Introduction
Approximately 300,000 Canadians are living with epilepsy, half of whom are women, and the majority (75%) are diagnosed before age 30. 1,Reference Ng, Maxwell and Yates2 Seizure control during pregnancy remains, by far, the primary indication for antiepileptic drug (AED) usage in pregnancy, followed by pain and migraine management. Reference Tomson, Battino and Craig3,Reference Leong, Mamdani, Gomes, Juurlink, Macdonald and Yogendran4 Seizures during pregnancy have been associated with maternal physical injury, metabolic acidosis, fetal heart rate deceleration, and low birth weight. Reference Chen, Chiou, Lin and Lin5–Reference Liporace and D’Abreu8 In utero AED exposure, on the other hand, has been associated with a 2–3 fold increase in the incidence of major congenital malformations (MCM) such as neural tube defects, cleft palate, cardiac, and urogenital defects. Reference Nau, Zierer, Spielmann, Neubert and Gansau9–Reference Tomson, Battino and Bonizzoni11 Large population-based registries of pregnant women exposed to AED have been instrumental in helping clinicians discuss these competing risks with their patients.
The North American AED Pregnancy Registry (NAAPR) started enrolling pregnant women from Canada and the United States of America (USA) in 1997. NAAPR’s mission is to document the frequency of MCM among infants with in utero AED exposure.
Already, NAAPR has provided information that has been of great use to clinicians counseling women with epilepsy (WWE). An analysis of 7370 pregnancies exposed to AED monotherapy and 479 AED-unexposed pregnancies between 1997 and 2011 suggested that newer AEDs may have a lower risk of MCM. The risk of MCM varied between 9% for valproate (CI95%: 6–13) and 1% for gabapentin (CI95%: 0–4). A dose-effect relationship was furthermore found between the incidence of MCM and in utero exposure to valproic acid: the risk of MCM was 26% (CI95%: 8–35) with daily maternal intake of >1500 mg of valproic acid, compared with 4% (CI95%: 1–8) for a maternal daily intake of ≤500 mg. Reference Hernández-Díaz, Smith and Shen12
Canada does not have its own separate prospective registry of AED use in pregnancy. It is therefore crucial to have a significant proportion of Canadian pregnant women enrolled in NAAPR. There are several reasons why the population of women of childbearing age in Canada may be different from that in the USA. Healthcare delivery and access, geography, immigration, fertility, age composition, Reference Barbieri and Ouellette13 overall maternal mortality rates, Reference Hoyert, Danel and Tully14,15 rates of adolescent pregnancy, Reference Langille16 and comorbidities in pregnancy Reference Albrecht, Maloni, Thomas, Jones, Halleran and Osborne17 are indeed some of the factors that may lead to diverging populations of pregnant women between the two countries. Of note, these factors may also differ among the different Canadian provinces.
The objectives of this study were twofold: first, to estimate the contribution of Canadian women to NAAPR since its inception in 1997 and, second, to evaluate whether AED usage differed significantly between Canadian pregnant women and their American counterparts.
Materials and Methods
Patient Enrollment
Women taking one or more AED, residing in Canada or the USA, and became pregnant between January 1, 1997, and December 31, 2019, were eligible for enrollment into NAAPR. Enrollment for pregnant women on no AED (controls) was also introduced in 2003. Restrictions were not imposed on maternal age nor on indication for AED usage. Initially, recruitment relied mostly on physician advocacy – via association conference meetings, letters to neurologists and obstetricians, and newspaper advertisement targeted at women of childbearing age. Eligible women were asked to self-enroll by calling a toll-free number, which is still in use (1-888-233-2334). Reference Holmes and Wyszynski18 In the early 2000s, a website was created (www.AEDPregnancyRegistry.org). Currently, healthcare professionals cannot directly enroll their patients in the registry, but they can refer them to the aforementioned website or toll-free number. NAAPR also makes pamphlets and posters that can be displayed in healthcare providers’ offices. Over the past decade, NAAPR has made significant efforts towards diversifying its outreach approach: a communication consultant was hired to reach women of childbearing age, the website was upgraded, and content linked to the NAAPR webpage posted on social media regularly (i.e., Facebook, Instagram, Twitter, and LinkedIn). A Canadian Epilepsy Awareness Month email was created and sent to those located in Canada who have opted to receive communication via the NAAPR website: as of January 2021, it had 354 subscribers. Data on method of recruitment (i.e., healthcare professional referral, website, or social media) were collected starting in 2015. This allowed a comparison of the methods of recruitment between the USA and Canada, using a chi-squared test for trend for overall discrepancies, and proportional Z-tests for individual methods of enrollment.
