Introduction
Cacao (Theobroma cacao L.) is an undergrowth tree that is mostly grown by small-scale farmers in the main producing countries such as Cote d’Ivoire and Ghana (Koko et al., Reference Koko, Snoeck, Lekadou and Assiri2013; Jagoret et al., Reference Jagoret, Michel, Ngnogué, Lachenaud, Snoeck and Malézieux2017; Mattalia et al., Reference Mattalia, Wezel, Costet, Jagoret, Deheuvels, Migliorini and David2022; Sanial et al., Reference Sanial, Ruf, Louppe, Mietton and Hérault2022). Initially, it was grown under tree canopies (Jagoret et al., Reference Jagoret, Snoeck, Bouambi, Ngnogue, Nyassé and Saj2018; Sanial et al., Reference Sanial, Ruf, Louppe, Mietton and Hérault2022). Between 1961 and 2020, worldwide increasing cacao demand and economic interest resulted in an increase in cultivated area from 4 to 12 million hectares (FAO, 2022). This is particularly the case in Ivory Coast, the world’s largest cacao bean producer, where more than 70% of the cacao orchards were established following forest clearing with no overhead shade trees, except during the early years of plantation establishment (Assiri et al., Reference Assiri, René, Olivier, Ismaël, Jules and Amoncho2009). The monoculture cropping system, known to produce high cacao bean yields, called the ‘full sun’ system, was the main advocated system during this period (Koko et al., Reference Koko, Snoeck, Lekadou and Assiri2013; Jagoret et al., Reference Jagoret, Snoeck, Bouambi, Ngnogue, Nyassé and Saj2018; Mattalia et al., Reference Mattalia, Wezel, Costet, Jagoret, Deheuvels, Migliorini and David2022). The monoculture cropping system relies also on improved varieties of cacao and increased chemical soil fertilisation. As a result, almost 80% of the country’s forest area was lost in the second half of the last century, partly due to cacao culture expansion. This heavy deforestation in favour of monoculture cropping systems combined with a lack of sustainable soil management practices has led to biodiversity loss, soil degradation, plant disease outbreaks (such as swollen shoot virus), and decreased production capacity. Also, it has contributed to increased carbon emissions in the atmosphere (Somarriba et al., Reference Somarriba, Cerda, Orozco, Cifuentes, Dávila, Espin, Mavisoy, Ávila, Alvarado, Poveda, Astorga, Say and Deheuvels2013; Abou Rajab et al., Reference Abou Rajab, Leuschner, Barus, Tjoa and Hertel2016; Panja, Reference Panja2021). Cacao agroforestry, which involves the temporal and/or spatial association of one or more woody species with cacao, has been proposed as a solution for tackling biodiversity loss, reducing human pressure on remaining forests, and mitigating climate change effects (Sanial et al., Reference Sanial, Ruf, Louppe, Mietton and Hérault2022). Trees in cacao plantations provide shade for cacao plants, contribute to carbon sequestration in tree biomass, recycle nutrients to maintain soil fertility, accumulate soil organic matter (SOM), and conserve soil biodiversity (Beer et al., Reference Beer, Muschler, Kass and Somarriba1997; Somarriba et al., Reference Somarriba, Cerda, Orozco, Cifuentes, Dávila, Espin, Mavisoy, Ávila, Alvarado, Poveda, Astorga, Say and Deheuvels2013; Batsi et al., Reference Batsi, Sonwa, Mangaza, Ebuy and Kahindo2021). Several studies of traditional cacao-growing systems in Cameroon and Ghana have shown that shade trees favour cacao tree establishment and survival and reduce physiological stress by protecting cacao trees from increased temperatures (Beer et al., Reference Beer, Muschler, Kass and Somarriba1997; Abou Rajab et al., Reference Abou Rajab, Leuschner, Barus, Tjoa and Hertel2016; Blaser et al., Reference Blaser, Oppong, Hart, Landolt, Yeboah and Six2018). Furthermore, companion trees in cacao plantations generate additional income through the production of wood products (e.g., timber, firewood, fruit, resins, and medicines), and cultural and aesthetic services (Beer et al., Reference Beer, Muschler, Kass and Somarriba1997; Somarriba et al., Reference Somarriba, Cerda, Orozco, Cifuentes, Dávila, Espin, Mavisoy, Ávila, Alvarado, Poveda, Astorga, Say and Deheuvels2013; Batsi et al., Reference Batsi, Sonwa, Mangaza, Ebuy and Kahindo2021). Many studies have shown that intercropping shade trees with cacao improves cacao growth and bean yield (Koko et al., Reference Koko, Snoeck, Lekadou and Assiri2013; Asitoakor et al., Reference Asitoakor, Vaast, Ræbild, Ravn, Eziah, Owusu, Mensah and Asare2022). However, others have reported a neutral or even negative effect on cacao beans in terms of yield (Abou Rajab et al., Reference Abou Rajab, Leuschner, Barus, Tjoa and Hertel2016; Abdulai et al., Reference Abdulai, Vaast, Hoffmann, Asare, Jassogne, Van Asten, Rötter and Graefe2018; Sauvadet et al., Reference Sauvadet, Saj, Freschet, Essobo, Enock, Becquer, Tixier and Harmand2020; Blaser-Hart et al., Reference Blaser-Hart, Hart, Oppong, Kyereh, Yeboah and Six2021). The interactions between two layers of trees in a plantation may involve competition for light, water, or nutrient resources. However, facilitation processes such as a favourable microclimate and the supply of nutrients from litter to the soil could also occur (Beer et al., Reference Beer, Muschler, Kass and Somarriba1997; Abdulai et al., Reference Abdulai, Vaast, Hoffmann, Asare, Jassogne, Van Asten, Rötter and Graefe2018). Trebissou et al. (Reference Trebissou, Tahi, Munoz, Sanchez, N’Guetta, Cilas and Ribeyre2021) reported several factors affecting cacao growth and productivity, such as the characteristics of neighbouring trees, resource limitations (nutrients and water), and physiological conditions (photoinhibition, radiation load, and low leaf water potential) inducing a competitive effect and/or affecting production mechanisms. These interactive effects are mainly related to the associated tree species, notably the quantity and quality of their leaf litter (Sauvadet et al., Reference Sauvadet, Saj, Freschet, Essobo, Enock, Becquer, Tixier and Harmand2020). These authors also reported that shade trees could be expected to improve soil parameters even if cacao yields remain unchanged.
