Introduction
Cancer is one of the most important causes of death worldwide. The total number of new cases of cancer has been reported about 14 million in 2012 and 8.8 million in 2015, respectively [1]. Furthermore, a report relies that approximately 300 000 cases of cancer are diagnosed in children and adolescents under the age of 19 years old every year and about 80 000 deaths per annum from the childhood cancer are estimated throughout the world [2]. The most common neoplasms in children are haematological malignancies, which represent about 45% of all newly diagnosed paediatric cancers [Reference Hoell3]. In Iran, the incidence rate of haematological neoplasia is estimated as 661 per million in children under 14 years old and the most common cancer is leukaemia [Reference Mousavi, Pourfeizi and Dastgiri4].
However, certain microbial infections have been identified as strong risk factors for specific cancers and there is a higher mortality rate among infection-related cancers, in comparison with other malignancies [Reference de Martel5]. For example, the association between the hepatitis C virus and the development of different types of malignancies has been reported frequently [Reference Torres6]. There is also a link between parasite infections and cancers [Reference Machicado and Marcos7]. Furthermore, comparative epidemiological studies have suggested a connection between infectious agents and blood cancers in young individuals [Reference Benamrouz8].
Toxoplasma gondii, an obligate intracellular coccidian parasite which is widely distributed in the world, is the most common parasitic infection in humans and all warm-blooded animals [Reference Bayani9, Reference Dubey and Jones10]. Toxoplasmosis occur in humans through the ingestion tissue cysts via the undercooked meat of intermediate hosts and/or by the ingestion of water or food contaminated by oocysts from the definitive hosts [Reference Dubey and Jones10, Reference Daryani11]. This infection is generally self-limiting due to efficient immune control, which limits the dissemination of the rapidly multiplying stage of the parasite. In contrast, it is life threatening in immunosuppressed patients [Reference Saadatnia12]. Several studies have highlighted the fact that T. gondii infection may be associated with some specific malignancies such as brain tumours [Reference Yazar13–Reference Wang16]. An ecological study demonstrated that the risk of brain cancer in humans increases in patients with T. gondii infection [Reference Thomas17]. The parasite DNA was found in breast and lymph node tissues of the breast cancer patients [Reference Kalantari18] and also in lymphoma cells from patients with intraocular B-cell lymphoma [Reference Shen19]. Furthermore, a recent study showed a relatively high prevalence of acute and chronic toxoplasmosis in children with cancer in southwest of Iran, the results of which were obtained by serological and molecular techniques [Reference Saki, Tavakoli and Pedram20]. The mechanisms of how T. gondii initiates tumorigenesis are not clear. Latent infection of T. gondii may increase the host inflammation responses which boosts mutations and may affect the cancer development. Also like other intracellular pathogens, may disturb cellular barriers by oncogenic products gradually accumulating inside the cells [Reference Thomas17]. More recent reports show that T. gondii can export its own miRNAs into host cell, which may affect the regulation of the hosts’ gene expression, and therefore lead to the start cancer [Reference Huang21].
The possible link between T. gondii infection and blood cancer including leukaemia in children has not been well understood [Reference Huang21]. Therefore, the present study aimed to investigate the possible association between the Toxoplasma infection and haematological neoplasia, and associated risk factors in children and adolescents.
Methods and materials
Study design
In a case–control study format, seroprevalence and the associated risk factors of T. gondii infection were evaluated in 101 patients suffering from haematological neoplasms (case group) who were treated in the haematology–oncology ward of Amirkola Pediatric Hospital, Amirkola, Iran, and 138 children without any history of malignancy who were admitted to the outpatient clinic of the hospital were included in the study as a control group. The control subjects were frequency-matched with cancer patients by age, gender and residence. The current study was carried out from March 2016 to August 2017. All participants in both case and control groups were included in our study under the supervision of a paediatrician. Inclusion criteria for enrolment of cases were: (i) patients suffering from haematological malignancies in the affiliated Hospital of Babol University of Medical Sciences in Amirkola, Amirkola, Iran; (ii) aged under 18 years old; and (iii) who agreed to contribute in the study.
Ethical aspects
The purpose of this study was explained to the parents of each participant and they gave their written and informed consent. This work was approved by the ethical committee of Babol University of Medical Sciences, Babol, Iran (MUBABOL.REC.1395.24).
Sample collection
Two millilitres of venous blood was taken from each participant and the serum was separated after clot formation by centrifugation at 2500 rpm for 5 min. The sera were collected in Eppendorf tubes and then transported in an icebox to Parasitology–Mycology Department, Faculty of Medicine, Babol University of Medical Sciences, where they were kept at −20 °C until tested.