Data Collection
Pregnant women enrolled into NAAPR were asked to complete a questionnaire at three different time points: at time of enrollment, at 7 months of gestation, and 8–12 weeks after delivery. Data on country and province of residence and method of recruitment were collected at the time of enrollment. Data on medication usage (including AED and other potential teratogenic agents) were obtained at each time point. Through interviews conducted by the research coordinator at 7 months of gestation and at 8–12 weeks of life, pregnancies that resulted in miscarriage, stillbirth, or neonatal deaths were identified. MCM identification was performed by the study teratologist (LBH) who reviewed the primary physician’s clinical notes recorded at all office visits through age 12 weeks. Consultation reports from disciplines such as cardiology, urology, orthopedics, or genetics were also reviewed. Malformations were defined as a structural abnormality with surgical, medical, or cosmetic importance. To ensure consistency in the classification of specific physical findings, a “Decision Tree” has been maintained since NAAPR was established in 1997. If signs or symptoms of a possible MCM (e.g., heart murmur, urinary tract infection) were identified within the first 12 weeks of life, but a diagnosis of MCM was not yet reached, copies of imaging and other diagnostic studies’ reports were obtained, and contact with the mother maintained until a diagnosis of MCM could be excluded or confirmed.
Enrollment Rate Ratio to NAAPR Among Canadian Women: Comparison with the USA
An enrollment rate to NAAPR was calculated for both Canada and the USA. These “national” enrollment rates consisted of the number of subjects enrolled into NAAPR from the corresponding country, on the numerator, and the population of females aged 15–44 years old of that country, on the denominator. These “national” enrollment rates were calculated for the entire study period and for each individual year of study between 1997 and 2019. The eligible population of each country (i.e., the population of women aged 15–45) was obtained using age-stratified annualized person-years from the US Census Bureau and Statistics Canada. 19–23 Canadian Enrollment rate ratios (ERR) to NAAPR were then measured by dividing the national enrollment rate from Canada by that of the USA. A Canadian ERR of 1.0 signified that Canadian women of childbearing age were as likely as their American peers to be enrolled into NAAPR. Poisson confidence intervals were used to assess whether the Canadian ERR diverged significantly from that null value of 1. Reference Fay24
An expected annual number of Canadian enrollees into NAAPR could also be calculated by multiplying the Canadian population eligible for enrollment (i.e., Canadian females aged 15–45 on a given year) by the American enrollment rate into NAAPR for that same year. This yielded an expected value of Canadian enrollees to NAAPR which assumed an identical rate of enrollment between Canada and the USA. The expected number of Canadian enrollees was compared with the observed number of Canadian enrollees to NAAPR. To facilitate this comparison, a graphical representation of the annualized expected number of Canadian enrollees into NAAPR was juxtaposed with the observed value.