It should be stressed that most of these results were not obtained via an experimental design. These studies were mostly based on the comparisons between farmers’ orchards where the species and number of companion trees varied (Abou Rajab et al., Reference Abou Rajab, Leuschner, Barus, Tjoa and Hertel2016; Abdulai et al., Reference Abdulai, Vaast, Hoffmann, Asare, Jassogne, Van Asten, Rötter and Graefe2018; Sauvadet et al., Reference Sauvadet, Saj, Freschet, Essobo, Enock, Becquer, Tixier and Harmand2020; Blaser-Hart et al., Reference Blaser-Hart, Hart, Oppong, Kyereh, Yeboah and Six2021; Asitoakor et al., Reference Asitoakor, Vaast, Ræbild, Ravn, Eziah, Owusu, Mensah and Asare2022). Other factors, such as soil or farmer practices, were not always under control. The shade trees in these studies involved mainly forest and fruit tree species maintained after forest clearing (Asitoakor et al., Reference Asitoakor, Vaast, Ræbild, Ravn, Eziah, Owusu, Mensah and Asare2022).
Fast-growing tree legumes, due to their ability to fix atmospheric nitrogen in symbiotic association with microorganisms, provide benefits and have been tested in agroforestry systems over the last few decades. Several authors have reported improved productivity when tree legumes are combined with other tree plantations, such as coconut or cacao plantations (N’guessan et al., Reference N’guessan, Dupuy, Assa and N’Goran2006; Somarriba et al., Reference Somarriba, Cerda, Orozco, Cifuentes, Dávila, Espin, Mavisoy, Ávila, Alvarado, Poveda, Astorga, Say and Deheuvels2013; Abou Rajab et al., Reference Abou Rajab, Leuschner, Barus, Tjoa and Hertel2016; Koutika and Richardson, Reference Koutika and Richardson2019; Sauvadet et al., Reference Sauvadet, Saj, Freschet, Essobo, Enock, Becquer, Tixier and Harmand2020). However, very few of these studies have assessed the long-term influence of specific shade tree species in cacao-based agroforestry systems (Asitoakor et al., Reference Asitoakor, Vaast, Ræbild, Ravn, Eziah, Owusu, Mensah and Asare2022), especially regarding cacao productivity and the achievement of broader carbon sequestration objectives.
Tree characteristics, such as the quality and quantity of litter produced, influence soil fertility and net primary production. Indeed, there is a close relationship between foliage and litter characteristics and soil nutrient availability (Hobbie, Reference Hobbie2015). According to the agroecological performance evaluation tool promoted by the Food and Agriculture Organization of the United Nations (FAO), the SOM content is the most relevant indicator of soil health (Mottet et al., Reference Mottet, Bicksler, Lucantoni, De Rosa, Scherf, Scopel, López-Ridaura, Gemmil-Herren, Bezner Kerr, Sourisseau, Petersen, Chotte, Loconto and Tittonell2020). Indeed, SOM is widely recognised for its significance in nutrient exchange within the soil environment, as a carbon sink, and for its influence on soil fertility. Consequently, it is closely related to plant productivity, particularly that of cacao (Oldfield et al., Reference Oldfield, Bradford and Wood2019; Minasny et al., Reference Minasny, McBratney, Wadoux, Akoeb and Sabrina2020; Rangel-Mendoza and Silva-Parra, Reference Rangel-Mendoza and Silva-Parra2020).
In 1998, the Centre National de Recherche Agronomique (CNRA) launched an experiment at the Divo Research Station to test the growth and production of cacao plants planted with two types of tree legumes, Albizia lebbeck (L.) Benth. (Fabaceae) and Acacia mangium Willd. (Fabaceae), against a control [cacao without associated tree legume (ATL)]. After 20 months of intercropping, Konan and Koffi (Reference Konan and Koffi2003) reported positive effects of A. lebbeck and negative effects of A. mangium on the growth and productivity parameters of young cacao trees. After 20 years, the trial was still ongoing, enabling the positive or negative impact of the two legumes on cacao productivity to be evaluated experimentally over a longer period. Consequently, this study evaluated the biomass of 20-year-old cacao plants and their bean yield in the presence or absence of tree legumes using observed and measured data.
Materials and methods
Study area
This study was carried out in the centre-west of Ivory Coast at the CNRA experimental station in Divo (5° 47.528280' N/-5° 15.041462' W). The natural vegetation of this region is the Guinean semideciduous forest. The climate is subequatorial, with an annual average temperature of 26 °C and an average relative humidity of 85%. The rainfall regime is bimodal, with two dry seasons and two wet seasons. The annual average precipitation is more than 1200 mm (Ehounou et al., Reference Ehounou, Kouamé, Tahi, Kassin, Kotaix, Dékoula, Yao, Kouadio, N’guessan and Soro2019). Soils are highly weathered, as in most intertropical regions of the world. These soils have diffuse horizon boundaries, a clay assemblage dominated by low-activity clays (mainly kaolinite), and a high sesquioxide content. They have great soil depth (> 100 cm), good permeability, and stable microstructure. However, these soils are chemically poor and characterised by extremely low native fertility resulting from very low nutrient reserves (WRB, Reference WRB2015).
