Skeletal muscle is an essential tissue for whole-body energy metabolism and is an important site for insulin action. Skeletal muscle fatty acid metabolism is subject to extensive in vivo regulation, in particular by the control of fatty acid entry into the cell, transfer of fatty acids into the mitochondria and the capacity of the β-oxidation cycle(Reference Kraegen, Cooney and Turner1). The balance between the uptake and utilisation of fatty acids will ultimately determinate the magnitude of lipid accumulation in muscle cells. Whether muscle lipid accumulation causes insulin resistance still requires definitive proof, but highly plausible mechanisms support this possibility(Reference Kraegen, Cooney and Turner1).
Not only accumulation of muscle TAG content but also muscle NEFA pattern seems to affect insulin-mediated glucose uptake, as increased SFA in skeletal muscle TAG directly correlate with insulin resistance(Reference Manco, Mingrone and Greco2). Moreover, the fatty acid composition of skeletal muscle phospholipids has also been related to peripheral insulin sensitivity(Reference Borkman, Storlien and Pan3–Reference Clore, Li and Gill5). It is well known that fatty acid composition of the diet can influence membrane fatty acid composition(Reference Abbott, Else and Hulbert6), and many reports have shown that a high intake of dietary saturated fat significantly worsens insulin resistance, in particular through modifications in the composition of cell membrane phospholipids(Reference Riccardi, Giacco and Rivellese4).
Polyphenols may modulate lipid metabolism, in particular in muscle. In fact, some studies have demonstrated that polyphenols have muscle lipid-lowering properties(Reference Mulvihill, Allister and Sutherland7), down-regulate cardiac fatty acid translocase/cluster of differentiation 36 (CD36) gene expression(Reference Qin, Polansky and Harry8) and modulate liver desaturase activity(Reference Ajmo, Liang and Rogers9–Reference Ogino, Osada and Nakamura11). Nevertheless, whether polyphenols may modulate skeletal muscle fatty acid composition has never been addressed. The present study was thus designed to determine whether a grape polyphenol extract (PPE) modulates the skeletal muscle fatty acid composition of phospholipids, NEFA and TAG content. Muscle plasmalemmal and mitochondrial fatty acid transporters, GLUT4, lipid metabolism pathways and desaturase activities were also explored.
Materials and methods
Animals and diets
All animal experiments were performed according to European directives (86/609/CEE) and approved by ‘Comité d'Ethique en matière d'Expérimentation Animale: Région Languedoc-Roussillon’. A total of eighteen male Wistar rats (Charles River Laboratories, L'Arbresle, France) aged 6 weeks (200 g) were used in the present study. Rats were housed under conditions of constant temperature (20–22°C), humidity (45–50 %) and a standard dark cycle (20.00–08.00 hours). Our institutional guidelines for the care and use of laboratory animals were observed. The rats were randomised into three groups of six animals: a control group (control) was fed a control semi-purified diet for 6 weeks; a high fat–high sucrose (HFHS) group was fed a HFHS diet for 6 weeks; a grape polyphenol (HFHS/PPE) group was fed a HFHS diet containing 2 g PPE (Provinols™; Société Française de Distillerie, Vallon Pont d'Arc, France) per kg diet for 6 weeks. The control diet contained the following (per kg): 200 g casein; 660·7 g starch; 40 g soyabean oil; 50 g cellulose; 35 g(Reference Reeves, Nielsen and Fahey12) mineral mix; 10 g(Reference Reeves, Nielsen and Fahey12) vitamin mix; 1·8 g l-cystine; 2·5 g choline bitartrate. The HFHS diet contained the following (per kg): 200 g casein; 100·7 g starch; 300 g sucrose; 40 g soyabean oil; 110 g olive oil; 150 g coprah oil; 50 g cellulose; 35 g(Reference Reeves, Nielsen and Fahey12) mineral mix; 10 g(Reference Reeves, Nielsen and Fahey12) vitamin mix; 1·8 g l-cystine; 2·5 g choline bitartrate. The detailed fatty acid composition of the diets is given in Table 1. Rats were given free access to distilled water and food. Body growth and diet consumption were determined weekly, and energy intake was calculated. Provinol™ is an alcohol-free powder PPE obtained from red wine produced in the Languedoc-Roussillon region. The Provinol™ powder contains a minimum of 95 % of total polyphenols (46 % proanthocyanidols, 21 % prodelphinidol, 6·1 % total anthocyanes, 3·8 % catechin, 3 % epicatechin gallate, 1·8 % OH cinnamic acid, 1·4 % flavanol, 0·15 % resveratrol and 0·095 % free anthocyane; Société Française de Distillerie, Vallon Pont d'Arc, France).