Provincial Enrollment Rate Ratios: Comparison with the Canadian Average
To evaluate for inter-provincial differences in enrollment rates to NAAPR, a provincial ERR was calculated for each province, using the provincial enrollment rate on the numerator and the national Canadian enrollment rate on the denominator. This calculation was performed for the last 5 years under study (2015–2019), as this was felt to be the most relevant period to guide future actions aimed at increasing enrollment rates from provinces with the lowest relative contribution to NAAPR. A province with an ERR of 1.0 therefore has the same rate of enrollment to NAAPR, on average, as the rest of Canada. A province with an ERR above 1.0 had a higher enrollment rate than the Canadian average and vice versa for a provincial ERR below 1.0. A Poisson distribution was used to provide 95% confidence intervals and assess for statistically significant difference in rates of enrollment between the different Canadian provinces and the national average. For ease of interpretation, the provincial ERRs were represented geographically, color-coded, and grouped by quintiles.
Comparing AED Usage in Canada and the USA
Differences in the proportion of pregnant women on polytherapy between the two countries country were assessed for using a chi-square test. For pregnant women on monotherapy, a chi-squared test for trend was performed to analyze for overall differences in AED prescription patterns between the two countries. To look for differences in the prescription of specific AEDs, two-sample proportional Z-tests were performed for each individual AED. The study’s ability to detect a difference in the proportion of women on a given AED was estimated by calculating Cohen’s h coefficient, assuming a power of 80% and a risk of alpha-error of 0.05. Reference Cohen25
Data Accessibility and Statistical Analysis
The complete deidentified dataset used in this study and the statistical analysis R code file is available upon request to the corresponding author. R statistics was used for statistical analysis. 26–Reference Teucher and Russell31 Statistical significance was defined as p < 0.05 unless otherwise specified.
Ethics
This study was approved by the Institutional Review Board of the MassGeneral Hospital for Children (Protocol number 1999P008168) and reviewed annually.
Results
During the study period, 10,215 pregnant women from both countries were enrolled into NAAPR. While 9220 pregnant women were exposed to AEDs, 995 were unexposed and acted as controls. Of the total, 432 (4.1%) were enrolled in Canada (Figure 1). Three-hundred-sixty-two pregnant Canadian women were exposed to AED while 70 were controls. The total observed number of Canadian women enrolled into NAAPR (n = 432) was significantly lower than expected based on the relative population weight of Canadian women of reproductive age within North America (expected n = 1081 women). This translated into a calculated Canadian national ERR of 0.39 (CI95% [0.35–0.43], p < 0.01). A pattern of low relative enrollment from Canada was observed for every year under study (Figure 2).

Figure 1: Observed enrollment to NAAPR by country (1997–2019). Observed enrollment to NAAPR by country between 1997 and 2019. Two landmark events are emphasized with dotted lines: the opening of enrollment to pregnant women unexposed to AEDs (controls) in 2003 and the inauguration of a social medial campaign in 2016. NAAPR: North American Antiepileptic drug Pregnancy Registry. Made using R statistics. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/.

Figure 2: Observed and expected Canadian enrollment to NAAPR (1997–2019). The expected number of Canadian women enrolled to NAAPR based on enrollment rates among American women of childbearing age is marked by the blue line. The observed number of Canadian women enrolled to NAAPR is marked by red bars. Population data were obtained from the US Census Bureau and Statistics Canada. NAAPR: North American Antiepileptic drug Pregnancy Registry. Made using R statistics. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/.
For the 5-year period spanning 2015–2019, geographic variations in enrollment rates to NAAPR were seen within Canada (Table 1; Figure 3). No women from the northern territories (North-Western Territories, Nunavut, and Yukon) or Prince Edward Island were enrolled during that time period. Quebec, with 16 women enrolled during that time, made a contribution to NAAPR below its population weight among the Canadian population (provincial ERR = 0.35[CI95%: 0.19–0.58], p < 0.001). At the other end of the spectrum, Nova Scotia had the highest relative enrollment rate within Canada (provincial ERR = 1.55, [CI95%: 0.66–3.11], p = 0.32), followed by Saskatchewan (provincial ERR = 1.33, [CI95%: 0.60–2.58], p = 0.49). In Ontario, the most populous Canadian province, a trend could also be seen towards a contribution that was above the Canadian average (ERR = 1.24, [CI95% 0.97–1.57], p = 0.08).