The soil at the experimental site (pedo-horizon A: 0 to 15 cm depth) had the following characteristics: (i) clay content of 224–447 g kg−1; (ii) soil organic carbon (SOC) of 6.4–9.7 g kg−1; (iii) total nitrogen (N) of 0.8 to 1.0 g kg−1; (iv) total phosphorus (P) of 94.5–138.8 mg kg−1; (v) available P of 0.8–2.4 mg kg−1; (vi) exchangeable potassium (K+) 0.1–0.2 cmol kg−1; (vii) magnesium (Mg2+) of 0.4–1.1 cmol kg−1; (viii) calcium (Ca2+) of 2.1–3.3 cmol kg−1; and (ix) pHwater ranging from 5.1 to 5.9 (CNRA, unpublished technical report). These soils are generally considered suitable for cacao cultivation (Ehounou et al., Reference Ehounou, Kouamé, Tahi, Kassin, Kotaix, Dékoula, Yao, Kouadio, N’guessan and Soro2019).
Experimental design
An experimental cacao agroforestry system (cAFS) was set up in 1998 on a former experimental cacao plantation site that was left fallow and colonised by Chromolaena odorata for more than 10 years. The tree legumes A. mangium and A. lebbeck were planted after clearing, and two years later, the cacao seedlings were planted underneath. The trial was thus considered to be 20 years old at the time of data collection in 2020. Although the plants were maintained for 20 years, the whole experiment was supposedly conducted according to the same protocols. Indeed, the site has been managed more akin to many cacao plantations in Côte d’Ivoire, especially those operated by farmers, despite being on an experimental station. However, the trial plots exhibited no signs of infestation or disease.
The experiment involved four blocks arranged perpendicular to the slope (< 1%) over a 2-ha area. Initially, each block was subdivided into five plots of 726 m2 (33 m × 22 m) with five ATL factor levels: one unshaded cacao monoculture plot (Control), two plots with A. lebbeck (Cacao-Alb), and two plots with A. mangium (Cacao-Aca). Four-metre-wide alleys separated blocks and plots. The cacao trees were planted at a spacing of 2.5 m between trees in a row and 3 m between rows, i.e., a density of 1333 plants ha−1. ATLs were planted at a density of four trees per plot, i.e., 55 trees ha−1. For 20 years, the trial was maintained using small farmers’ traditional cacao plantation management practices. Few data were collected in the early years, but all parameters were unfortunately no longer evaluated due to difficulties during and after the internal armed conflict that ended in 2011. In 2020, considering the mortality of ATLs and cacao trees over time, three plots per block were selected, corresponding to Cacao-Alb, Cacao-Aca, and the Control group, for the purpose of the present study. In subplots delineated around the ATLs within these plots, the ATLs were assessed for biomass, while the cacao trees were assessed for biomass and bean yield along two 10 m transect diagonals.
Three distance classes delineated between the trunks of the cacao trees and ATL were defined according to the rows of planted cacao trees: D1 was defined as the first row of cacao trees closest to the ATL (1.75 m), D2 was defined as those between 3.25 and 5 m from the ATL, and D3 was defined as those between 7 and 9 m from the ATL.
Data collection
Cacao tree biomass production
Cacao trees and ATLs were counted, and the number of missing trees was assessed by the difference from the initial planting layout. The circumference of the trunk at a height of 50 cm was measured on the current cacao trees, from which the diameter at 50 cm (D50) was determined. The diameter at breast height (DBH) of the ATLs was also determined from the circumference at 1.30 m. The cacao and ATL heights at their tops were measured using a clinometer (Suunto, type PM-5/360PC). The extent of canopy cover of the ATLs was estimated from crown diameter measurements using a decametre. To test the effect of the distance between the ATL and the cacao, tree measurements at three distance classes (D1, D2, and D3) were considered. The cacao count was used to calculate the current density per unit area and compare it to the initial planting density. Cacao density under the ATL canopy was also specifically recorded.
The total biomass of each cacao plant, including both aboveground (AGB) and belowground (BGB) biomass, was estimated using the allometric equation developed by Somarriba et al. (Reference Somarriba, Cerda, Orozco, Cifuentes, Dávila, Espin, Mavisoy, Ávila, Alvarado, Poveda, Astorga, Say and Deheuvels2013) for AGB (Eq 1) and that of Cairns et al. (Reference Cairns, Brown, Helmer and Baumgardner1997) for BGB (Eq 2), assuming a 50 cm diameter. The ATL total biomass was estimated using the allometric equations of Chave et al. (Reference Chave, Réjou-Méchain, Búrquez, Chidumayo, Colgan, Delitti, Duque, Eid, Fearnside, Goodman, Henry, Martínez-Yrízar, Mugasha, Muller-Landau, Mencuccini, Nelson, Ngomanda, Nogueira, Ortiz-Malavassi, Pellissier, Ploton, Ryan, Saldarriaga and Vieilledent2014) for AGB (AGBi; Eq 3) and that of Cairns et al. (Reference Cairns, Brown, Helmer and Baumgardner1997) for BGB (BGBi; Eq 4). For ATL wood density, we referred to the International Centre for Agroforestry African wood density database produced by (Carsan et al., Reference Carsan, Orwa, Harwood, Kindt, Stroebel, Neufeldt and Jamnadass2012).
where AGB = cacao aboveground biomass (kg tree−1); BGB = cacao belowground biomass (kg tree−1); H = cacao tree height (m); D50 = cacao diameter at 50 cm height (cm); AGBi = ATL aboveground biomass (kg ha−1); BGBi = ATL belowground biomass (kg ha−1); Hi = ATL tree height (m); DBHi = ATL diameter at breast height (cm); and Wi = ATL wood density (g cm−3).