Socio-demographic, clinical and behavioural data
Socio-demographic data including age, gender, residence and parents’ education level were obtained for all patients using a questionnaire form. Clinical data explored in patients included the type of blood cancer and a history of blood transfusion. Behavioural data included consumption of raw/undercooked meat, eating of raw vegetables and fruit, exposure to soil and animal contact. These variables were selected based on the literature [1]. Data were obtained from the parents of the patients, physicians and medical examination records.
Serological analysis
First, the sera were evaluated for the presence of IgG antibodies against T. gondii by commercially available enzyme-linked immunosorbent assay (ELISA) (EUROIMMUN, Germany) based on the manufacturer's instructions. Second, all IgG-positive serum samples were assessed for IgM antibodies to T. gondii using an ELISA kit (EUROIMMUN). The optical density of IgG and IgM antibody titres was read at 450 nm by an automatic ELISA reader and the results were interpreted according to the kit's guidelines. The negative samples had IgG titres of <8 IU/ml. A sera IgG titre higher than 11 IU/ml and between 8 and 11 IU/ml were considered positive and border line, respectively. The sensitivity and specificity of the test are 99.9% and 100%, in that order.
Statistical analysis
SPSS v. 22.0 software (SPSS Inc., Armonk, NY, USA) was used to analyse the results. Age values and IgG titre were reported as median values and interquartile ranges (IQRs), and were compared by Mann–Whitney U test. The association between the characteristics of the subjects and T. gondii infection was carried out by χ 2 test and Fisher's exact test. Odd ratios (OR) and 95% confidence intervals (CIs) were calculated by logistic regression and P < 0.05 was considered statistically significant. Furthermore, multivariable analyses were applied to evaluate the association between the features of the studied population and the T. gondii infection.
Results
The median and IQR of age among case and control groups were (6 (IQR 4–9)) and (5 (IQR 3–7)), respectively, indicating no significant difference between the two groups (P = 0.154). The demographic factors including age, gender and place of residence were frequency-matched between groups but the level of parents education was not matched. The demographic characteristics of the participants included in this study are shown in Table 1. These data showed that 81.2% of the cases reported a blood transfusion, while only 2.9% of the subjects in the control group reported a blood transfusion (P < 0.0001). Animal and soil contact were more prevalent in the control group than the case group (P = 0.045 and P = 0.047, respectively).
Table 1. Socio-demographic characteristics and T oxoplasma gondii exposure among children with blood cancers and without any history of malignancy attending to Amirkola Children Hospital, Amirkola, Iran

Anti-T. gondii antibodies (IgG) were detected in the sera of 37 out of 101 cancer patients, with an overall seroprevalence of 36.6%. Twelve out of 138 (8.7%) cases in the control group were positive for anti-T. gondii antibodies (IgG). This difference was statistically significant and showed that the chance of haematological malignancies was higher in seropositive cases (OR 6.07, 95% CI 2.963–12.437, P < 0.0001). The median and IQR of IgG titre from the case group (7.7 (IQR 0.25–13.5)) was higher than the control (0.2 (IQR 0.1–0.5)) (P < 0.0001) (Table 1).
The frequencies of IgG antibodies against T. gondii and the associated risk factors among case and control groups are presented in Table 2. Among the case group, the frequency of IgG antibodies against T. gondii had a higher rate in patients aged under 4 years and those living in rural areas. Statistical analysis using χ 2 test showed that no significant difference was observed between the presence of anti-T. gondii antibodies and behavioural and demographic characteristics among the case and control groups.