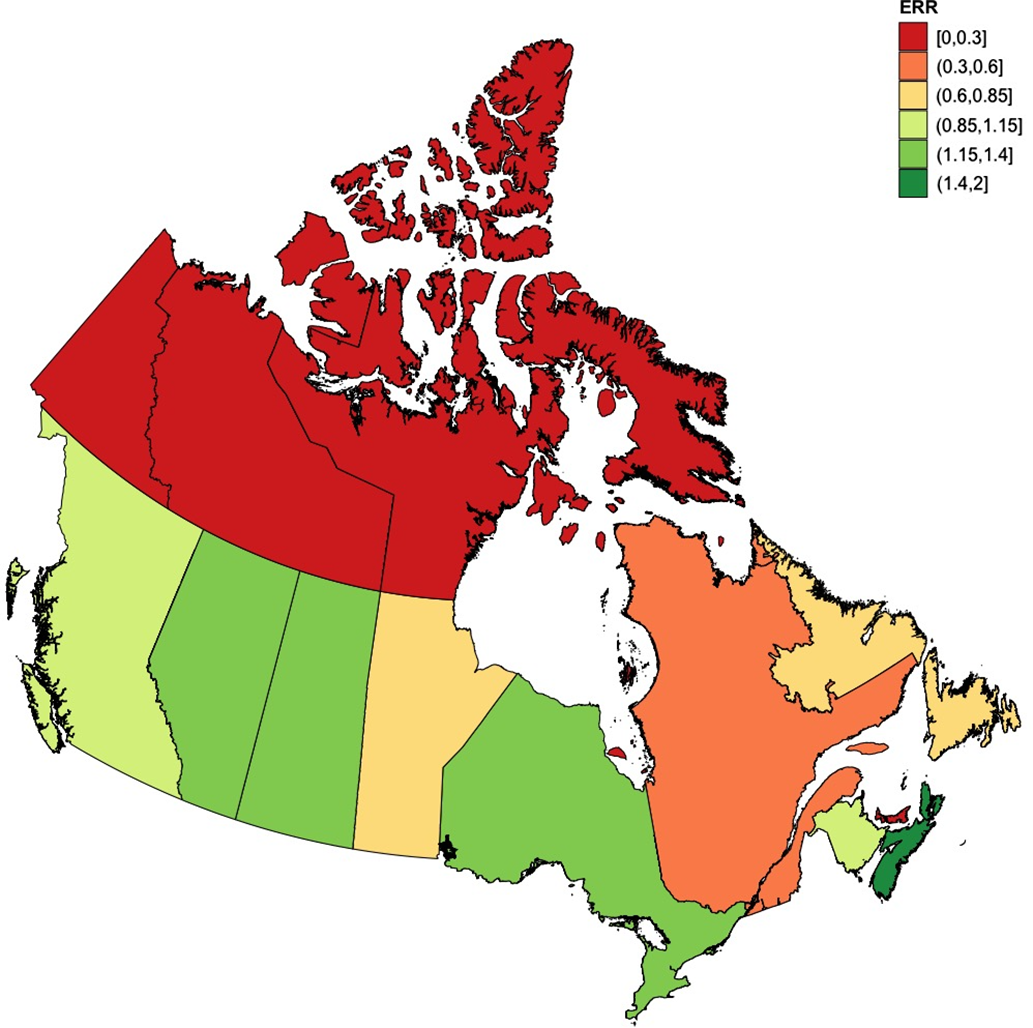
Figure 3: NAAPR enrollment rate ratios by Province and Territory (2015–2019). Enrollment rate ratios to NAAPR by province and territory, using the average incidence rate of enrollment of Canada as comparator. Red denotes absence of participant enrollment, yellow denotes enrollment close to the Canadian average, and green signifies an enrollment rate above the Canadian average. Population data were obtained from Statistics Canada. ERR = Enrollment rate ratio; NAAPR = North American Antiepileptic drug Pregnancy Registry. Made using R statistics. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/.
