Introduction
Nodular worms (Oesophagostomum spp.) are frequent parasites of the large intestine in pigs, ruminants, primates, and rodents after hosts ingest infective third stage rhabditiform larvae that develop from first-stage larvae hatching from eggs shed in feces. Eleven species of this parasitic nematode have been described, some of which can be especially pathogenic in humans and domestic animals (Anderson Reference Anderson2000). It’s common practice to culture parasite eggs in feces to harvest infective larvae useful in inoculums for experimental animals or for the identification of species. Coproculture of helminths is also useful for metabolic, immunological, genetic, developmental, and ultrastructural studies, as well as tissue culturing and evaluation of anthelminthics (Silverman & Hansen Reference Silverman and Hansen1971). In coprocultures, feces from infected individuals are mixed with inert media to increase aeration and moisture for nematode parasite eggs to develop and hatch into larvae that molt twice before becoming infective. Several procedures used to culture infective Oesophagostomum spp. larvae from eggs in feces exist, but it’s not known whether they yield similar number of larvae. For example, Oesophagostomum spp. third stage larvae have been harvested from pig feces mixed with vermiculite (Sellers et al., Reference Sellers, Dipelou and Roy1975; Henriksen & Korsholm, Reference Henriksen and Korsholm1983; Roepstorff et al., Reference Roepstorff, Bjørn and Nansen1987). Coprocultures of feces mixed with charcoal have been used to quantify Oesophagostomum bifurcum in human populations in Africa (Polderman et al., Reference Polderman, Krepel, Baeta, Blotkamp and Gigase1991; Krepel et al., Reference Krepel, van der Velde, Baeta and Polderman1995), and quantify the effects of high temperature on the infective larvae of O. columbianum (Premvati & Lal, Reference Premvati and Lal1961). Procedures originally developed for other nematode parasite species have also been used to culture Oesophagostomum spp. infective larvae. For example, Oesophagostomum radiatum larvae have been cultured by mixing peat moss with feces (Douvres, Reference Douvres1962), as was used for Haemonchus contortus (Cauthen, Reference Cauthen1940) and Hyostrongylus rubidus (Kendall et al., Reference Kendall, Thurley and Peirce1969). One other possible method for culturing larvae that has not been frequently explored in the study of Oesophagostomum spp. is mixing infected feces with sawdust as was done for culturing feces from sheep with mixed strongyle infections, including Oesophagostomum spp. (Agyei, Reference Agyei1995). In this study we compare the number of infective larvae recovered from coprocultures of an organic sow naturally infected with Oesophagostomum spp. prepared with charcoal, sawdust, or vermiculite to determine which method yielded more larvae.
Methods
An experiment comparing the number of larvae recovered using four different culture media was set up at room temperature (~22 °C) with vermiculite, coconut shell granular activated carbon charcoal (effective size 0.5 - 0.7 mm), medium grit chemical-free hardwood sawdust, and water as a control. The experiment was repeated twice using five replicate Petri dishes (145mm x 20mm) per media type the first time, and 10 replicate dishes per media type the second time. Fresh feces were collected from a sow known to be naturally infected with Oesophagostomum spp. and managed organically at Rodale Institute (Kutztown, Pennsylvania, USA). Feces were brought to the lab on ice, and then for each replicate Petri dish, 15 g of feces was mixed with 15 g of culture media and 21 mL of tap water on Day 0 and subsequently sprayed with water every other day to remoisten the media as needed. Controls consisted of 3 g of feces evenly smeared on a 90 mm filter paper that was placed over the bottom half of an inverted 100 mm Petri dish placed inside a larger 145 mm dish that was then filled with water until the edges of the filter paper were immersed. On Day 9, larvae were harvested from each replicate dish using the Baermann technique (Baermann, Reference Baermann1917) and larvae from each dish stored in 20 mL of tap water in scintillation vials. The total number of larvae in each replicate was volumetrically estimated by mixing the contents of each vial with a magnetic stir bar and counting with replacement of the number of larvae under the 0.125 mL grid of three replicate McMaster slide chambers. The average of the three McMaster was then used to estimate the total number of larvae in 20 mL. Data were analyzed using R statistical software v.3.4.1 (R Core Team, 2017). A Shapiro-Wilk test on the number of larvae recovered showed data was significantly different from a normal distribution (W = 0.59, p < 0.0001). Therefore, statistical comparisons of the number of larvae recovered were done using non-parametric tests. A Wilcoxon rank sum test with continuity correction was used to assess differences in the number of larvae recovered between trial 1 and trial 2. A Kruskal-Wallis rank sum test was used to assess whether differences between culture media existed, and if they did, a Dunn (Reference Dunn1964) Kruskal-Wallis multiple comparison procedure was run with p-values adjusted using the Benjamini-Hochberg method (Benjamini & Hochberg, Reference Benjamini and Hochberg1995).The mean ± SEM (standard error of the mean) for each culture media type are reported for trials 1 and 2. All statistical analyses used an alpha level of 0.05.