Cacao bean yield
Cacao bean yields were determined using the counting methodology proposed by Jagoret et al. (Reference Jagoret, Michel, Ngnogué, Lachenaud, Snoeck and Malézieux2017). The pods were counted four times at regular intervals from May to November 2020, considering the gradual appearance and maturation of pods. The survey included all the pods and cherelles at least 10 cm long. These were assumed to reach maturity (Jagoret et al., Reference Jagoret, Michel, Ngnogué, Lachenaud, Snoeck and Malézieux2017). At each round of counting, immature pods were counted but not removed. However, they were marked to avoid double counting. All ripe pods were collected, classified, and counted as healthy, damaged, or rotten for each cacao tree. Each healthy pod was weighed. According to Tahi et al. (Reference Tahi, Trebissou, Guiraud, Ribeyre, Lachenaud, Pokou, Walet, Aka, Coulibaly, Kébé, Assi, Koné, Kassin and Cilas2017), the bean yield per tree was calculated from the number of pods and the average pod weight per tree. The potential bean yield (PBY) was estimated using the total number of pods (healthy or not), and the estimated marketable bean yield (MBY) was determined using only the number of healthy pods (Eq 5 and Eq 6). The difference between PBY and MBY was considered the estimated bean yield loss (BYL) due to unmarketable pods (Eq 7), and its relative proportion to the estimated PBY (BYLR) was also calculated for each treatment (Eq 8).
where PBY is the estimated potential bean yield (kg tree−1); MBY is the estimated marketable bean yield (kg tree−1); NP is the total pod number (pod tree−1); NHP is the healthy pod number (pod tree−1); HPW is healthy ripe pod weight average (kg tree−1); BYL is the estimated loss of bean yield from unhealthy pods (kg tree−1); BYLR is the estimated bean yield loss proportion relative to PBY; and CC is the pod weight-to-marketable cacao bean conversion coefficient for healthy ripe pods (0.0875).
Soil organic matter assessment
Soil was sampled in November 2019 in the subplot using a 6-cm diameter cylindrical auger every 10 cm to a depth of 30 cm. In the ATL subplots, sampling points were located 1.75 m, 3.25 m, 5 m, 7 m, and 9 m from the tree legume trunk within two diametrically opposed transects. Soil samples were then pooled from both transects for each distance from the ATL to obtain one composite sample per tree distance and soil depth. In the control plots, the same sampling protocol was applied considering a randomly chosen point on each subplot from which the sampling distances were defined. Sampling was performed after carefully removing the topsoil litter layer. The soil samples were air-dried and stored at ambient temperature. The soil samples were sieved at 2 mm. To assess the SOC content, infrared spectrometry and chemical analysis of soil C were performed according to the procedure of Malou et al. (Reference Malou, Moulin, Chevallier, Masse, Vayssières, Badiane-Ndour, Tall, Thiam and Chapuis-Lardy2021). Visible and near-infrared reflectance spectra of each sample (n = 1320 sieved at 2 mm) were acquired at 2-nm intervals between 350 and 2500 nm with a LabSpec 4 spectrophotometer (Analytical Spectral Devices, Boulder, CO, USA). The soil C content of the subset samples was determined by the Dumas method on 100 mg aliquots of soil (ground to < 0.2 mm) using a CHN elemental analyser (Thermo Finnigan Flash EA1112, Milan, Italy) (NF ISO 10694 1995). The numerical processing of spectral data was carried out using TheUnscrambler® X 10.4 software (Camo Software, Oslo, Norway). The SOM content was estimated from the SOC content (Eq 9). For this study, only the SOM data from 0 to 30 cm were considered to test the relationships between soil fertility and the cacao tree biomass and bean yield.
where SOM is the soil organic matter (g kg−1); SOC is the soil organic carbon content (g kg−1), and 1.724 is the conversion factor (Bemmelen factor).
Data analysis
Statistical analyses were performed to explore (i) the effects of ATLs on cacao biomass, bean yield parameters, and SOC content vs the Control (no ATL) and (ii) the extent of these effects according to the distance (D1, D2, and D3) of the cacao tree to the ATL. These statistical analyses were conducted using linear mixed models (Lmer), which included the block effect as a random effect. The assumptions of Lmer were checked by inspecting residual plots for homogeneity and quantile–quantile plots for normality. A post hoc test (lmerTest R package, Kuznetsova et al., Reference Kuznetsova, Brockhoff and Christensen2017) was used to compare the means of different factor levels for each factor among treatments, revealing a significant effect at the 5% level. We used a linear regression model to determine the relative importance of SOM, especially in the upper soil layer (0–10 cm) on cacao pod production and bean yields. All the statistical analyses were performed using R statistical software (version 4.3.0, R Core Team, 2023), under its R studio interface.
Results
Associated tree legume characteristics and cacao tree density
After 20 years of growth and intercropping with cacao trees, the density of ATLs (A. lebbeck and A. mangium) showed no significant variation (p > 0.05) between the Cacao-Alb and Cacao-Aca associations (Table 1). However, the height and trunk diameter of A. mangium were significantly greater (p < 0.01) than those measured for A. lebbeck. In contrast, the crown diameters of these tree legumes were not significantly (p > 0.05) different (Table 1). Compared to the initial planting density (1333 tree ha−1), the cacao density was reduced by 51%, 39%, and 32% in the Cacao-Aca, Cacao-Alb, and Control treatments, respectively. The cacao tree density in Cacao-Aca (653 ± 206.3 tree ha−1) was significantly lower (p < 0.001) than that in Cacao-Alb (817 ± 174.4 tree ha−1), which in turn was lower than that in the Control (912 ± 267.5 tree ha−1).