Table 1: Provincial enrollment rate ratios to NAAPR (2015–2019)

CI = confidence interval; ERR = enrollment rate ratio; NAAPR = North American Antiepileptic drug Pregnancy Registry; NWT = Northwest Territories; PEI = Prince Edward Island; PY = person-years.
a Female population aged 15–44 years old between 2015 and 2019.
** p < 0.001.
From 2015 to 2019, methods of patient enrollment into NAAPR differed significantly between Canada and the USA (χ2=48, p < 0.01) (Table 2). Compared with their American peers, Canadian women were significantly less likely to be enrolled into NAAPR via their healthcare professional (20.3% vs. 44.0; χ2=20.8; p < 0.01) and more commonly enrolled via the organization website (34.9% vs. 21.4%; χ2=11.5; p < 0.01) or through social media (44.8% vs. 34.6%; χ2=8.5; p = 0.04).
Table 2: Methods of enrollment to NAAPR by country (2015–2019)
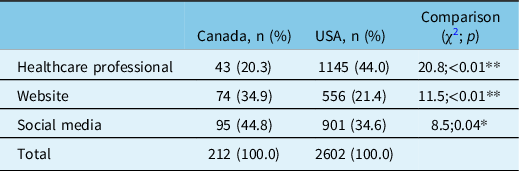
NAAPR = North American Antiepileptic drug Pregnancy Registry.
p < 0.05; **p < 0.05.
Twenty-five percent of Canadian pregnant women were on polytherapy (n = 90), compared with 21% of American pregnant women (n = 1873, χ2=2.3, p = 0.13) (Table 3). The study was, however, underpowered (power = 43%, alpha = 0.05) to detect a difference of this scale between the two countries. For women on monotherapy, the overall AED prescription pattern was not statistically different between Canada and the USA (χ2=0.26, p = 0.61). Nonetheless, when assessing specific AED usage, differences could be discerned between the two countries. Compared with their American peers, Canadian pregnant women on monotherapy were indeed more likely to report taking carbamazepine (24% vs. 15%; χ2=12.0; p < 0.01) or valproic acid (8% vs. 4%; χ2=5.4; p = 0.02) and less likely to report taking lamotrigine (25% vs. 33%; χ2=7.4; p < 0.01) (Table 3).
Table 3: Antiepileptic drug usage among pregnant women in Canada and the USA (1997–2019)

USA = United States of America.
a Percentages of patients on polytherapy are expressed as a proportion of total women enrolled from each country.
b Percentages for the different agents used as monotherapy are as a percentage of total women under monotherapy for each country.
*p < 0.05; **p < 0.01.
Interpretation
This subgroup analysis of Canadian women enrolled into NAAPR suggests that Canada under-contributed, relative to its population size, to NAAPR’s enrollment for every year between 1997 and 2019. The transborder discrepancy in enrollment rates to NAAPR seems unlikely to be explained by differences in prevalence of epilepsy alone. Previous studies have actually found that the proportion of Canadians living with epilepsy (330,000, or 1.0% of the population) 1 appears similar to that seen in the USA (3,000,000, or 1.1%). Reference Zack and Kobau32
One possible explanation for this discrepancy in enrollment rates is that NAAPR, being established in the USA, has closer ties to a network of clinicians based in that country. This is supported by data on methods of enrollment which show that Canadian women were more likely to have been enrolled to NAAPR through social medial or directly through the website rather than via a healthcare professional. Whether this reflects lack of awareness or lack of engagement among Canadian healthcare providers is difficult to discern.