Table 1 Fatty acid composition of the experimental diets* (percentage of total fat)
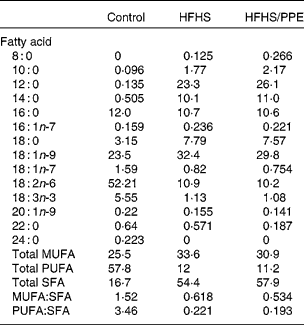
HFHS, high-fat–high-sucrose; PPE, polyphenol extract.
* Values are based on identifiable peaks. Determined by GLC (FOCUS GC; Thermo Electron Corporation, Vantaa, Finland).
Sampling and routine biochemical analyses
Non-fasted rats were anaesthetised with pentobarbital (Ceva Santé Animale, Libourne, France), and blood was obtained from the abdominal vein with a heparinised syringe (sodium heparinate; Panpharma SA, Fougères, France). Blood samples were centrifuged at 1000 g for 10 min at 4°C, and plasma was collected and stored at − 80°C until analysis. Gastrocnemius muscles were quickly removed and frozen in liquid N2 and kept at − 80°C.
Plasma glucose, total cholesterol, TAG and NEFA concentrations were measured by enzymatic techniques (Konelab; Thermo Electron Corporation, Vantaa, Finland). Plasma insulin and leptin levels were quantified with ELISA kits (Linco Research, St Charles, MO, USA). Protein level in the tissue homogenate was measured by Bradford's technique(Reference Bradford13).
Muscle mitochondrial enzymatic activity measurement
Muscle homogenates were prepared on ice (1 g of wet tissue in 9 ml phosphate buffer, 50 mm, pH 7) using a Polytron homogeniser and centrifuged at 1000 g for 10 min at 4°C. β-Hydroxyacyl CoA dehydrogenase (β-HAD) was measured spectrophotometrically according to Yang et al. (Reference Yang, He and Schulz14). Citrate synthase activity was measured spectrophotometrically according to Srere(Reference Srere15). Complex II and complex II+III activities were determined spectrophotometrically according to Rustin et al. (Reference Rustin, Chretien and Bourgeron16). Complex IV (cytochrome c oxidase activity) was measured spectrophotometrically according to Wharton & Tzagoloff(Reference Wharton and Tzagoloff17).
Muscle total lipid extraction
Skeletal muscle was homogenised in NaCl (9 g/l), using an Ultra Turax homogeniser (Ultra-Turrax TP 18/10, Janke and Kunkel, KG, IKA-Werk, Staufer, Germany), and lipids were extracted from the homogenate using the method of Folch et al. (Reference Folch, Lees and Sloane Stanley18). Phosphorus was quantified on the chloroform–methanol extract in order to determine total phospholipid quantity as described previously(Reference Bartlett19). The chloroform–methanol lipid extract was used for neutral and polar lipid separation on a TLC plate in order to analyse the fatty acid composition of each lipid class by GC after transesterification.
Lipid class analyses and fatty acid preparation
Separation of muscle lipid classes (phospholipids, TAG and NEFA) on the TLC plate was carried out essentially as follows: 1 ml (100 mg tissue) of the total lipid extracts was evaporated, diluted in 60 μl of Folch solution and applied to the origin of a Silica Gel 60 TLC plate 20 cm × 20 cm (Merck Chimie SAS, Fontenay sous Bois, France) with a Linomat IV (Camag, Chromacim SAS, Moirans, France). The dried lipid spots were developed with hexane–diethyl ether–glacial acetic acid (70:30:1, v/v/v). Plates were allowed to dry and then sprayed with dichlorofluorescein. Bands corresponding to phospholipids, TAG and NEFA were identified under UV illumination, and collected by scraping the silica into a glass tube. Fatty acid methyl esters were prepared by incubation with acidified methanol, according to the method of Lepage & Roy(Reference Lepage and Roy20), after the addition of an internal standard (17 : 0, 500 μg/ml) to lipids isolated by silica from TLC plates. Briefly, the methylation reagent was generated by mixing H2SO4 with methanol and butylated hydroxytoluene as an antioxidant (50 mg/l), and the sample was heated at 90°C for 45 min to get the fatty acid methyl esters. After the addition of sodium bicarbonate, distilled water and hexane, the sample was vortexed and centrifuged, and the upper hexane layer was transferred to a glass vial, evaporated under a N2 stream at 37°C and dissolved in iso-octane for GC analysis. Individual fatty acid methyl esters were identified according to the retention times of standards by GLC (FOCUS GC; Thermo Electron Corporation, Thermo Fisher Scientific, Courtaboeuf, France), with a VARIAN Cpsil88 capillary column (50 m × 0·32 mm inner diameter × 0·20 μm df), an AS-3000 autosampler and a flame ionisation detector (Thermo Electron Corporation, Thermo Fisher Scientific). Fatty acid methyl esters were quantified using the chromatographic peak area according to the internal standard method.
Muscle TAG and NEFA levels
Muscle TAG and NEFA levels were then calculated from the total fatty acid methyl ester molarity in TAG and NEFA bands, respectively, isolated on TLC plates.