Table 2. Seroprevalence and encountering particular risk factors of Toxoplasma infection in 101 patients with haematological malignancies and 138 individuals without cancer
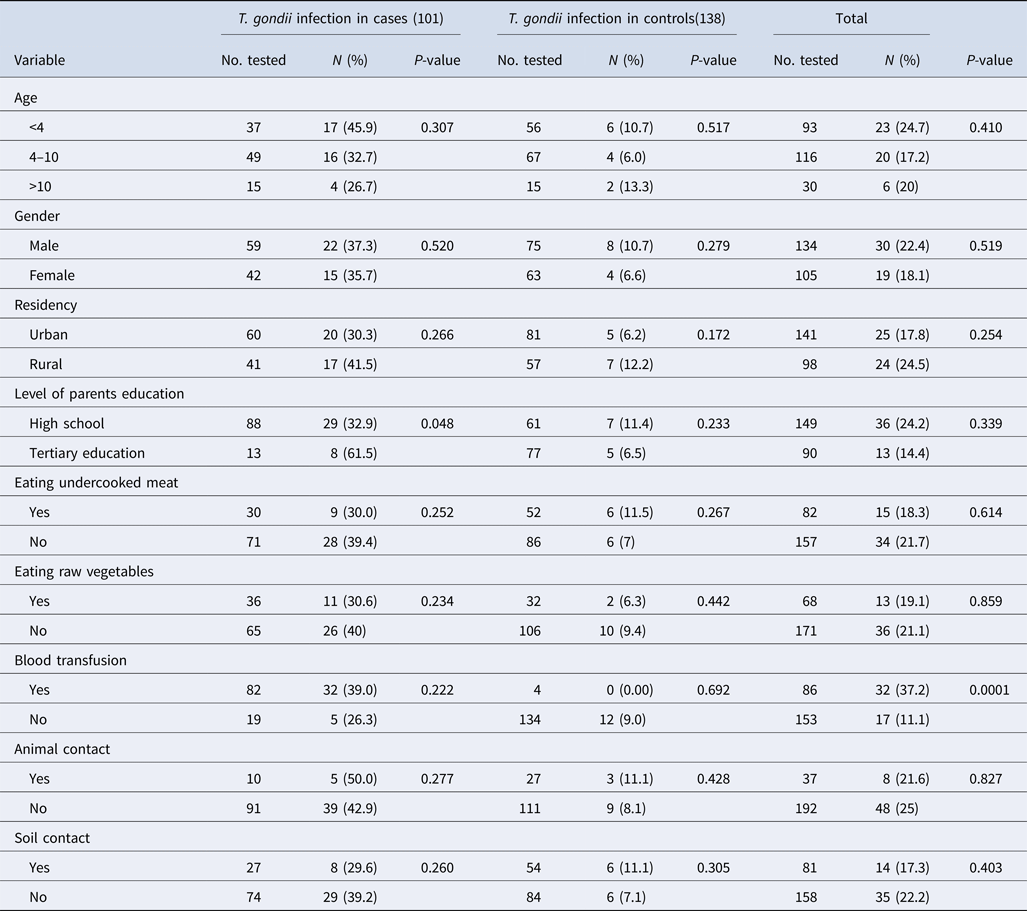
Statistical analysis was performed by χ 2 test.
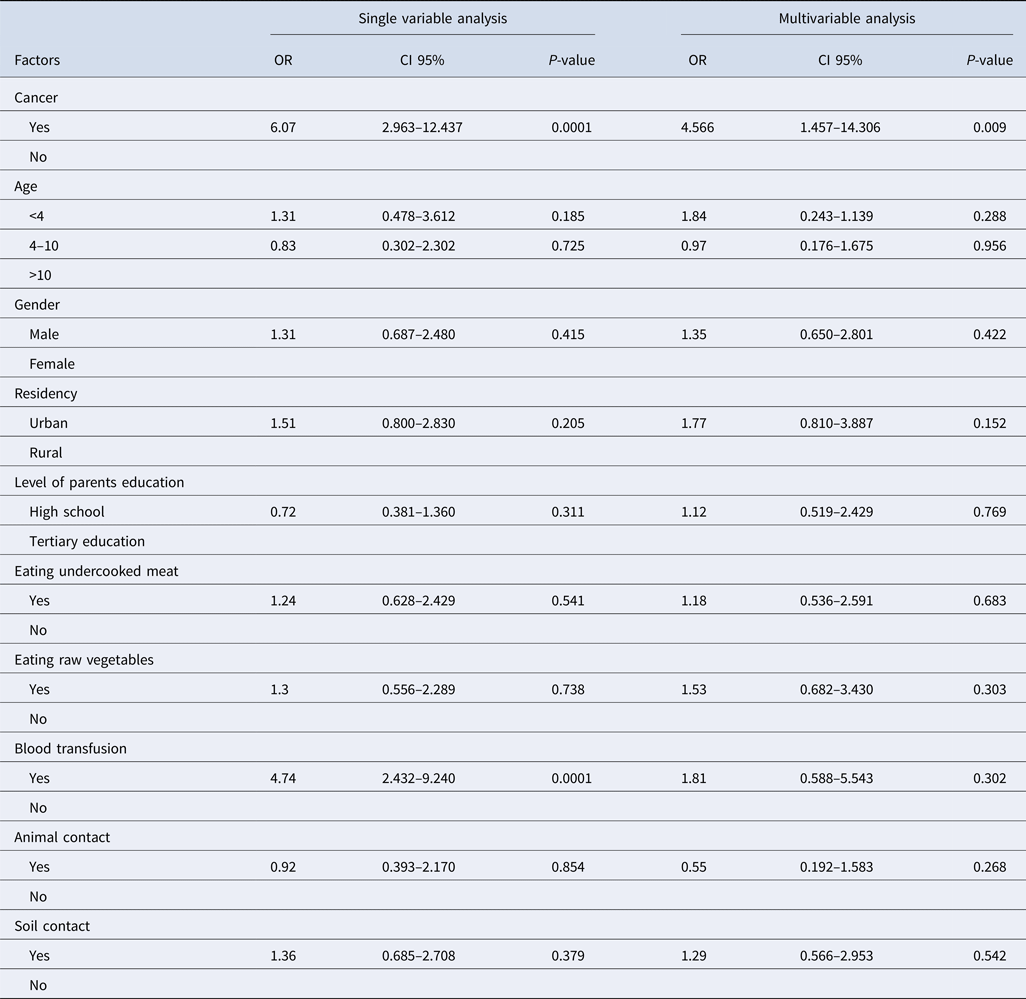
Our findings showed that toxoplasmosis was more prevalent in patients with a history of blood transfusion than cases without a history of blood and/or blood product transfusion. The results of the single variable analysis showed that toxoplasmosis was more prevalent in patients with a history of blood transfusion than cases without a history of blood and/or blood product transfusion (OR 4.74, 95% CI 2.43–9.24, P < 0.0001) and also in patients with cancer (OR 6.07, 95% CI 2.97–12.44, P < 0.0001). In the multivariable analysis, only cancer was significantly related to toxoplasma infection (OR 4.57, 95% CI 1.46–14.31, P = 0.009). None of the behavioural characteristics and demographic data were significant (Table 3).
Table 3. The logistic regression analysis for the factors that influence Toxoplasma infection in cancer patients compared with control subjects
The frequency of anti-T. gondii antibodies (IgG) in lymphoblastic leukaemia (acute lymphoblastic leukaemia), Hodgkin's lymphoma and T-cell lymphoma was 33 (31.9%), 3 (50%) and 1 (100%), in that order (Table 4).
Table 4. The rates of seropositivity of anti-T. gondii antibodies (IgG) in different haematological cancer paediatric
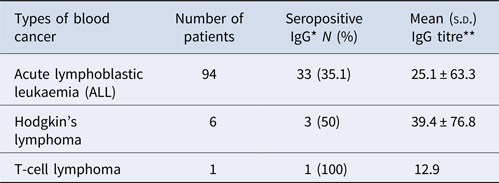
*χ2 = 1.27, P = 0.59; **df = 100, P = 0.85
Anti-T. gondii IgM was not detected in IgG-positive patients in the case group. In the control group, the frequency of positive and borderline IgM antibodies against T. gondii were 1 (8.3%) and 3 (25%), respectively.
Discussion
