Candida auris is a globally emerging pathogen that is often resistant to multiple antifungal agents.Reference Vallabhaneni, Kallen and Tsay 1 – Reference Larkin, Hager and Chandra 4 In several studies, C. auris has been recovered from environmental surfaces in healthcare facilities, suggesting that contaminated surfaces may be an important source of acquisition.Reference Vallabhaneni, Kallen and Tsay 1 – Reference Calvo, Melo and Perozo-Mena 3 However, it is not clear whether C. auris has a greater propensity to survive on surfaces than other Candida species. Although relatively little information is available on the extent of Candida contamination of surfaces in healthcare facilities, several Candida species can survive for prolonged periods on surfaces,Reference Kramer, Schweke and Kampf 6 and some studies have demonstrated recovery of Candida species other than C. auris from surfaces in hospitals.Reference Pfaller 7 Here, we compared the survival rates of Candida auris with other Candida species on surfaces, and we conducted a culture survey to determine the frequency of contamination of hospital environmental surfaces with Candida species.
METHODS
The study protocol was approved by the institutional review board of the Louis Stokes Veterans Affairs Medical Center. We examined survival of 8 C. auris strains and 3 strains each of C. albicans, C. glabrata, and C. parapsilosis on dry and moist surfaces for up to 7 days. The 8 strains of C. auris have been described previously: MRL 31102 and 31103 and CBS# 10913, 12372, 12373, 12772, 12776, and 12777.Reference Larkin, Hager and Chandra 4 The C. albicans strains were American Type Culture Collection strains (ATCC) SC5314, MBL32249, and MBL 32708. The C. glabrata strains were ATCC MBL31820, 34870, and 9542. The C. parapsilosis strains were clinical isolates. The moist surfaces were 10-mm-diameter sections of moist nonnutrient agar inside a Petri dish sealed with parafilm to prevent desiccation. The dry surfaces were 10-mm-diameter, circular, nonporous steel disks. In preliminary experiments, the survival rates of C. auris were similar on multiple different dry surfaces, including steel disks, ceramic or plastic laminate tiles, and sections of privacy curtain material (data not shown).
The surfaces were inoculated with 106 colony-forming units (CFU) of washed Candida species from an overnight culture suspended in 10 µL of phosphate-buffered saline (PBS). The inoculum was allowed to air dry. At 2 hours and at 1, 2, and 7 days, the inoculated surfaces were sampled by transferring to plastic tubes containing 1 mL of PBS and vortexing for 2 minutes. Quantitative cultures were performed by plating serially diluted specimens on Sabouraud dextrose agar (Becton Dickinson, Sparks, MD) that were incubated at 37°C for 72 hours to determine the presence and concentration of Candida species. The percent recovery at each time point was calculated in comparison to the CFU recovered immediately after inoculation. The experiments were performed in triplicate.
In the hospital, we used BBL CultureSwabs (Becton Dickinson, Cockeysville, MD) to sample moist surfaces (ie, sinks and shower drains) and dry surfaces in patient rooms; for dry surfaces the swabs were premoistened with sterile PBS. For large surfaces, 5×10-cm areas were sampled; for small objects, the entire surface area was sampled. For dry surfaces, 1 swab was used to sample multiple sites inside the patient room (bed rails, bedside tables, call buttons, and telephones) and 1 swab was used to sample sites in the bathroom (toilet seats and toilet hand rails). The swabs were vortexed for 1 minute in sterile PBS, and serially diluted aliquots were plated on Sabouraud dextrose agar. Colonies consistent with Candida species were subjected to identification using API 20C AUX for yeast identification (BioMerieux, Lombard, IL) and the Bruker Biotyper matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) system (Bruker, Billerica, MA) including a research-use-only library containing C. auris.Reference Mizusawa, Miller and Green 8 For comparison, aliquots from the swab samples were plated on selective media for methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and fluoroquinolone-resistant gram-negative bacilli.Reference Nerandzic, Cadnum, Pultz and Donskey 9 For selection of fluoroquinolone-resistant gram-negative bacilli, MacConkey plates containing 1 µg/mL of ciprofloxacin were used.