Table 1. Means (± standard deviation) of density of associated tree legumes, diameter at breath height (DBH), and height and crown diameter in a 20-year-old cocoa-agroforest in Divo (Ivory Coast)

* For each variable, values followed by the same letter are not significantly different (p < 0.05).
Tree legume effects on cacao tree biomass and bean yield among treatments
The cacao trunk circumference did not significantly differ (p > 0.05) between the Cacao-Aca or Cacao-Alb ATL systems and the cacao monoculture (Control). Moreover, the height of the cacao trees associated with the tree legumes did not significantly differ (p > 0.05) from that of the Control plants, regardless of the ATL species (Table 2). The mean total biomass of the cacao plants varied between 55.3 and 63.4 kg tree−1, with 82% of the total biomass being from aboveground materials (Table 2). The total biomass of cacao (AGB + BGB) in the Cacao-Aca group was slightly lower than that in the Control (−12.4%), although these differences were not statistically significant (p > 0.05; Table 2). Cacao–Alb association also had no significant effect on cacao biomass compared to that in the Control group. Generally, the cacao stand biomass was not significantly different between the Cacao-Alb group and the Control group after 20 years of intercrop (Table 2, Table 3). In contrast, the presence of A. mangium significantly reduced the cacao biomass compared to the Control (Table 3; −40%).
Table 2. Means (± standard deviation) of cacao cropping systems characteristics when associated with the tree legumes Albizia lebbeck (in Cacao-Alb) or Acacia mangium (in Cacao-Aca), or without (Control) in a 20-year-old experiment in Divo (Ivory Coast). Cacao tree variables: Circumference: the circumference of the trunk was measured at 50 cm above ground
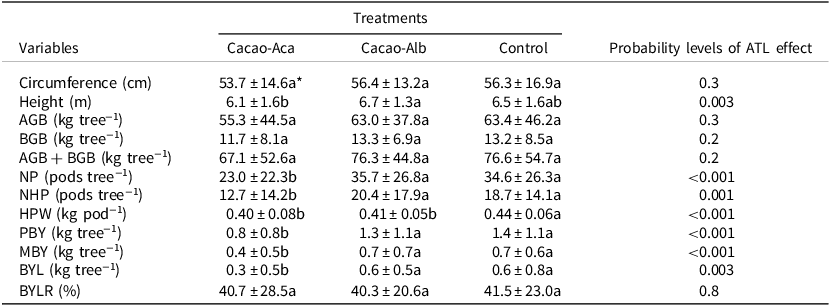
AGB = Aboveground biomass; BGB = Belowground biomass; NP = total pods number; NHP = healthy pods number; HPW = healthy pods weight; PBY = potential bean yield; MBY = marketable bean yield; BYL = bean yield loss due to unhealthy pods; BYLR = proportion of bean yield lost relative to PBY.
* Values followed by the same letter in a row are not significantly different (p < 0.05).
Table 3. Means (± standard deviation) of cacao tree biomass and bean yields at the plot level in 20-year-old cacao plantations associated with the tree legumes Acacia mangium (in Cacao-Aca) or Albizia lebbeck (in Cacao-Alb) compared with a conventional 20-year-old cacao monoculture (Control)

AGB = Aboveground biomass; BGB = Belowground biomass; PBY = potential bean yield; MBY = marketable bean yield.
* Values followed by the same letter in a row are not significantly different (p < 0.05).
There were as many as 36 pods tree−1 in Cacao-Alb. The number of pod tree−1 was significantly lower (p < 0.001) in the Cacao-Aca plot than in the Cacao-Alb or Control plots (Table 2). The introduction of ATL resulted in a significant (p < 0.001) decrease in pod weight in both the Cacao-Aca and Cacao-Alb plots (Table 2). Cacao PBY was estimated to vary between 535 and 1163 kg ha−1 among the treatments. Furthermore, the yield was lower in Cacao-Aca plot (−43%; p < 0.001) than in the Control and Cacao-Alb plots, which showed similar values. The proportion of unmarketable beans (BYLR) resulting from unhealthy pods ranged from 40.7% to 41.5%, and the proportion did not differ among the treatments (Table 2). Finally, cacao stand bean production was not significantly different between the Cacao-Alb group and the Control group after 20 years of intercrop (Table 3). In contrast, the presence of A. mangium significantly reduced cacao PBY (−54%) and MBY (−57%) compared to those observed in the other treatments.
Effect of the distance between cacao trees and the associated tree legumes
The mean cacao height measured close to the ATL (at distance D1) in Cacao-Aca was significantly lower (p < 0.01) than that measured at distances D2 and beyond (Table 4). Trunk circumference did not differ significantly with distance to the ATL regardless of the species. Likewise, the distance to the ATL did not significantly impact the calculated cacao biomasses.