Muscle desaturase activity indices
The activity of Δ9-desaturase, an enzyme that converts stearic acid (18 : 0) and palmitic acid (16 : 0) to oleic acid (18 : 1n-9) and palmitoleic acid (16 : 1n-7), respectively, was estimated by the ratio (18 : 1n-9+16 : 1n-7)/(18 : 0+16 : 0). A decrease in this ratio in each of the lipid classes can be related to a decrease in the Δ9-desaturase activity and vice versa. The activity of Δ5-desaturase, an enzyme that converts dihomo-γ-linoleic acid (20 : 3n-6) to arachidonic acid (20 : 4n-6), was estimated by the ratio 20 : 4n-6/20 : 3n-6(Reference Biggemann, Laryea and Schuster21). The activity of Δ6-desaturase, an enzyme that converts linoleic acid (18 : 2n-6) to γ-linolenic acid (18 : 3n-6), was estimated by the ratio 18 : 3n-6/18 : 2n-6(Reference Stefan, Peter and Cegan22).
Immunoblotting
Frozen gastrocnemius muscle samples were homogenised using an Ultra Turax homogeniser in an ice-cold extraction buffer containing 20 mm-Tris–HCl, 150 mm-NaCl, 1 mm-EDTA, 0·5 % Triton X-100, 0·1 % SDS, 1 mm-phenylmethylsulfonyl fluoride, 10 μm-leupeptin and 1 μm-pepstatin. Proteins (50 μg) were separated with 10 % SDS-PAGE and then transferred to a nitrocellulose membrane (45 min, 200 V). The membranes were blocked in 5 % fat-free milk for 1 h at room temperature. Then, the membranes were incubated overnight with a primary antibody against phospho-acetyl-CoA carboxylase (Cell Signaling Technology, Inc., Danvers, MA, USA), fatty acid synthase (Cell Signaling Technology, Inc.), stearoyl-CoA desaturase 1 (SCD1; Santa Cruz Biotechnology, Santa Cruz, CA, USA), CD36 (Abcam, Cambridge, UK) and carnitine palmitoyltransferase 1 (m-CPT1; Santa Cruz Biotechnology) in blocking buffer. After washes in Tris-buffered saline/Tween under gentle agitation, the membranes were incubated for 1 h with a horseradish peroxidase-labelled antibody. After further washes, blots were treated with enhanced chemiluminescence detection reagents (Thermo Fisher Scientific). β-Actin was used as a loading reference, and blot intensities were measured using ImageJ software (NIH, Bethesda, MD, USA).
Muscle mRNA expression
Real-time quantitative PCR (RT-qPCR) was used to measure the mRNA expression of target genes in gastrocnemius muscle. Total RNA was extracted with Trizol reagent (Invitrogen Life Technologies, Cergy Pontoise, France). The reverse transcriptase reaction was performed with 5 μg of total RNA using SuperScript II Reverse Transcriptase (Invitrogen) and oligo (dT) primers. The RT-qPCR analysis was performed using IQ™ SYBR Green Supermix (Biorad, Hercules, CA, USA) with a MiniOpticon detection system (Biorad). Results were normalised with the gene encoding RPS9 that was used as reference. After normalisation by RPS9, all results are expressed as a percentage of control as means and standard deviations. The primer sequences used for RT-qPCR are shown in Table 2.
Table 2 Primer sequences used for real-time quantitative-PCR

CD36, cluster of differentiation 36; SCD1, stearoyl-CoA desaturase 1.
Statistical analysis
The number of animals required for this experiment was calculated from the expected difference in β-HAD activity between the control group and the HFHS group. As the starting hypothesis was that the fat diet would increase muscle mitochondrial β-oxidation, we expected a 30 % increase in β-HAD activity in the HFHS group than in the control group. A standard deviation of 20 % of the higher value could be allowed, as observed by Benton et al. (Reference Benton, Holloway and Campbell23). For a type 1 risk α of 0·05 and a power (1 − β) of 80 %, the number of subjects required was six per group.
Results are expressed as means and standard deviations. Statistical analysis was based on one-way ANOVA followed by Fisher's multiple comparison test. The limit of statistical significance was set at P < 0·05. Statistical analyses were performed using the StatView program (SAS Institute, Cary, NC, USA).
Results
Characterisation of the rats with high-fat–high-sucrose and high-fat–high-sucrose/polyphenol extract diets
The mean dietary intake was lower in HFHS or HFHS/PPE diet-fed rats compared with control rats, while energy intake was not modified significantly among the three groups (Table 3). Weight gain increased significantly in HFHS diet-fed rats compared with control rats (Table 3). Plasma leptin level was significantly increased in HFHS and HFHS/PPE diet-fed rats compared with control rats (Table 3). Plasma NEFA, TAG and total cholesterol levels were not different among the control, HFHS and HFHS/PPE diet-fed rats (Table 3).
