INTRODUCTION
Influenza A viruses are important pathogens in both mammalian and avian hosts. Previous experimental studies have shown that dogs are susceptible to influenza A virus infection, but do not develop any clinical signs [Reference Houser and Heuschele1]. However, in recent years, some influenza viruses have been found to adapt to, and circulate efficiently in, sustained transmission chains in canine populations and cause severe, even fatal, clinical disease [Reference Harder and Vahlenkamp2]. In Florida in January 2004, canine influenza was reported as an emerging disease having been discovered in racing greyhounds. Canine influenza virus (CIV) subtype H3N8 appears to have spread from horses to dogs [Reference Crawford3]. A further study demonstrated that this virus was able to spread between dogs [Reference Jirjis4]. In 2007, a different influenza virus, subtype H3N2, caused an outbreak of canine respiratory disease in Korea [Reference Song5]. It appeared to be entirely of avian origin, but was able to be transmitted between dogs [Reference Song6]. Very recently, Li et al. [Reference Li7] isolated four strains of H3N2 avian-origin CIVs in dogs in Guangdong Province, China and demonstrated that all eight genes of these viruses were phylogenetically close to one H3N2 Korean CIV. Subsequently, six strains of H3N2 CIV were isolated from sick dogs in Jiangsu Province, China, but these were most similar to the feline isolate in Korea and different from the Guangdong isolates which were most similar to the canine isolates in Korea [Reference Lin8].
Canine influenza is an emerging disease in dogs and there is only limited information on the clinical signs and histopathological lesions. Results from Jung et al. [Reference Jung9] have shown that dogs experimentally infected with the Korean CIV only developed respiratory tract diseases, and no extrapulmonary lesions or viral antigens were detected. However, the H3N2 CIV isolates from Jiangsu acquired broad tissue tropism in mice [Reference Lin8]. Here, to gain a complete understanding of the disease caused by CIV Jiangsu isolates, we analysed in detail the pathobiological behaviour of one Jiangsu isolate in canine hosts.
METHODS
Animals
Twelve healthy beagle dogs aged 7–8 weeks old were housed in a BSL-2 isolation facility under standard husbandry conditions. The dogs were allowed 1 week for acclimatization purposes before the experiment. Food and water were provided as needed. All the experiments were performed in a BSL-2 isolation facility. The dogs were confirmed to be negative for current circulating influenza viruses by serology as determined by haemagglutination inhibition (HI) assays as described by Jirjis et al. [Reference Jirjis4]. Prior to this study, we obtained the approval of the Animal Ethics Committee of Nanjing Agricultural University. All animal experiments complied with the guidelines of the Animal Welfare Council of China.
Virus
A/canine/Jiangsu/06/2010 was isolated in our previous study [Reference Lin8]. The nucleotide sequences for eight genes of the virus have been deposited in GenBank (accession numbers JN247616–JN247623). Stocks of CIV were produced in 9- to 10-day-old specific pathogen-free (SPF) embryonated chicken eggs by standard methods [Reference Woolcock10] to a viral titre of 106·95 50% egg infectious dose (EID50)/0·1 ml as determined by the Reed & Muench method [Reference Reed and Muench11].
Experimental infection
Nine dogs were randomly chosen and assigned to the experimentally infected group. A 1-ml aliquot of virus stock (106·95 EID50/0·1 ml) was used to inoculate each of nine dogs intranasally. The other three dogs were controls and were inoculated with 1 ml sterile phosphate-buffered saline (PBS). To avoid contact transmission, dogs were housed singly in individual cages.
Clinical observations, sample collection
Clinical observations were recorded daily from 0 to 9 days post infection (p.i.), using a record sheet. Body temperature, body weight, eating and drinking, nasal and eye secretions, coughing, sneezing and mental state were recorded. Some clinical signs were assigned scores to evaluate the severity of the respiratory disease, as described by Jirjis et al. [Reference Jirjis4], and given in Table 1. All dogs were checked twice each day for clinical signs of respiratory disease throughout the experiment. The average clinical scores were calculated based on the daily score.
Table 1. Assessment of scores for the evaluation of respiratory disease

Nasal and rectal swab samples were collected individually to detect virus shedding from days 1–9 p.i. The swab was removed from the nose or anus and the tip of the swab was immersed carefully in an Eppendorf tube containing 1 ml virus transport medium (PBS containing 10 000 U/ml penicillin, 10 000 μg/ml streptomycin, 250 μg/ml gentamicin).