Table 4. Means (±standard deviation) of growth characteristics and bean production of cacao trees at three distances to the associated tree legumes Albizia lebbeck (in Cacao-Alb) or Acacia mangium (in Cacao-Aca), in a 20-year-old experiment in Divo (Ivory Coast). Distance between cocoa trunk and ATL trunks D1:0 to 1.75 m, D2:3.25 to 5 m, D3:7 to 9 m. The circumference of the trunk was measured at 50 cm above ground
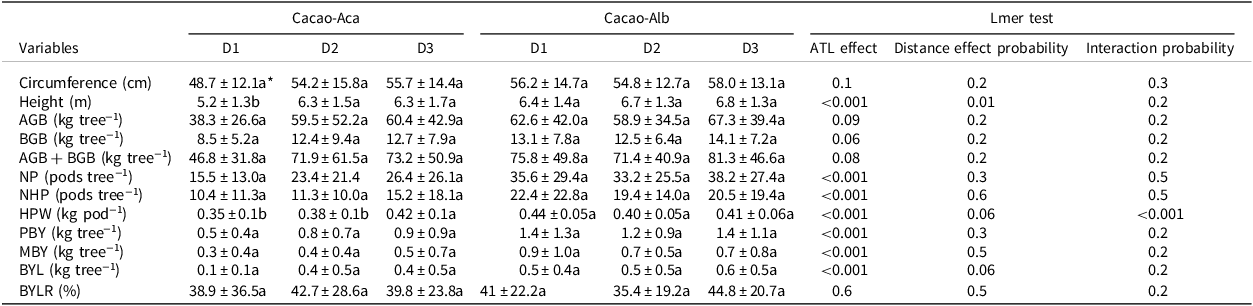
AGB = Aboveground biomass; BGB = Belowground biomass; NP = total pods number; NHP = healthy pods number; HPW = healthy pods weight; PBY = potential bean yield; MBY = marketable bean yield; BYL = bean yield loss due to unhealthy pods; BYLR = proportion of bean yield lost relative to PBY; Lmer = linear mixed models.
* For each variable and within the same agroforest, values followed by the same letter are not significantly different (p < 0.05).
The healthy pod weight (HPW) and PBY decreased when the cacao plants were growing close to the A. mangium plants (at D1 and D2). Conversely, these variables were significantly greater in cacao trees close to A. lebbeck (p < 0.001; Table 4).
Tree legume effects on the soil organic matter pool
The SOM content in the 0–10 and 0–30 cm soil layers significantly differed among the treatments (p < 0.001; Table 5). In both soil layers, Cacao-Alb and Control groups showed similar SOM values. However, in comparison to those in the control group, the SOM of the Cacao–Aca group exhibited a significant decrease (−22%) in both soil layers. Overall, in terms of absolute values, the Cacao-Alb treatment had the highest SOM content compared to the other two treatments. Specifically, there was a 12% and 9% increase in SOM at 0–10 cm and 0–30 cm soil depths, respectively, compared to those in the Control. Additionally, there was 44% and 40% more SOM at 0–10 cm and 0–30 cm soil depths, respectively, in the Cacao-Alb group than in the Cacao-Aca group.
Table 5. Mean (± standard deviation) of soil organic matter (SOM) in 20-year-old cacao agroforests at the plot level and at three distances to the associated tree legumes (ATL) Acacia mangium (in Cacao-Aca) or Albizia lebbeck (in Cacao-Alb) compared to a conventional 20-year-old cacao monoculture (Control). Distance between cocoa trunk and ATL trunks D1:0 to 1.75 m, D2:3.25 to 5 m, D3:7 to 9 m. Probability levels derived from the linear mixed models (Lmer) test on SOM for comparison of effects considering [A] presence or absence of ATL, [B] distance from the ATL, and [A
![]() ${\rm{\, \times\, }}$
B] interaction of ATL presence with distance to the cacao tree
${\rm{\, \times\, }}$
B] interaction of ATL presence with distance to the cacao tree

* For each variable, plot-level values followed by the same capital letter across treatments are not significantly different (p < 0.05).
† For each variable and within the same agroforest, values at the distances to the ATL followed by the same lowercase letter are not significantly different (p < 0.05).
The distance to the ATL did not significantly impact the estimated SOM content in any of the Cacao-Aca and Cacao-Alb plots (p > 0.05; Table 5).
Discussion
In this study, the estimated potential cacao bean yields of the studied systems fall within the production range typically observed in Ivory Coast (250–1251 kg ha−1), as reported by Assiri et al. (Reference Assiri, René, Olivier, Ismaël, Jules and Amoncho2009, Reference Assiri, Abel, Arnaud, Kacou, Florent and Yves2012). Cacao circumferences and heights measured at the Divo experimental station were within the range of values reported by Borden et al. (Reference Borden, Anglaaere, Adu-Bredu and Isaac2019) in Ghana and Jagoret et al. (Reference Jagoret, Michel, Ngnogué, Lachenaud, Snoeck and Malézieux2017, Reference Jagoret, Snoeck, Bouambi, Ngnogue, Nyassé and Saj2018) in Cameroon. The experimental cacao plantations in this study could be considered representative of non-intensive cacao plantations, as are most smallholder plantations on Ivory Coast. However, it is important to note that the yield evaluation covered only an 8-month period rather than the generally recommended 2-year period.