A quasibinomial logit model was used to compare survival of the different Candida species on inoculated surfaces at 1 and 7 days. The Fisher exact test with adjusted P values for post hoc pairwise comparisons was used to compare percentages of positive hospital environmental cultures for Candida species versus the bacterial pathogens. Data were analyzed using R version 3.2.2 software (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
As shown in Figure 1, on dry steel disks the percent recovery of each of the Candida species in comparison to the initial inoculum decreased over time. However, for each strain tested, organisms remained detectable after 7 days. Recovery rates of C. auris at days 1 and 7 were significantly greater than recovery rate of C. albicans but significantly less than the recovery rate of C. parapsilosis (P<.05). In addition, recovery rates of each of the 8 strains of C. auris were similar. On moist nonnutrient agar, no significant decrease in the recovery rate of the Candida species was observed.
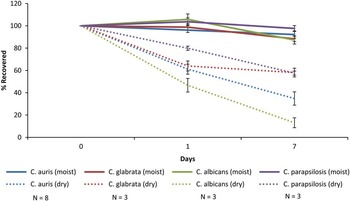
FIGURE 1 Survival of 8 strains of Candida auris, 3 strains of C. glabrata, 3 strains of C. parapsilosis, and 3 strains of C. albicans on dry steel disks and on moist nonnutrient agar. The surfaces were inoculated with 106 colony-forming units (CFU) of the Candida species and quantitative cultures were performed at 2 hours and at 1, 2, 4, and 7 days after inoculation. The percent recovery at each time point was calculated in comparison to the CFU recovered immediately after inoculation. The experiments were performed in triplicate.
Figure 2 shows the frequencies of recovery of Candida species and the bacterial pathogens from hospital surfaces. There were no significant differences in the percent recovery of Candida species versus the other pathogens from dry surfaces (P≥.05). However, Candida species were recovered significantly more often than the other pathogens from moist surfaces (P<.01). The Candida species that recovered included 7 C. glabrata strains, 7 C. parapsilosis strains, 1 C. tropicalis strain, 1 C. albicans strain, 1 C. metapsilosis strain, and 1 C. lusitaniae strain.

FIGURE 2 Rate of recovery of Candida species, methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and carbapenem-resistant gram-negative bacilli from dry and moist hospital surfaces.
DISCUSSION
Overall, 8 strains of C. auris survived on moist or dry surfaces for 7 days. Candida auris exhibited a greater propensity to survive on surfaces than C. albicans, but not C. parapsilosis or C. glabrata. In comparison to common bacterial pathogens, Candida species were recovered with similar frequencies from dry surfaces and were recovered significantly more often from moist areas such as sinks. These results provide support for the hypothesis that contaminated surfaces could be an important source for transmission of Candida auris.Reference Vallabhaneni, Kallen and Tsay 1 – Reference Calvo, Melo and Perozo-Mena 3
The CDC recommends thorough daily and terminal disinfection of room surfaces and shared medical equipment in rooms of patients with C. auris infection. 5 Although many disinfectants have an Environmental Protection Agency registration against Candida species, it is recommended that a disinfectant effective against C. difficile spores be used. 5 Further data are needed regarding the efficacy of different disinfectants against C. auris. Given that we frequently recovered Candida species from moist surfaces, the potential for spread of C. auris from moist sites, such as sinks that have been implicated in dissemination of multidrug-resistant gram-negative bacilli, should also be clarified.Reference Kotay, Chai and Guilford 10
The high recovery rate of non-albicans Candida species from hospital surfaces suggests that the environment might be an underappreciated reservoir for spread of Candida species other than C. auris. Non-albicans Candida species, including C. lusitaniae, C. parapsilosis, and C. glabrata, have been recovered from the hospital environment.Reference Pfaller 7 , Reference Mizusawa, Miller and Green 8 Further studies are warranted to investigate the role of contaminated surfaces in transmission of Candida species.
Our study has some limitations. We only studied 3 to 8 strains of each Candida species. However, survival rates on surfaces were similar for different strains. We studied survival on surfaces in a laboratory setting, and we studied only 2 types of surfaces. As noted previously, preliminary experiments demonstrated that the survival rates of C. auris were similar on multiple types of dry surfaces. We only studied survival on surfaces for 7 days. Further studies are needed to evaluate longer time periods and to identify other factors that may impact survival. Finally, we did not compare the survival rates of the Candida species to those of bacterial pathogens.
ACKNOWLEDGMENTS
Financial support: This work was supported by the Department of Veterans Affairs.
Potential conflicts of interest: C.J.D. has received research grants from Merck, GOJO, STERIS, and EcoLab, and he serves on an advisory board for 3M. All other authors report no conflicts of interest relevant to this article.