Table 3 Characteristics of the rats and glucose homeostasis
(Mean values and standard deviations, n 6)*

HFHS, high-fat–high-sucrose; PPE, polyphenol extract.
* One-way ANOVA was used followed by Fisher's multiple comparison test.
† Mean values were significantly different compared with control diet-fed rats (P < 0·05).
‡ Mean values were significantly different compared with HFHS diet-fed rats (P < 0·05).
Glucose homeostasis
Plasma glucose level was significantly increased in HFHS and HFHS/PPE diet-fed rats (Table 3). Plasma insulin level was non-significantly increased in HFHS diet-fed rats compared with control rats. Interestingly, plasma insulin level was significantly decreased in HFHS/PPE diet-fed rats compared with HFHS diet-fed rats (Table 3). Moreover, GLUT4 mRNA expression was non-significantly decreased in HFHS diet-fed rats compared with control rats, but it was significantly up-regulated in HFHS/PPE diet-fed rats compared with HFHS diet-fed rats (Fig. 1).

Fig. 1 mRNA and protein expression of glucose and fatty acid transporter in muscle. (A) Real-time quantitative PCR analysis of mRNA expression of GLUT4 and cluster of differentiation 36 (CD36) in the muscle of control (![]() ), high-fat–high-sucrose (HFHS,
), high-fat–high-sucrose (HFHS, ![]() ) and HFHS/polyphenol extract (PPE) (
) and HFHS/polyphenol extract (PPE) (![]() ) diet-fed rats. Results were normalised with the gene encoding RPS9 that was used as the reference. (B) Western blot was performed on proteins extracted from the muscle of control, HFHS and HFHS/PPE diet-fed rats using anti-CD36, anti-carnitine palmitoyltransferase 1 (CPT1) antibodies. Anti-β-actin was used to confirm equal loading. (C) Quantification of CD36 and CPT1 protein expression. Western blot was quantified using ImageJ software (NIH, Bethesda, MD, USA). Values are means, with standard deviations represented by vertical bars (n 4–6). One-way ANOVA was used followed by Fisher's multiple comparison test. * Mean value was significantly different compared with control rats (P < 0·05). † Mean value was significantly different compared with HFHS diet-fed rats (P < 0·05).
) diet-fed rats. Results were normalised with the gene encoding RPS9 that was used as the reference. (B) Western blot was performed on proteins extracted from the muscle of control, HFHS and HFHS/PPE diet-fed rats using anti-CD36, anti-carnitine palmitoyltransferase 1 (CPT1) antibodies. Anti-β-actin was used to confirm equal loading. (C) Quantification of CD36 and CPT1 protein expression. Western blot was quantified using ImageJ software (NIH, Bethesda, MD, USA). Values are means, with standard deviations represented by vertical bars (n 4–6). One-way ANOVA was used followed by Fisher's multiple comparison test. * Mean value was significantly different compared with control rats (P < 0·05). † Mean value was significantly different compared with HFHS diet-fed rats (P < 0·05).
Muscle lipid content and fatty acid composition
Muscle TAG content was increased by 1·6-fold in HFHS diet-fed rats compared with control rats and returned to control values with PPE supplementation (Table 4). Muscle NEFA and phospholipid levels were not different among the control, HFHS and HFHS/PPE diet-fed rats. Moreover, composition of muscle NEFA remained unchanged in HFHS and HFHS/PPE diet-fed rats (data not shown).
Table 4 Muscle lipid content, β-hydroxyacyl CoA dehydrogenase (β-HAD), citrate synthase activities and mitochondrial respiratory complex activities*
(Mean values and standard deviations, n 6)

HFHS, high-fat–high-sucrose; PPE, polyphenol extract.
* One-way ANOVA was used followed by Fisher's multiple comparison test.
† Mean values were significantly different compared with HFHS diet-fed rats (P < 0·05).
‡ Mean values were significantly different compared with control diet-fed rats (P < 0·05).
§ Cytochrome c oxidase.
However, fatty acid composition of the muscle TAG fraction was modulated by fatty acid composition of the diet. Indeed, the proportions of muscle palmitic acid (16 : 0) and palmitoleic acid (16 : 1n-7), and major PUFA, decreased in HFHS and HFHS/PPE diet-fed rats compared with control rats. Moreover, the proportions of stearic acid (18 : 0) and oleic acid (18 : 1n-9) levels increased in HFHS and HFHS/PPE diet-fed rats compared with control rats (Table 5). Nevertheless, we observed that the PPE had no effect on the fatty acid composition of muscle TAG, except for palmitoleic acid that was lower with PPE than without PPE.
Table 5 Muscle fatty acid composition of TAG (percentage of total fatty acids in the TAG fraction)*
(Mean values and standard deviations, n 6)

HFHS, high-fat–high-sucrose; PPE, polyphenol extract; ND, not determined.
* One-way ANOVA was used followed by Fisher's multiple comparison test.