Intercropping A. mangium and A. lebbeck in a cacao stand induced no significant variation in the biomass of individual cacao trees compared to that in the cacao monoculture, regardless of the ATL species. However, Cacao-Aca negatively affected the productivity parameters calculated at the stand level (p < 0.05). In contrast, when used as a companion tree, A. lebbeck did not result in a reduction in cacao biomass or bean production compared to the unshaded cacao trees (Control). This difference may be due to litter quality and SOM content in addition to differences in cacao stand density per plot compared with those of the Control, which were lower in the Cacao-Aca plots than in the Cacao-Alb plots. Therefore, the density of cacao trees appeared to be the main determinant of biomass production per unit of surface area. SOM is widely known to be important for nutrient exchange in the soil environment, as a carbon sink, and for soil fertility and is therefore related to the productivity of plants, notably cacao (Oldfield et al., Reference Oldfield, Bradford and Wood2019; Rangel-Mendoza and Silva-Parra, Reference Rangel-Mendoza and Silva-Parra2020). Furthermore, it is widely known that in a tree and a shrub plantation such as a cAFS, soil fertility is restored over time by the transformation of the litter produced by cacao and/or ATL into SOM. Litter decomposition and mineralisation in the soil are determined by the activities of decomposers and soil microorganisms. The chemical and physical qualities of the litter also play major roles in litter transformation and nutrient availability. A. mangium phyllodes (10–15 cm in length and 8 cm in width) are known to be rich in tannins, lignin, and polyphenols, making their leaf litter difficult to decompose; their annual decomposition rate was found to be slow at 40–60%, (Ngoran et al., Reference Ngoran, Zakra, Ballo, Kouamé, Zapata, Hofman and Van Cleemput2006; Castellanos-Barliza and Peláez, Reference Castellanos-Barliza and Peláez2011; Gnahoua et al., Reference Gnahoua, Oliver, Nguessan and Balle2014). In contrast, A. lebbeck leaves have very small leaflets (< 2 cm in diameter) with a lower C/N ratio (12) than do the leaves of A. mangium (18) according to Gnahoua et al. (Reference Gnahoua, Oliver, Nguessan and Balle2014). The litter produced by A. lebbeck was thus assumed to decompose faster than that produced by the other species, resulting in mass losses of 60–70% annually (Singh et al., Reference Singh, Raghubanshi and Singh2004; Ochire-Boadu et al., Reference Ochire-Boadu, Abunyewa, Kaba, Twum-Ampofo, Dawoe, Agbenyega and Barnes2020). Cacao leaves, which have lower annual decomposition rates, lose 30–40% of their mass (Yao et al., Reference Yao, Koné, Otinga, Kassin and Tano2021; Bai et al., Reference Bai, Gallart, Singh, Hannet, Komolong, Yinil, Field, Muqaddas and Wallace2022). Soil nutrient improvement through litter decomposition would therefore be limited by A. mangium compared to A. lebbeck.
In the studied system, the cacao trees were relatively small (7 m), covering the whole ground, shedding, and producing new leaves and pods that were harvested for approximately five months in a year. These small trees were overhead by higher tree legumes, which induced shade, produced new leaves annually, and potentially fixed atmospheric N2. Thus, the growth and production of the two tree layers resulted in interactions between the cacao trees and the ATLs. These interactions imply competition for water or nutrient resources. However, facilitation processes such as a favourable microclimate and continuous nutrient supply from the litter to the soil could also occur. These interaction effects are mainly related to the associated tree species, notably the quantity and the quality of their leaf litter (Sauvadet et al., Reference Sauvadet, Saj, Freschet, Essobo, Enock, Becquer, Tixier and Harmand2020). Shaded trees can therefore be expected to improve soil parameters even if cacao yields remain unchanged as reported by Sauvadet et al. (Reference Sauvadet, Saj, Freschet, Essobo, Enock, Becquer, Tixier and Harmand2020). Trebissou et al. (Reference Trebissou, Tahi, Munoz, Sanchez, N’Guetta, Cilas and Ribeyre2021) reported several factors affecting cacao growth and productivity, such as the characteristics of neighbouring trees, resource limitations (soil nutrients and water), and physiological stress conditions (photoinhibition and shading effect), which can induce a competitive effect and/or affect production mechanisms. Wibaux et al. (Reference Wibaux, Konan, Snoeck, Jagoret and Bastide2017) indicated that the average cacao production is proportional to the resources available to each tree. Thus, the drivers of cacao production included the area used by each cacao in the plot, i.e., the density of cacao, the resources available in this area (water, nutrients, and radiation), and ultimately, the technical management applied, which could have an impact on the above factors. In the present study, the management methods used were the same for all plots and can therefore be excluded as an explanation for the differences between treatments.
Cacao trees close to A. mangium had smaller trunk diameters and shorter heights, which could also reflect a greater competition between the two tree species. This difference between the cacao trees in the Cacao-Aca plots and those in the Cacao-Alb plots and the Control was reflected more in the cacao tree height than in the trunk diameter. Blaser-Hart et al. (Reference Blaser-Hart, Hart, Oppong, Kyereh, Yeboah and Six2021) showed in the same environmental context in Ghana that shade trees with low crowns caused larger reductions in incoming light than shade trees with more elevated crowns, which was associated with lower yield. However, in the present study, we observed that a negative impact occurred under A mangium, which had the most elevated crown. This suggested that the difference in light incidence between the two tree legumes could explain the difference in tree cacao growth and density, which was less relevant in this cacao tree plantation experiment. Trebissou et al. (Reference Trebissou, Tahi, Munoz, Sanchez, N’Guetta, Cilas and Ribeyre2021) reported that greater density of cacao trees increased the inter-tree competition (cacao vs. cacao; cacao vs. ATL): the closer the trees were to each other, the more they would try to grow taller to obtain more light. This competition between cacao trees occurred gradually, intensifying over time as resources were depleted (Trebissou et al., Reference Trebissou, Tahi, Munoz, Sanchez, N’Guetta, Cilas and Ribeyre2021). For Rijkers et al. (Reference Rijkers, Pons and Bongers2000), increased tree size (height) under conditions of shade and high density is a mechanism for ensuring plant photosynthetic efficiency. Therefore, the difference in tree density under the different treatments could also explain the difference in cacao tree size and consequently in biomass and bean production. However, the greater cacao density under A. lebbeck after 20 years of intercropping favoured cacao height growth in contrast to that under A. mangium where the cacao density was lower. Therefore, tree legumes would have limited available resources, especially nutrients, to a degree in the context of this study, resulting in competition between and with cacao, as noted by Nygren et al. (Reference Nygren, Leblanc, Lu and Gómez Luciano2013).