Differing access to subspecialized epilepsy care is a second potential factor that may explain the discrepancies in enrollment rates between the two countries. Although such disparities in access to care are not clearly supported by the scarce data published on this topic, Reference Burneo, Shariff, Liu, Leonard, Saposnik and Garg33,Reference Burneo, Jette and Theodore34 patients with epilepsy in Canada may be more likely to receive care as part of a multidisciplinary care model, which includes nurse practitioners and general practitioners, who may be less aware of subspecialized registries such as NAAPR. Access to specialized care as a potential contributor to lower Canadian enrollment rate is supported by a loose correlation between the provincial ERR and the provincial number of physicians per capita. For instance, Alberta, Ontario, and Nova Scotia are provinces with high ERR and a relatively high number of physicians per capita. 35 In contrast, the three Northern territories and Prince Edward Island have the lowest numbers of physicians per capita in Canada, mirroring their lack of enrollment into NAAPR. 35
One strategy employed in the USA to increase healthcare professional engagement has been to identify “champions” – front-line healthcare providers with a particular interest in identifying side effects of AED during pregnancy – who are frequently updated on the progress of NAAPR via emails and in-person meetings at scientific conferences. In the future, a similar strategy could be adapted by identifying Canadian healthcare providers willing to act as such “champions.” We hope that creating a core group of advocates for enrollment will be further facilitated by the recent creation of the WWE subcommittee at the Canadian League Against Epilepsy. Other venues to enhance Canadian healthcare professionals’ engagement could include outreach efforts that reflect the multidisciplinary nature of caring for WWE in pregnancy and are aimed more broadly at nurse practitioners, general practitioners, obstetricians, midwives, social workers, patient advocate groups, and neurologists who do not specialize in epilepsy.
A third possible cause for the relatively lower enrollment rate from Canada pertains to differences in language, as suggested by the lower enrollment rates from Quebec, the only primarily francophone province in Canada. In addition to French speakers, Canada’s multilingual population may also struggle to access the translation services that are made available by NAAPR. 36 There is currently the option of using a translation service when completing the questionnaire on the phone, but this is not clearly indicated on the website. NAAPR’s plans to incorporate a multilingual interface on the website, clearly indicating that a translation service is available over the phone, and to make the promotional materials available to be on display in physicians’ office translated may help increase enrollment among non-English speaking Canadian pregnant women.
In addition to linguistic factors, cultural and technical barriers may also contribute to a lower enrollment rate from Canada. This is supported by the absence of enrollment from the Canadian northern territories. These territories may lack healthcare professionals with culturally appropriate training in providing care to Native-Canadian patients and educate them on the possibilities of enrolling in registries such as the NAAPR. Reference Redvers and Blondin37,Reference Arnaert and Schaack38 Northern and remote communities also face challenges with differing access to broadband internet, another possible cause of lower enrollment. 39 Despite these challenges, reaching the Canadian aboriginal and far north populations is necessary to provide a more accurate and representative sample of the Canadian population of women of childbearing age. Interestingly, over the last 3 years of this study, as NAAPR increased its online presence, the gap between Canadian and American enrollment rates appeared to be narrowing (see Figure 2). This suggests that Internet-based methods that transcend borders are a promising path to increase Canadian participation into NAAPR.
