Article contents
Using differential scanning calorimetry to characterize the precipitation and dissolution of V(CN) and VC particles during continuous casting and reheating process
Published online by Cambridge University Press: 26 June 2018
Abstract
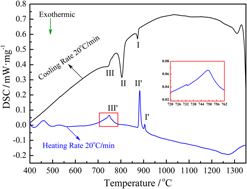
In this work, differential scanning calorimetry (DSC) was used to characterize and analyze the precipitation/dissolution kinetics of second phase particles during the cooling/reheating process in a vanadium microalloyed steel. The results indicated that three obvious exothermic peaks were detected on the cooling DSC curve. Furthermore, three corresponding endothermic peaks were also detected on the heating DSC curve. Combined with thermodynamic calculation and transmission electron microscopy analysis, these three exothermic peaks along cooling DSC curve were defined as the precipitation reaction of V(CN), the reaction of austenite transformation into ferrite and the precipitation reaction of VC, respectively. Meanwhile, three corresponding reverse reactions for cooling were also defined along the reheating DSC curve. The linear regression result revealed that the precipitation activation energies for V(CN) and VC were identified as 311.2 kJ/mol and 167.6 kJ/mol, respectively. The dissolution activation energies for VC and V(CN) were identified as 255.4 kJ/mol and 592.6 kJ/mol, respectively.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 1
- Cited by