The roots of the trees determined competition or facilitation processes for nutrients and water uptake between ATL and cacao. A. mangium and A. lebbeck are reported to have a very extensive lateral root system with approximately 80% of the length concentrated in the first 60 cm of soil (Orwa et al., Reference Orwa, Mutua, Kindt, Jamnadass and Simons2009; Saifuddin et al., Reference Saifuddin, Osman and Khandaker2022). Cacao plants also have a surface-concentrated root system that can cause significant competition for nutrients (Nygren et al., Reference Nygren, Leblanc, Lu and Gómez Luciano2013). Therefore, ATL and cacao could draw the water and nutrients they need from the same soil pool. A. mangium presented greater dendrometric parameters and consequently greater biomass. This indicated higher nutrient and water requirements for this tree legume than for Cacao-Alb and could explain the negative impacts on cacao stands. The negative impact of A. mangium on cacao trees, particularly those closer to their trunk, suggests the occurrence of a possible allelopathic process limiting the survival and productivity of cacao trees (Notaro et al., Reference Notaro, Collado, Depas, Dumovil, Denis, Deheuvels, Tixier and Gary2021; Asitoakor et al., Reference Asitoakor, Vaast, Ræbild, Ravn, Eziah, Owusu, Mensah and Asare2022). The same explanation could hypothetically apply to the greater mortality of cacao trees in the Cacao-Aca plots, given that, as reported by Konan and Koffi (Reference Konan and Koffi2003), the same trend was observed 20 months after planting the cacao trees at the start of the experiment. This tree legume may have limitations in improving soil fertility and agrosystem productivity as reported by Koutika and Richardson (Reference Koutika and Richardson2019). These authors reported the negative impacts of A. mangium on nutrient concentrations in the soil and in neighbouring trees. ATLs produce considerable quantities of litter annually and are thus able to rapidly return a substantial amount of mineral matter, particularly nitrogen, to the soil (Gnahoua et al., Reference Gnahoua, Oliver, Nguessan and Balle2014). However, the phosphorus and potassium contents of litter can be quite low in this type of system, and external fertiliser inputs are required to compensate for these deficiencies if the soils are cultivated.
The decreased cacao PBY in response to A. mangium may also be related to the relatively small size of the plants. Trebissou et al. (Reference Trebissou, Tahi, Munoz, Sanchez, N’Guetta, Cilas and Ribeyre2021) reported greater pod production for taller hybrid cacao plants, such as those under A. lebbeck and those in Control plots (Table 2). For Niklas (Reference Niklas2005), this could be explained by a more developed cacao root system in correlation with the AGB of the cacao plants. This would lead to a greater nutrient acquisition capacity relative to a greater soil volume exploration capacity. Furthermore, small trees would have lower carbohydrate reserves, thus limiting their ability to regulate their growth and pod production as rainfall variability between years and competition effects increase (Tosto et al., Reference Tosto, Zuidema, Goudsmit, Evers and Anten2022).
Blaser et al. (Reference Blaser, Oppong, Yeboah and Six2017) showed that shade trees reduced cacao biomass and pod production in farmer plots in Ghana. These authors mainly argued for a tree-shading effect instead of a soil fertility effect. In contrast, Mensah et al. (Reference Mensah, Ræbild, Asare, Amoatey, Markussen, Owusu, Asitoakor and Vaast2023), in the same country, reported a beneficial effect of shading on cacao tree physiology, growth, and yield. In this study, the benefits of shade trees were related to the tree legume species. A. mangium showed reduction but A. lebbeck did not. However, distinguishing the shading effect on the soil fertility effect of tree companions was difficult in these long-term cacao experiments because we did not evaluate the shading ratio of the two ATLs.
Conclusion
This study showed that, 20 years after planting, the cacao biomass and bean production were significantly lower under A. mangium shading than under full-sun cacao cropping systems. The presence of A. lebbeck maintained similar cacao tree biomasses and PBYs. These results showed the importance of tree species even within the same plant family. If plant traits such as leaf and litter characteristics explain this divergent effect, the density of cacao trees under shade conditions seems to be the main determinant of cacao tree productivity. Therefore, planting cacao trees under a shade tree does not necessarily impede biomass or bean production relative to a full-sun cacao tree system. A. lebbeck could be a beneficial species for cacao agroforest establishment.
Data availability statement
The datasets generated during and/or analysed during the current study are available in open access with controlled access and digital object identifier (DOI)
Acknowledgements
The authors would like to express special thanks to Lucette ADET and Joachim KOUAME, who helped with the selection of the plots and organisation of the fieldwork. Furthermore, they are grateful to all field staff members of the Divo CNRA Center for their contribution to the data collection, particularly to Narcisse MILOGO. In addition to supporting the research work, the SoCa project (BNP Paribas Foundation) granted a 4-year doctoral fellowship to Brahima SILUE.
Author contributions
Brahima K. SILUE: Investigation, conceptualisation, methodology, formal analysis, visualisation, writing – original draft, and writing – reviewing and editing. Armand W. KONE: Conceptualisation, methodology, visualisation, writing – reviewing and editing, and supervision. Jacques A. KOTAIX: Writing – reviewing and editing. Klotioloma COULIBALY: Writing – reviewing and editing. Sékou AIDARA: Conceptualisation, formal analysis, visualisation, and writing – reviewing and editing. Lydie CHAPUIS-LARDY: Writing – reviewing and editing, supervision, funding acquisition, and project coordination. Dominique MASSE: Conceptualisation, methodology, visualisation, writing – reviewing and editing, and supervision.
Funding statement
This research was supported by the SoCa project granted by the BNP Paribas Foundation through its 2017 Climate initiative.
Competing interests
The authors declare that they have no conflicts of interest.