Three dogs were chosen randomly on days 3, 6, and 9 p.i. from the experimentally infected group and euthanized with an intravenous overdose of pentobarbital sodium (80 mg/kg body weight) for necropsy and fresh tissue samples. The heart, liver, spleen, lung, kidney, brain and duodenum were collected for virus detection and histopathology examinations. The three dogs that comprised the control group were euthanized at the end of the experiment. Lesion scores per lobe were performed to determine the severity of lung lesions as follows: 0, no lesion; 1, mild lesion; 2, moderate lesion; 3, severe lesion.
RNA extraction and cDNA synthesis
Virus Nucleic Acid Extraction kit II (Geneaid, Taiwan) was used to extract viral RNA from 200 μl supernatant of swab and tissue homogenates. RNA was eluted in 50 μl RNase-free water. Sterile water was used instead of the specimen as the negative control. Reverse transcription was performed using primer Uni12 [matrix (M) gene] 5′-AGCRAAAGCAGG-3′ and Moloney murine leukaemia virus reverse transcriptase (M-MLV RT) RNase H– (200 U/μl) (TaKaRa, China).
Virus detection and quantitative PCR
Nested PCR for the conserved region of the M gene was performed to detect viral shedding, as described by Ellis & Zambon [Reference Ellis and Zambon12]. PCR amplification was performed on a TaKaRa PCR Thermal Cycler Dice (TP600). Amplicons (413 bp) were visualized by GelSafe nucleic acid dye (10 000 × solution; YuanPingHao-bio, China) added to 2% agarose gels after electrophoresis was complete. Sterile water was used instead of the specimen as the negative control in every test. Real-time PCR for quantitation of viral loads was performed as described by Lin et al. [Reference Lin8].
Histopathological examination and immunohistochemistry (IHC)
Tissue samples from the experimentally infected dogs were fixed by submersion in Bouin's solution and embedded in paraffin wax. Serial 4-μm sections were prepared for haematoxylin and eosin (H&E) staining and IHC. For the IHC assay, tissue sections were prepared on slides and stained using an immunoperoxidase test. Briefly, a 1:1000 dilution of rabbit-derived polyclonal antibody (produced in our laboratory using the purified influenza virus described in this study) was applied as the primary antibody, followed by the application of biotinylated goat anti-rabbit IgG as secondary antibody (DingGuo, China), which was then detected by horseradish peroxidase (HRP)-conjugated streptavidin (DingGuo). An enhanced HRP-DAB chromogenic substrate kit (TianGen, China) was used for chromogenic detection according to the manufacturer's instructions, the slides were then counterstained with haematoxylin.
RESULTS
Clinical signs and evaluations
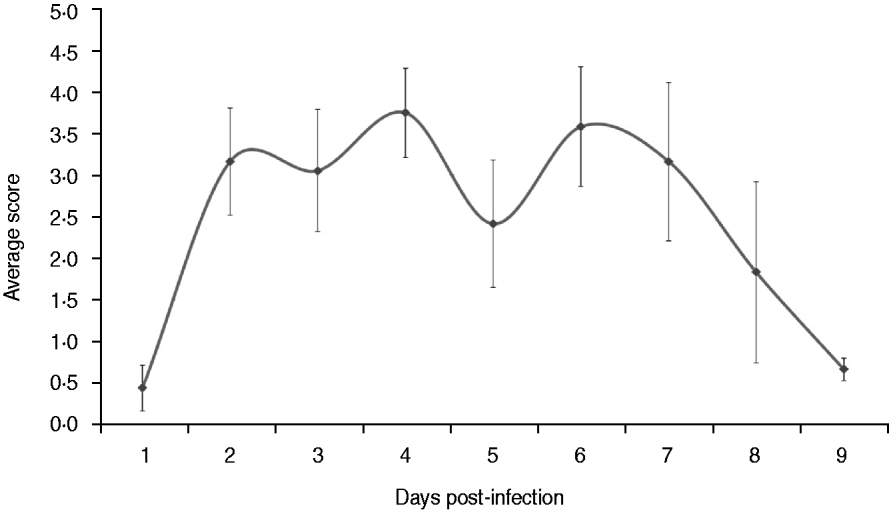
The dogs in the control group had a normal body temperature throughout the experiment (38·6 ± 0·2°C). Three out of nine dogs in the experimentally infected group developed fever on day 2 p.i. (body temperature ⩾39·5°C) and another two dogs developed fever 1 day later. The body temperature of the experimentally infected dogs peaked on day 4 p.i. (mean body temperature 39·8°C) and recovered on day 8 p.i. (body temperature <39·5°C).