† Mean values were significantly different compared with control diet-fed rats (P < 0·05).
‡ Mean values were significantly different compared with HFHS diet-fed rats (P < 0·05).
Fatty acid composition of the muscle phospholipid fraction was also modulated by fatty acid composition of the dietary lipids (Table 6). Moreover, PPE treatment exhibited profound effects on the phospholipid fatty acid composition (Table 6), where the PUFA:SFA ratio was significantly increased. The increased PUFA content (10 %) in HFHS/PPE diet-fed rats was mainly due to the increased contents of n-3 PUFA, particularly the 22 : 6n-3 fatty acid.
Table 6 Muscle fatty acid composition of phospholipids (percentage of total fatty acids in the phospholipid fraction)*
(Mean values and standard deviations, n 6)

HFHS, high-fat–high-sucrose; PPE, polyphenol extract; ND, not determined.
* One-way ANOVA was used followed by Fisher's multiple comparison test.
† Mean values were significantly different compared with control diet-fed rats (P < 0·05).
‡ Mean values were significantly different compared with HFHS diet-fed rats (P < 0·05).
Stearoyl-CoA desaturase 1 gene and protein expression and desaturase activities
SCD1 mRNA and protein expression levels were similar among the experimental groups (data not shown). The Δ9 desaturase activity index in the TAG and the phospholipid fractions was higher in HFHS or HFHS/PPE diet-fed rats compared with control rats (Tables 5 and 6), however, non-significantly in the TAG fraction in HFHS diet-fed rats. The Δ5 desaturase activity index in the phospholipid fraction was significantly decreased in HFHS diet-fed rats compared with control rats (Table 6). The PPE did not modify the Δ9 and Δ5 desaturases activity indices in both fractions by comparison with HFHS diet-fed rats.
Fatty acid transporters, lipogenesis and mitochondrial β-oxidation
Fatty acid transporter CD36 (gene and protein expression) was significantly increased in HFHS diet-fed rats compared with control rats and significantly decreased in HFHS/PPE diet-fed rats compared with HFHS diet-fed rats (Fig. 1). Mitochondrial fatty acid transporter CPT1 (protein expression) decreased significantly in HFHS/PPE diet-fed rats compared with control and HFHS diet-fed rats (Fig. 1).
Phosphorylated acetyl-CoA carboxylase and fatty acid synthase protein expression was unchanged among the groups (data not shown). Sterol regulatory element-binding protein-1c and PPAR α gene expressions were also similar in all groups (data not shown).
Muscle β-HAD activity increased significantly, and citrate synthase activity increased but not significantly in HFHS diet-fed rats compared with control rats, but both activities returned to the control level in HFHS/PPE diet-fed rats (Table 4). Muscle β-HAD activity was highly significantly correlated with muscle citrate synthase activity (r 0·893, P < 0·001).
Mitochondriogenesis and mitochondrial activity
PPARγ co-activator 1-α (PGC-1α) gene expression was slightly but significantly decreased (approximately − 20 %) in HFHS and HFHS/PPE diet-fed rats compared with control rats (Fig. 2). Nuclear respiratory factor 1 (NRF1) gene expression was slightly ( − 30 %) but significantly decreased in HFHS diet-fed rats and was significantly increased (30 %) in HFHS/PPE fed rats compared with control rats. Mitochondrial transcription factor A gene expression remained unchanged in all groups (Fig. 2). Moreover, mitochondrial respiratory complex activities (CII, CII+CIII and CIV) were not significantly modified whatever the group (Table 4).
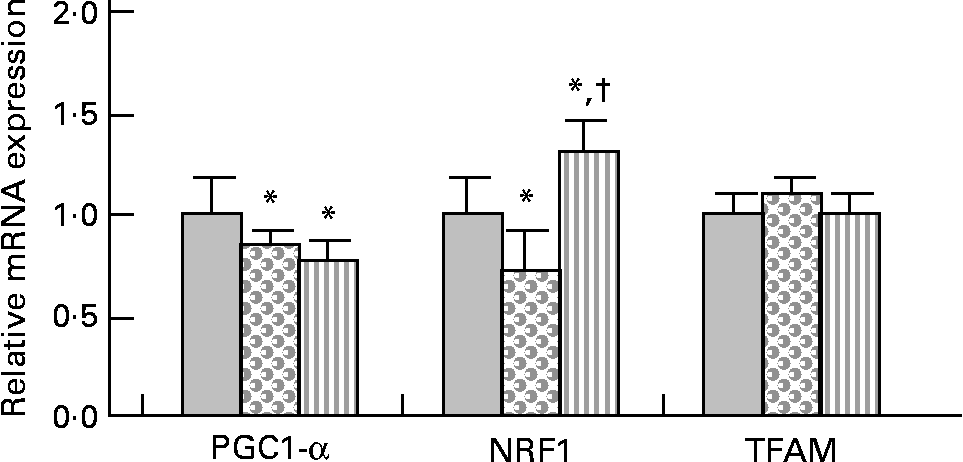
Fig. 2 Mitochondriogenesis factor in muscle. Real-time quantitative PCR analysis of mRNA expression of PPARγ co-activator 1-α (PGC1-α), nuclear respiratory factor 1 (NRF1) and transcription factor A, mitochondrial (TFAM) in muscle of control (![]() ), high-fat–high-sucrose (HFHS;
), high-fat–high-sucrose (HFHS; ![]() ) and HFHS/polyphenol extract (
) and HFHS/polyphenol extract (![]() ) diet-fed rats. Results were normalised with the gene encoding RPS9 that is used as the reference Values are means, with standard deviations represented by vertical bars (n 4–6). One-way ANOVA was used followed by Fisher's multiple comparisons test. * Mean value was significantly different compared with control rats (P < 0·05). †Mean value was significantly different compared with HFHS diet-fed rats (P < 0·05).