Results and Discussion
The number of Oesophagostomum spp. larvae recovered was higher in trial 1 than trial 2 (W= 740.5, p< 0.0001; Figure 1). As a result, statistical comparison between the different media types was done separately for each trial. There were differences in the number of Oesophagostomum spp. larvae recovered between different media types in trial 1 (KW = 13.753, df = 3, p = 0.003), and in trial 2 (KW = 19.677, df = 3, p = 0.0002). In both trials, sawdust media produced a numerically higher number of larvae relative to the other three media types (Figure 1). However, in trial 1 only the sawdust vs. vermiculite (Z = 2.62, p = 0.026), charcoal vs. water (Z = 2.51, p = 0.024) and sawdust vs. water comparisons were different (Z = 3.1, p = 0.011), while in trial 2 the sawdust vs. charcoal (Z = −2.33, p = 0.04), sawdust vs. vermiculite (Z = 3.93, p = 0.0005) and the sawdust vs. water (Z = 3.72, p = 0.0006) comparisons were different (Figure 1).
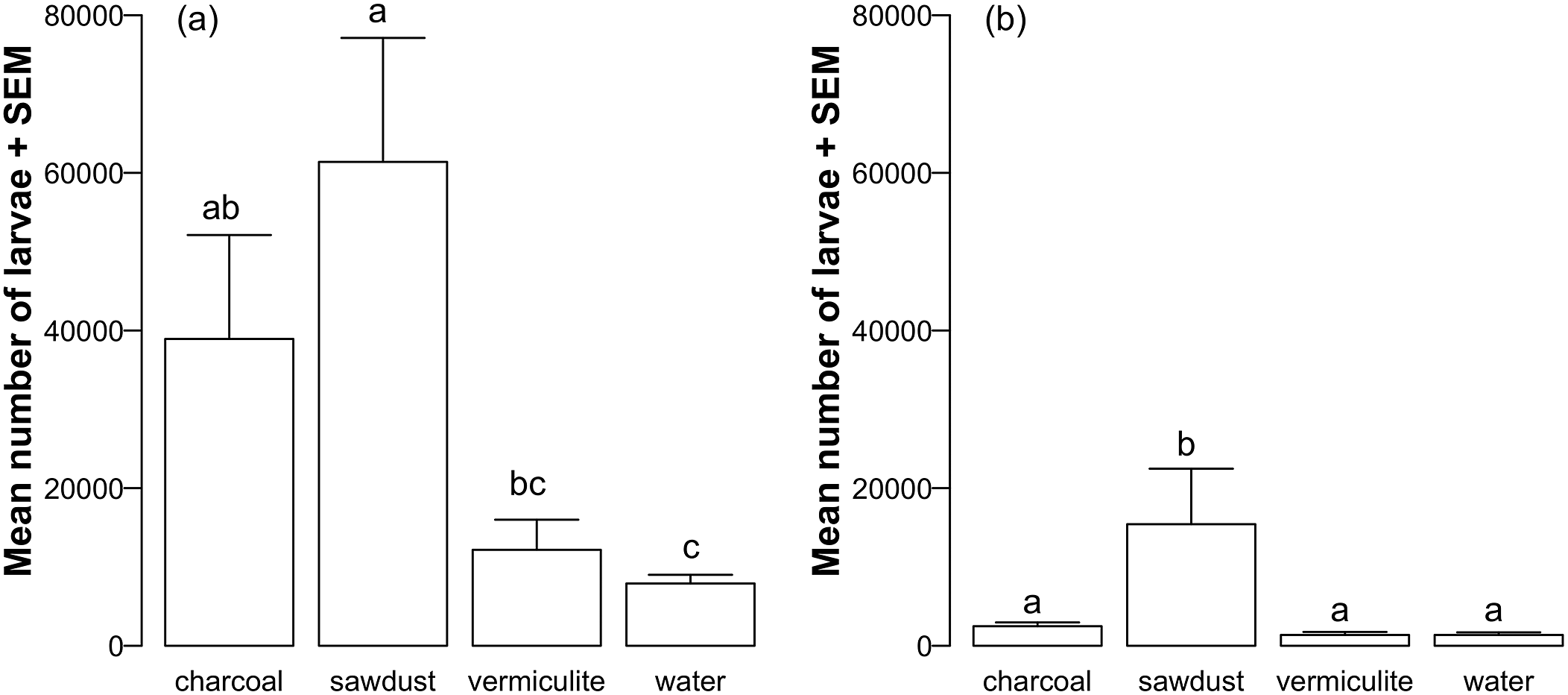
Figure 1. The mean number of Oesophagostomum spp. larvae (+SEM) cultured in feces mixed with charcoal, sawdust, vermiculite, or water (control) for 9 days at room temperature (22 °C) after (a) trial 1 (n= 5 Petri dishes per media) and (b) trial 2 (n= 10 Petri dishes per media). Bars with distinct letters indicate statistically significant pairwise comparison differences (Dunn’s post hoc tests, p<0.05).