Clinical signs in the experimentally infected dogs were detected about 36 h p.i., when two dogs developed fever, following a range of signs that included visible nasal discharge, coughing, sneezing, depression, anorexia, difficulty breathing and ocular discharge. Clinical scores of the experimentally infected dogs peaked on days 4 and 6 p.i. (Fig. 1). The dogs in the control group remained healthy during the experiment (data not shown). Six out of nine dogs exhibited sneezing in the experimentally infected group on day 2 p.i. and various degrees of visible nasal discharge could be observed in most of the dogs from days 2–9 p.i. Obvious depression was only observed in one dog between days 4 and 6 p.i. with severe mucopurulent nasal discharge and fever. Mild to moderate coughing was exhibited by all dogs of the experimentally infected group on day 5 p.i. In addition, ocular discharge was found in two dogs on day 4 p.i. On day 8 p.i., clinical signs of the last three dogs were relieved, although light coughing occurred occasionally in one dog.

Fig. 1. Average clinical scores of dogs infected experimentally. The dogs were observed twice every day throughout the experiment for clinical signs of respiratory disease that included body temperature, body weight, eating and drinking, nasal and eye secretions, coughing, sneezing and mental state. The average scores were calculated based on the daily score.The data are expressed as the mean ± standard error.
Lung congestion and consolidation were the predominant gross lesions observed from the pathology autopsy. There were no apparent gross lesions in other tissues. Three dogs that were euthanized on day 3 p.i. exhibited more congestion than consolidation in the lobes of the lung. Lung consolidation was characterized as hepatization and appeared on more lung lobes on day 6 p.i., showing that the pathological changes in the lung had become more severe. Gross lesions in the lung seen in animals autopsied on day 9 p.i. were characterized by mild consolidation and diffuse congestion. The mean lung lesion scores for experimentally infected dogs that were euthanized on days 3, 6, and 9 p.i. were 13, 13·5 and 7·7, respectively.
Virus shedding
The nasal and rectal swabs that were collected from all dogs 1 day before infection were CIV negative. CIV-infected dogs showed a high detection rate of nasal virus shedding throughout the experiment. All nasal specimens from CIV-infected dogs were positive except for one specimen collected on day 1 p.i. and one on day 9 p.i. There was a high detection rate (8/9) in rectal swabs from CIV-infected dogs on day 1 p.i. However, the number of positive results for rectal swabs decreased gradually with the duration of infection. No rectal specimen was positive for CIV after day 6 p.i. (Table 2).
Table 2. Virus shedding detection in nasal and rectal swabs of dogs in the experimentally infected groups

□, Euthanized on study day 3; ![]() , euthanized on study day 6;
, euthanized on study day 6; ![]() , euthanized on study day 9.
, euthanized on study day 9.
+/+, +/−, −/+, −/−: viral detection results of ‘nasal/rectal’ swab samples.
‘+’ indicates that this type of sample was positive, and ‘–’ indicates a negative sample.
Real-time PCR for quantitation of viral loads
Nine dogs were inoculated intranasally with 107·95 EID50 of the virus (7·7 × 1011 copies of viral RNA load). A real-time PCR assay was performed to determine viral loads in the main organs from CIV-infected dogs (Fig. 2).