) diet-fed rats. Results were normalised with the gene encoding RPS9 that is used as the reference Values are means, with standard deviations represented by vertical bars (n 4–6). One-way ANOVA was used followed by Fisher's multiple comparisons test. * Mean value was significantly different compared with control rats (P < 0·05). †Mean value was significantly different compared with HFHS diet-fed rats (P < 0·05).
Discussion
The present study was conducted to determine the biochemical effects of a PPE on both the quantity and quality of muscle lipids in HFHS diet-fed rats. The PPE was given mixed to the diet at a concentration that provided about 30 mg polyphenol/kg per d, which is equivalent to a high value within the normal dietary range of polyphenol intake in human subjects, and hence reflective of human nutrition.
Polyphenol extract prevented muscle TAG accumulation without modifying lipogenesis
Muscle TAG content was increased in HFHS diet-fed rats, and the PPE completely prevented their accumulation in the muscle. Sterol regulatory element-binding protein-1c mRNA expression, a transcriptional factor that activates the expression of key enzymes involved in lipogenesis(Reference Mulvihill, Allister and Sutherland7), and fatty acid synthase protein expression, a target of sterol regulatory element-binding protein-1c(Reference Wakil, Stoops and Joshi24), were not modified in HFHS or HFHS/PPE diet-fed rats compared with control rats. Moreover, phosphorylated acetyl-CoA carboxylase(Reference Kiens25) that controls lipid synthesis and lipid catabolism in mitochondria was unchanged in all groups. These results suggest that a direct action of the PPE on lipogenesis was not responsible for its observed lipid-lowering effect.
Fatty acid composition of muscle TAG was modulated by the HFHS diet but not by PPE supplementation. In fact, muscle TAG composition partly mirrors the quality of the fatty acids in the studied diets. Indeed, stearic acid (18 : 0) and oleic acid (18 : 1n-9) proportions in muscle TAG reflect well those of the diet in the present study. In line with this observation, previous studies have also reported that the fatty acid composition of serum, liver and muscle lipids is influenced by the type of fatty acids in the administered diet(Reference Ma, Folsom and Shahar26–Reference Lee, Pinnamaneni and Eo28).
SCD1 is a Δ9 desaturase that catalyses the conversion of SFA into MUFA, which serve as the preferred substrate for the synthesis of stored lipids(Reference Dobrzyn and Ntambi29). Although it is known that PUFA reduce and SFA increase SCD1 expression in the liver(Reference Ntambi30), there are few data describing SCD1 regulation by dietary fatty acids in the skeletal muscle. In the present study, elevated SCD1 activity index, calculated in the TAG fractions as the Δ9 desaturase activity index, was associated with increased fat accumulation in the skeletal muscle. However, Δ9 desaturase activity index remained similar in HFHS and HFHS/PPE diet-fed rats.
Polyphenol extract increased n-3 PUFA percentage in the phospholipid fraction
Phospholipid fatty acid composition of the cell membrane greatly influences membrane function, notably membrane fluidity, membrane protein incorporation and enzyme activities, receptor activation and, more generally, skeletal muscle function(Reference Hulbert, Turner and Storlien31, Reference Andersson, Nalsen and Tengblad32). For example, the binding of insulin to its receptor is significantly affected by the lipid environment around the receptor(Reference Liu, Baracos and Quinney33), and a high proportion of SFA in the cell membrane may impair insulin action(Reference Vessby34, Reference Janovska, Hatzinikolas and Mano35). On the contrary, the presence of PUFA (in particular n-3 fatty acids) in the muscle membrane has been associated with improved insulin sensitivity(Reference Storlien, Pan and Kriketos36, Reference Storlien, Jenkins and Chisholm37). Nevertheless, the mechanism by which n-3 fatty acids prevent insulin resistance in the skeletal muscle remains unclear.