Our results show that swine coprocultures using sawdust resulted in the production of a higher number of Oesophagostomum spp. larvae relative to the larva number produced using vermiculite or water controls. The number of larvae produced in charcoal coprocultures did not differ from sawdust in trial 1 but was significantly lower in trial 2 when a higher number of replicate dishes was used. Charcoal and vermiculite are the two most frequently cited media used in Oesophagostomum spp. coprocultures (e.g. Premvati & Lal, Reference Premvati and Lal1961; Sellers et al., Reference Sellers, Dipelou and Roy1975, Henriksen & Korsholm, Reference Henriksen and Korsholm1983; Roepstorff et al., Reference Roepstorff, Bjørn and Nansen1987; Polderman et al., Reference Polderman, Krepel, Baeta, Blotkamp and Gigase1991; Krepel et al., Reference Krepel, van der Velde, Baeta and Polderman1995). Yet, there are few published assessments of which technique leads to a higher yield of larvae for this nematode parasite. Studies comparing multiple culturing techniques have been done for nematodes infecting sheep (Agyei, Reference Agyei1995; Muchiut et al., Reference Muchiut, Fernandez, Dominguez, Riva, Rodriguez, Steffan, Bernat and Fiel2021), cattle (Berrie et al., Reference Berrie, East, Bourne and Bremner1988; Steffan et al., Reference Steffan, Henriksen and Nansen1989), and goats (Nwosu et al., Reference Nwosu, Iwuoha, Torru and Mohammed2006). However, a similar comparison of swine parasitic nematode coproculture techniques does not exist.
The higher number of Oesophagostomum spp. larvae recovered in this study using sawdust relative to vermiculite are unlike the results from sheep coprocultures which found no difference in the number strongyle larvae produced between sawdust and vermiculite media used (Agyei, Reference Agyei1995). Feces from calves experimentally infected with either Ostertagia ostertagi or Cooperia oncophora both yielded higher numbers of larvae in vermiculite and polystyrene pellet coprocultures when compared to coprocultures with no media added, and there were no differences between vermiculite and polystyrene pellet coprocultures (Steffan et al., Reference Steffan, Henriksen and Nansen1989). In contrast, incubation of whole sheep fecal pellets instead of mixing with polystyrene pellets or vermiculite yielded more Haemonchus contortus larvae (Muchiut et al., Reference Muchiut, Fernandez, Dominguez, Riva, Rodriguez, Steffan, Bernat and Fiel2021). Vermiculite in this study produced Oesophagostomum spp. larva in numbers not significantly different than charcoal or water in both trials, but significantly less when compared to the number of larvae that sawdust yielded.
The purpose of mixing feces with inert media is to increase aeration and suitable moisture for nematode eggs to hatch and larvae to develop, thus improving larval yields. This study shows that coprocultures of swine infected with Oesophagostomum spp. yield higher number of larva when sawdust is used relative to other media such as charcoal or vermiculite. Although we did not measure the proportion of eggs that hatched, coprocultures were all mixed with the same fecal sample on the same day, making comparisons between media appropriate. The relative fewer number of larvae recovered in trial 2 relative to the first trial is likely related to natural variability in the number of Oesophagostomum spp. eggs shed by hosts over time (Roepstorff & Murrell, Reference Roepstorff and Murrell1997).
Our comparison of coproculture media type used fecal material from a breeding sow singly infected with Oesophagostomum spp., yet concomitant infections in nature are the rule (Cox, Reference Cox2001). It’s therefore not known whether similar numbers of Oesophagostomum spp. larvae will be produced if sawdust is used in coprocultures mixed with feces from hosts with multiple parasite species infections. However, coprocultures using feces from cattle with mixed parasite infections have been shown to produce fewer larvae than when feces come from single infected host individuals (Berrie et al., Reference Berrie, East, Bourne and Bremner1988). Nonetheless, our data support the idea that use of sawdust may be a preferable media to use in coprocultures to harvest larvae of Oesophagostomum spp. infective to pigs or humans.
Financial support
This work was supported by the United Stated Department of Agriculture, National Institute of Food and Agriculture (Y.L., A.D.H., R.C., grant number 2017-51106-27129). Additional funds were provided by the Biology Department at Kutztown University.
Conflict of interest
The authors declare none.



