widespread survey efforts and very poor ecological knowledge of most species, means that estimates of conservations status are only as robust as the available information.
Although lowland forest loss (Buchanan et al. Reference Buchanan, Butchart, Dutson, Pilgrim, Steininger, Bishop and Mayaux2008) is the key threat to most birds in New Britain, emerging threats include logging at higher altitudes (observed by the authors) facilitated by the use of helicopters to access remote terrain, hunting of all bird species with guns and catapults, fragmentation of old-growth forest by logging access and village development roads, and the loss of important habitat trees (e.g. nesting hollows) due to local timber extraction. Overall rates of forest loss have declined in New Britain and this may be attributable to accessible forests being logged out as well as responsible oil palm companies addressing consumer concerns about forest loss. Conversely, forests are being re-logged at short intervals and with high extraction rates, and significant areas are being categorised as Special Agricultural Business Leases and clear-felled (Nelson et al. Reference Nelson, Gabriel, Filer, Banabas, Sayer, Curry, Koczberski and Venter2014, Bryan and Shearman Reference Bryan and Shearman2015).
In the face of ongoing habitat loss and these emerging threats, we recommend targeted surveys and threat assessments for enigmatic species that were not recorded in our study: Golden Masked-owl, New Britain Bronzewing, New Britain Flyrobin and New Britain Thicketbird. Besides sightings of these species, information about Slaty-backed Goshawk and New Britain Sparrowhawk should also be solicited from tour operators and visiting birdwatchers. More importantly, the legal validity and equity of Special Agricultural Business Leases needs to be addressed (Nelson et al. Reference Nelson, Gabriel, Filer, Banabas, Sayer, Curry, Koczberski and Venter2014), re-logging needs to be much more closely monitored and regulated, and the remaining large areas of unlogged lowland forest need serious consideration for legal protection against logging.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270917000156
Acknowledgements
The authors are grateful to two anonymous referees for their constructive comments. GD and RD gratefully acknowledge logistic support from Walindi Plantation Resort. D. Stojanovic, H. Cook, G. Barnett and E. Wagner also assisted RD with surveys. RD undertook surveys under ECU Animal Ethics permit 4664.