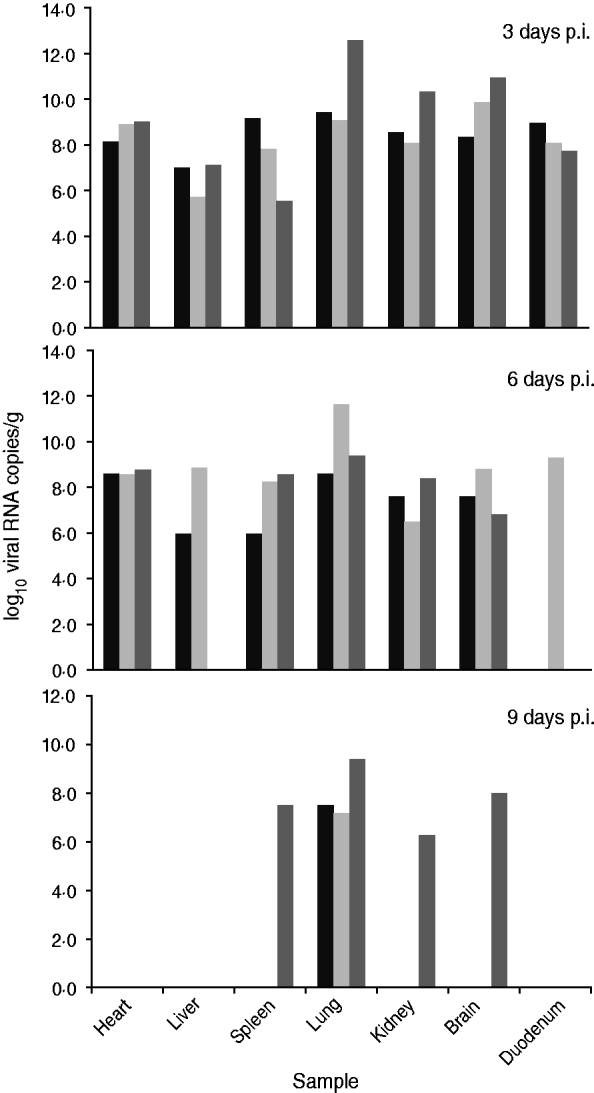
Fig. 2. Viral load in the main organs of dogs infected experimentally. Three dogs were chosen randomly and euthanized on days 3, 6, or 9 p.i. Each column represents viral load in an organ sample from one dog. Viral loads are expressed as log10 RNA copy numbers per gram of sample.
On day 3 p.i., all tested samples were positive and mean viral RNA loads were >108 copies/g, except for liver (3·98 × 106) and spleen (3·16 × 107). Lung was the tissue with the highest viral RNA load (2·51 × 1010 copies/g). Three days later, on day 6 p.i., the number of positive samples decreased. In the liver, 2/3 dogs were positive with viral RNA loads of 106 and 109 copies/g, respectively. In the duodenum, 1/3 dogs was positive with a viral RNA load of 2·51 × 109 copies/g. Moreover, the viral RNA load in the lungs decreased slightly, while there were noticeable decreases in the kidney and brain. In the spleen, there was a slight peak in the viral RNA level. However, viral RNA was detected at a constant level in the heart.
On day 9 p.i., all the lung samples were positive, but the viral RNA loads were significantly reduced (108 copies/g). Only a single sample of brain, spleen and kidney from one dog was positive with viral RNA loads of 108, 3·16 × 107 and 2·00 × 106 copies/g, respectively. In addition, the heart, liver and duodenum samples were negative for viral RNA.
Histopathology and IHC
Considering that the peak body temperature and clinical score occurred between days 4 and 6 p.i. and the gross lung lesions changed in severity on day 6 p.i., tissue samples on day 6 p.i. were collected for histopathological examination (Fig. 3) and IHC staining (Fig. 4). All three dogs showed similar histopathological lesions and viral antigen staining in every specific tissue, except for histopathological lesions in the brain.
In the heart, there were no typical or extensive histopathological lesions; IHC showed viral antigens stained brown in myocardial fibres (Fig. 4a).
In the liver, extensive degenerated hepatocytes were swollen and rounded with vacuoles of varying sizes in the cytoplasm and nuclei mostly on one side of the cytoplasm. The central veins of the hepatic lobule were dilated and packed with agglutinated and dissolved erythrocytes. Moreover, the hepatic sinusoids were hyperaemic with erythrocytes (Fig. 3a). The IHC stain showed viral antigens in the cytoplasm of degenerated hepatocytes (Fig. 4b).

Fig. 3 [colour online]. Histopathological appearances of H&E-stained liver, trachea, lung, kidney, brain and duodenum of dogs infected with A/Canine/Jiangsu/06/2010 at day 6 p.i. (a) Liver, showing extensive degenerated hepatocytes, dilatation and hyperaemia of the central vein in hepatic lobules (*), hyperaemia of the hepatic sinusoid (arrow), 100×. (b) Spleen, showing the periarterial lymphatic sheath significantly thickened (p), hyperaemia in the splenic trabecular artery (h), haemorrhage in the marginal zone (arrows), 100×. (c) Trachea, showing necrosis in tracheal epithelia (n), haemorrhage in lamina propria (arrows), 400×. (d) Lung, showing bronchioles and alveoli infiltrated with a large number of inflammatory cells, alveolar septum thinned or ruptured, alveolus compensatorily enlarged, 100×. (e) Lung, showing many inflammatory cell infiltrations and connective tissue proliferation, 400×. (f) Kidney, showing glomerular hyperplasia and swelling, degeneration in renal tubule epithelium and necrosis, 400×. (g) Brain, showing satellitosis (arrows) and neuronophagia (arrow after m); 400×. (h) Brain, showing lymphocytes around the cerebral blood vessels which were dilated (*), 100×. (i) Duodenum, showing necrosis of mucous membrane (arrow), 100×.