In the present study, phospholipid content remained unchanged in all groups, while the fatty acid composition of phospholipids was modified by the HFHS diet. Some desaturase activity indices were also modified. In fact, the Δ9 desaturase activity index, calculated in the phospholipid fraction, was significantly increased in HFHS diet-fed rats compared with control rats, and the Δ5 desaturase activity index was significantly decreased. In human subjects, a reduced activity of Δ5 desaturase is associated with insulin resistance(Reference Vessby34). PPE supplementation increased n-3 PUFA proportion in skeletal muscle membrane phospholipids, but it had no effect on the desaturase activity indices. To our knowledge, this is the first study reporting a significant impact of a polyphenol supplementation on the n-3 PUFA content of muscle membrane phospholipids. Resveratrol has been reported to be efficient in protecting PUFA from oxidation(Reference Fremont, Belguendouz and Delpal38). A protective effect of the PPE against PUFA oxidation during absorption or directly on muscle membrane phospholipids could be advanced. It is interesting in this context to note also that insulin sensitisers such as bezafibrate(Reference Matsui, Okumura and Kawakami39) increase the unsaturation of membrane lipids, and that part of their beneficial action may be a membrane lipid-mediated effect on the insulin receptor. Although impaired insulin action in the skeletal muscle is generally related to muscle lipid accumulation(Reference Storlien, Jenkins and Chisholm37), long-chain n-3 fatty acids in skeletal muscle phospholipids may be important for an efficient insulin action and for an efficient glucose transport.
Polyphenol extract down-regulated membrane fatty acid transporter cluster of differentiation 36
The uptake of fatty acids by parenchymal cells has long been considered to occur by passive diffusion, but it has been clear in the past decades that fatty acid transporters facilitate the cellular entry of fatty acids, which are then recruited by cytoplasmic fatty acid-binding proteins(Reference Glatz, Luiken and Bonen40). Among them, fatty acid transporter CD36 has an important role in the metabolic adaptation to changes in nutrient availability(Reference Su and Abumrad41), and it has been reported that an increased plasmalemmal CD36 accounted for the increased rate of fatty acid transport into obese muscle(Reference Holloway, Benton and Mullen42). In accordance, we have observed an increased mRNA and protein expression of muscle CD36 in HFHS diet-fed rats. Interestingly, CD36 expression returned to control values in HFHS/PPE diet-fed rats. Such effect of polyphenols has already been observed on cardiac CD36(Reference Qin, Polansky and Harry8, Reference Monagas, Khan and Andres-Lacueva43, Reference Kaul, Sikand and Shukla44), but it is the first time that down-regulation of CD36 gene and protein expression by a PPE is described in the skeletal muscle. Decreased CD36 expression may participate in reducing plasma fatty acid entry into skeletal muscle cells and consequently lowering intracellular lipid accumulation in muscle in HFHS/PPE diet-fed rats, as it has also been observed that reducing plasmalemmal CD36 reduces the severity of intramuscular lipid accumulation(Reference Smith, Mullen and Junkin45). Whether polyphenols modulate directly or indirectly CD36 expression, as CD36 is highly responsive to insulin stimulation(Reference Glatz, Luiken and Bonen40), needs further investigation.
Polyphenol extract reduced mitochondrial fatty acid β-oxidation
CPT1 has a crucial role in the movement of fatty acid across the mitochondrial membrane and thus regulates mitochondrial fatty acid β-oxidation(Reference Kerner and Hoppel46). We observed that CPT1 protein expression was significantly decreased in HFHS/PPE diet-fed rats compared with control and HFHS diet-fed rats. In accordance, the increased β-oxidation in HFHS diet-fed rats (β-HAD activity) returned to normal values with PPE treatment. The surprising decrease in CPT1 protein expression observed in the present study with the PPE may result from the decrease in the fatty acid flux into the muscle following the decrease in CD36 protein expression. Literature evidence from different models has shown that increased fatty acid entry into the mitochondria may cause insulin resistance(Reference Koves, Ussher and Noland47, Reference Muoio and Newgard48). Koves et al. (Reference Koves, Ussher and Noland47) have recently proposed that elevated fatty acid oxidation during high-fat feeding results in a build-up of potentially toxic incomplete lipid oxidation products that may contribute to reducing insulin sensitivity. Moreover, Holloway et al. (Reference Holloway, Benton and Mullen42) demonstrated that intramuscular TAG depots were increased when the rate of fatty acid uptake exceeded the capacity of mitochondrial oxidation. Thus, the paradoxical decrease in CPT1 protein expression with PPE supplementation may be beneficial to insulin sensitivity. Consistent with the present results, several studies have shown that pharmacological inhibitors of CPT1 improve systemic insulin sensitivity(Reference Deems, Anderson and Foley49–Reference Zarain-Herzberg and Rupp51).