With regard to the preliminary analysis of differences in AED usage between the two countries, there is a (nonstatistically significant) trend towards a greater proportion of pregnant women taking polytherapy in Canada than in the USA. This study was underpowered to detect this degree of difference (25% vs. 21%) between the two countries, and further studies with larger sample sizes may be required to confirm this finding. This study also suggests that pregnant Canadian women were more likely to be exposed to carbamazepine and valproic acid and less likely to be taking lamotrigine, than their American peers. Of note, smaller discrepancies in AED usage between the two countries may have escaped detection due to insufficient sample sizes, especially for newer, less commonly prescribed AED. When newer drugs are approved for usage in the Canadian market, publicly funded drug insurance programs often contain costs with several measures which generally encourage clinicians to prescribe lower-cost generic drugs before considering newer, more onerous drugs. Reference Wang, Li, Sweetman and Hurley40–Reference Holtkamp and Theodore42 It is possible that such constraints on non-generic newer AED use may be less prevalent in the USA or that a smaller proportion of women purchase AEDs in the USA via publicly funded medication insurance programs. Data from the Organization for Economic Co-operation and Development indeed suggest that the USA spends more, per capita, on pharmaceuticals than Canada. 43
There are some limitations with this study. For ERR calculations, the true population at risk is the annualized population of pregnant women from each jurisdiction, since pregnancy is a sine qua none condition for inclusion into NAAPR. Due to the lack of reliable and readily available such population data, we used the annualized population of women of childbearing age of each jurisdiction as a surrogate measure of the population eligible for enrollment. It is debatable whether “birth rate” would represent a better estimate of eligible population. Although Canada has a somewhat lower fertility rate than the USA (e.g., 1.47 births per woman vs. 1.64 in 2019), 44,45 this difference alone is unlikely to explain the significantly lower relative enrollment rate to NAAPR noted in Canada. Furthermore, there are other issues with using birth rate as a surrogate to number of pregnancy, including the fact that only viable pregnancies are considered in birth rates and that it does not account for multiple gestations. Regarding the rates of AED usage between Canada and the USA, potential confounders not captured into NAAPR (e.g., socioeconomic status, comorbidities, epilepsy severity) may explain at least part of the discrepancy in AED usage between the two countries. The issue of incomplete exhaustivity noted in the different Canadian provinces furthermore precludes a more in-depth analysis of less commonly prescribed AED, many of which are more recently marketed AEDs.
The study can also count on several strengths. Data collection was performed on a prolonged period (22 years) and included a large number of patients from an understudied population (i.e., Canadian pregnant women taking AEDs). A relatively constant data collection method was used, thus minimizing the risks that year-to-year variations would affect the study’s generalizability. Women provided their location of residence, as opposed to location of care, therefore mitigating the risk of border effect that can occur when, for instance, large academic hospitals are located close to a provincial border.
Much work remains to be done to advance our understanding of the effects of AEDs on developing fetuses, especially in the Canadian context. Future studies are needed to investigate differences in rates of gestational AED usage, polytherapy, access to subspecialized epilepsy care, and MCM between the USA and Canada, and between the various Canadian provinces. We hope that by identifying the current gaps in enrollment to NAAPR, we can, through the framework already established by NAAPR, collect more inclusive comprehensive Canadian-specific data. With greater awareness of the potential teratogenicity of some agents among clinicians, clearer guidelines on caring for WWE, such as the Ontario 2015 Epilepsy Implementation Task Force guidelines, 46 and with increasingly newer AEDs being covered by the various Canadian public drug insurance programs, we are hopeful that positive changes are coming for Canadian women.
Disclosures
We would like to thank the clinicians and women who have been involved with NAAPR and contributed to improving the care of women with epilepsy. SNC and LBH receive salary support from funds provided each year to the Massachusetts General Hospital in Boston by the sponsors and contributors to the North American AED Pregnancy Registry.
Declaration of Author’s Competing Interests
SNC and LBH receive salary support from funds provided each year to the Massachusetts General Hospital in Boston by the sponsors and contributors to the North American AED Pregnancy Registry (NAAPR). NAAPR is sponsored by AbbVie pharmaceuticals, Advanz Pharma Corporation, Greenwich Biosciences, Janssen Pharmaceuticals, Pfizer Inc., Sunovion Pharmaceuticals Inc., UCB, and Zogenic Inc. Contributors to NAAPR are Apotex Inc., Aurobindo Pharma Limited, Cipla Limited, Dr Reddy’s Laboratories, GlaxoSmithKline, Teva Pharmaceutical Industries Ltd, and Validus Pharmaceuticals LLC. No other competing interests were declared.
Statement of Authorship
JH contributed to the conceptual design of the study, statistical analysis of the data, and writing of the manuscript. SNC contributed to data collection. LBH contributed to the conceptual design of the study and review of the manuscript. EB contributed to the conceptual design of the study, writing and review of the manuscript.