Fig. 4 [colour online]. Immunohistochemistry (IHC) detection of influenza viral antigen in collected tissues of dogs infected with A/Canine/Jiangsu/06/2010 at day 6 p.i. (a) Heart, viral antigens in myocardial fibres, 400×. (b) Liver, viral antigens in degenerated hepatocytes, 400×. (c) Spleen, viral antigens in marginal zone cells, 400×. (d) Trachea, viral antigens in epithelial cells and tracheal glands, 400×. (e) Lung, viral antigens in bronchiolar epithelium, type I alveolar cell, 400×. (f) Kidney, viral antigens in proximal tubule epithelium and renal glomerular podocyte, 400×. (g) Brain, viral antigens in neurons, 400×. (h) Duodenum, viral antigens in the columnar epithelium of the intestinal gland, 400×.
In the spleen, the number of lymphocytes in the periarterial lymphatic sheath was significantly increased and this area was thickened. Haemorrhage was observed in the marginal zone of the spleen. Hyperaemia in the splenic trabecular artery (Fig. 3b) may suggest inflammation. The IHC stain showed viral antigens in the marginal zone of the spleen. Some phagocytes were stained brown (Fig. 4c).
In the trachea, extensive necrosis appeared in the tracheal epithelial cells, lamina propria and tracheal glands, accompanied by haemorrhage in the lamina propria connective tissue interspaces (Fig. 3c). The IHC stain showed a large amount of viral antigen in epithelial cells and the tracheal glands of the tracheal submucous membrane (Fig. 4d).
In the lung, the alveolar septum was thickened or ruptured and the alveolar capillaries distended and filled with erythrocytes (Fig. 3d). Some alveolar spaces were enlarged in compensation and some adjacent alveoli fused into a large empty lumen. In some areas, the alveolar space was packed with a fibrinous network, erythrocytes and neutrophils. Some bronchioles and their alveoli were filled with a large number of inflammatory cells, infiltration in the alveoli and a small amount of connective tissue proliferation, showing hepatization (Fig. 3e). The IHC stain showed a large amount of viral antigens in the bronchiolar epithelium, lumen and type I alveolar cells (Fig. 4e).
In the kidney, histopathological examination showed acute glomerulonephritis. The glomerulus was swollen and filled Bowman's capsule. Endothelial and mesenchymal cells in the glomerulus showed proliferation. The epithelia of the renal tubules underwent degeneration and necrosis (Fig. 3f). The IHC stain showed a large amount of viral antigen in the proximal tubule epithelium and renal glomerular podocyte (Fig. 4f).
In the brain, histopathological examination revealed non-suppurative encephalitis. Glial cell hyperplasia with satellitosis (a neuron surrounded with oligodendrocytes and microgliocytes) was seen in one of three infected dogs, and neuronophagia (a degenerative or dead neuron engulfed by microgliocytes) in all three infected dogs (Fig. 3g). Lymphocytic infiltration around blood vessels was observed in the internal pyramidal layer of the cerebral cortex in one of the three infected dogs (Fig. 3h). The IHC stain showed viral antigens in neurons (Fig. 4g).
In the duodenum, epithelia of the duodenal mucosa underwent degeneration, necrosis and desquamation (Fig. 3i). The IHC stain showed viral antigens in the columnar epithelium of the small intestinal gland (Fig. 4 h).