Polyphenol extract increased GLUT4
Glucose uptake into the skeletal muscle is primarily mediated by the insulin-responsive GLUT4(Reference Klip52). GLUT4 mRNA expression was decreased but not significantly in HFHS diet-fed rats, while the PPE significantly up-regulated its expression by approximately 140 %. This suggests a positive effect of the PPE on the regulation of glucose transport. Previous studies have shown that some individual polyphenols or other PPE such as resveratrol(Reference Chi, Chen and Chi53, Reference Breen, Sanli and Giacca54) or green tea polyphenols(Reference Qin, Polansky and Harry8, Reference Cao, Hininger-Favier and Kelly55) had a beneficial effect on muscle glucose transport.
Polyphenol extract did not modify mitochondrial biogenesis
Mitochondria are the principal energy sources of the cell that convert nutrients into energy through cellular respiration(Reference Wallace56). In the skeletal muscle, PGC-1α has been shown to stimulate mitochondrial biogenesis via co-activation of the NRF1 and to regulate genes involved in the oxidative phosphorylation. PGC-1α also co-activates the PPAR, thereby regulating pathways of lipid metabolism(Reference Lin, Handschin and Spiegelman57). We observed that mRNA expression of PGC-1α was down-regulated with the HFHS diet, with or without the PPE. Consistent with the present results, it has been demonstrated that palmitic acid or high SFA down-regulate mRNA expression of PGC-1α(Reference Coll, Jove and Rodriguez-Calvo58, Reference Koves, Li and An59). In contrast, an activation of PGC-1α by resveratrol was demonstrated by Lagouge et al. (Reference Lagouge, Argmann and Gerhart-Hines60); however, it must be underlined that their diet contained 4 g resveratrol/kg diet, while the PPE (0·15 % resveratrol) was given at 2 g PPE/kg diet in the present study. Nevertheless, it must be noted that down-regulation of PGC-1α with the HFHS diet was low (only 20 % decreased). NRF1 is an intermediate transcription factor that stimulates the synthesis of mitochondrial transcription factor A, a final effector activating the duplication of mitochondrial DNA molecules(Reference Vina, Gomez-Cabrera and Borras61). The gene expression of NRF1 was slightly down-regulated with the HFHS diet and up-regulated with the PPE, while the mRNA expression of mitochondrial transcription factor A was unchanged among the three experimental groups. Moreover, the mitochondrial respiratory chain complex activities were not modified whatever the diet. PGC-1α also co-activates the PPAR, thereby regulating pathways of lipid metabolism(Reference Lin, Handschin and Spiegelman57). In the present study, the gene expression of PPAR-α was not modified whatever the diet. Regarding the slight modification of PGC-1α and NRF1 gene expression with the HFHS or HFHS/PPE diet, and the absence of modification of transcription factor A, mitochondrial and PPAR-α gene expression and of mitochondrial respiratory chain complex activities, the present results suggest that the lipid-lowering effect of the PPE in muscle did not result from either an effect on mitochondrial biogenesis or an effect on mitochondrial activity.
In conclusion, the PPE favourably modulated muscle lipid metabolism and membrane phospholipid fatty acid composition, with an increase in the proportions of n-3 PUFA in muscle phospholipids, in HFHS diet-fed rats. A diagram of the pathways affected by the PPE is provided in Fig. 3. The PPE lowered CD36 gene and protein expression, thus probably decreasing fatty acid transport and lipid accumulation in the skeletal muscle. It also increased muscle GLUT4 expression. However, an effect of the PPE on muscle mitochondrial biogenesis and mitochondrial activity did not seem to be implicated.

Fig. 3 Polyphenol extract (PPE) intake modified muscular membrane lipid and glucose transport in high-fat–high-sucrose diet-fed rats. High fat intake increased membrane fat transport, which is then stored as TAG or degraded in the mitochondria. The PPE lowered cluster of differentiation 36 (CD36) gene and protein expression, thus probably decreasing fatty acid transport and lipid accumulation (TAG and diacylglycerols (DAG)) in the skeletal muscle. The decrease in lipid entry in the cell or a direct effect of the PPE may be involved in the increase in carnitine palmitoyltransferase 1 (CPT1) protein expression and β-oxidation (due to lower β-hydroxyacyl CoA dehydrogenase activity). The PPE also increased muscle GLUT4 expression. Whether the effect of the PPE on membrane glucose uptake passes by an activation of insulin receptor needs to be investigated. FA, circulating fatty acids; IRS, insulin receptor substrate.
Acknowledgements
The authors thank L. Lepourry and B. Bonnafos for the use of the animal facilities. The authors acknowledge the Société Française de Distilleries (Mr David Ageron) that supplied the PPE powder. The authors declare no conflict of interest. The present study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. C. C. and C. F.-C. designed the study; M. A., G. F., V. O., A. S. and C. F.-C. conducted the study, M. A., F. M., J.-P. C., C. W.-C., M.-A. C., C. C. and C. F.-C. analysed the data; C. F.-C. wrote the manuscript and had responsibility for the final content. All authors read and approved the final manuscript.