DISCUSSION
Canine influenza is a new, contagious respiratory disease that has attracted the attention of veterinary practitioners and scientists in the fields of virology and epidemiology [Reference Harder and Vahlenkamp2]. Our studies demonstrated that A/canine/Jiangsu/06/2010 presented and replicated efficiently in multiple extrapulmonary organs and thus caused varying degrees of lesions. Most of the tissues analysed, including the heart, liver, spleen, lung, kidney, brain and duodenum, were shown to contain a high viral RNA load at an early stage of infection. However, the pathological observations reported by Song et al. [Reference Song5] were restricted mainly to the respiratory tract including whole tracheas and lungs. The significantly different results may be due to the host's immunity, as younger animals were used in our study. Additionally, it is possible that the virus disseminated more widely in this study due to strain variation. The H3N2 CIV isolate from Jiangsu has been demonstrated to acquire broad tissue tropism in mice [Reference Lin8]. Sequence analysis shows that a unique two amino-acid insertion in the NA stalk was found in the A/canine/Jiangsu/06/2010 (H3N2) isolate [Reference Lin8]. The long-stalked NA can efficiently cleave the receptor from which HA has been released and prevent re-binding [Reference Baigent and McCauley13]. Hence these viruses are released efficiently, which may contribute to the efficient replication in tissues. In order to determine the reasons for wide tissue tropism of the Chinese strain, further study is necessary to produce new viral strains by reverse genetics and site-directed mutagenesis and to compare their pathogenicity in dogs.
Quantitative detection showed that the lungs of CIV-infected dogs had higher viral RNA loads than seen in other organs, suggesting that the lung is the most susceptible organ in CIV infection. Furthermore, the mean viral RNA load in the lung remained at the 108 copies/g level on day 9 p.i., suggesting that CIV had more opportunity to be shed via the upper respiratory tract. The result was correlated with a high virus detection rate in nasal swabs from experimentally infected dogs. Therefore, the CIV-infected dogs that were ill for more than a week may also pose a threat to other healthy dogs.
Previous studies have concluded that CIV-infected dogs shed virus via nasal discharge with no virus being found in rectal swabs [Reference Song6, Reference Lee14]. Interestingly, in our study, the virus could be detected in rectal swabs and the detection results in rectal swabs correlated well with the viral RNA load in the duodenum. The difference may be related to the different characteristics of infecting viral strains. The infected dogs exhibited nasal discharge, coughing, sneezing, depression and fever, which were consistent with previous findings. Significantly, CIV-associated non-suppurative encephalomyelitis might have contributed to the depression seen in the dogs. In addition, the benign prognostic evidence of CIV-infected dogs on day 8 p.i. may be attributed to the enhanced immune response. This suggestion is supported by the histopathological and IHC findings. In the spleen, the periarterial lymphatic sheath attached to the thymus-dependent region was observed to be significantly thickened, suggesting the enhancement of cellular immunological responses in CIV-infected dogs. Strong IHC staining was found in the cells of the marginal zone surrounding the white pulp, representing the immune-related lymphocytes that captured the pathogens. Previous studies have demonstrated that the marginal zone contains a subset of B cells that are primed to respond to antigens delivered via the blood sinuses and to present the antigen to the immune-related cells such as follicular dendritic cells [Reference Browne15, Reference Ferguson, Youd and Corley16]. More work is needed on antiviral immunity.
Because the canine isolate was of avian influenza virus origin, we have investigated its pathogenicity in chickens. However, chickens do not appear to be a susceptible host for this avian-origin CIV (our unpublished data). This suggests that this virus has crossed species barriers and adapted to a new mammalian host.
Intraspecies variation of avian-lineage H3N2 CIV between dogs has been reported in South Korea [Reference Song6]. Jirjis et al. [Reference Jirjis4] demonstrated that CIV subtype H3N8 is easily transmissible among dogs by direct contact and causes respiratory disease in susceptible dogs. These findings strongly suggest that the transmission ability of CIV between dogs could play an important role in the epizootiology of the disease. To study the pandemic potential of Chinese avian-origin CIV, further investigation will be focused on dog-to-dog transmission.
CIV is a newly identified, highly contagious respiratory pathogen of dogs. Although there is no report of infection in humans caused by CIV H3N2, previous studies have demonstrated that the dog is susceptible to infection with other human influenza viruses both naturally and experimentally [Reference Gibbs and Anderson17]. Furthermore, H3 subtype viruses have been proven to be able to recruit avian, mammalian and human hosts [Reference Harder and Vahlenkamp2]. These facts highlight the potential for canine species to act as a ‘mixing vessel’ for the evolution of influenza A viruses. Considering the close companionship of dogs and people, the transmission of avian-origin CIV into the human population from infected dogs cannot be excluded. Ferrets are a better model for influenza infection and transmission in humans as they are naturally susceptible to the virus and have a similar distribution of sialic acid glycans in the respiratory tract [Reference Driskell18]. Therefore, further experiments using a ferret model should help to better understand the zoonotic potential of avian-origin CIV.
ACKNOWLEDGEMENTS
This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Program for New Century Excellent Talents in University (NCET-07-0440).
DECLARATION OF INTEREST
None.