The potential association between parasitic protozoan infections and cancer has been documented by clinical and epidemiological investigations [Reference Benamrouz8]. The Apicomplexan parasite, T. gondii is considered to be associated with ocular tumours, meningioma, leukaemia and lymphoma [Reference Benamrouz8, Reference Yazar13, Reference Chintakuntlawar14]. In this frequency-matched case–control study, the results showed a higher seroprevalence rate of anti-T. gondii IgG antibodies in patients with haematological neoplasms under 18 years of age (36.6%) in comparison with the control subjects (8.7%), with a statistically significant difference (P < 0.0001). The possible explanations are: (1) patients with cancer are more sensitive to this parasite because they are immunocompromised and T. gondii is the most common protozoan parasite causing opportunistic infections in immunocompromised individuals [Reference Wang22] and/or (2) possible role of this parasite in the induction of cancer in humans. Although, the mechanisms underlying the association between cancer and toxoplasmosis are not clearly understood, but T-cell exhaustion phenomenon may affect the inability of the host to control intracellular pathogen infections or tumours [Reference Bhadra and Khan23]. There is evidence that T. gondii is able to increase the motility of the host's dendritic cells and macrophages, which may affect both the spread of parasites and progression of tumorous diseases [Reference Lambert, Dellacasa-Lindberg and Barragan24]. Furthermore, T. gondii may manipulate gene expression in the host cell by miRNAs and thus could cause cancer onset [Reference Huang21]. However, toxoplasmosis has frequently been described to be linked with some particular neoplasms such as acute and chronic leukaemias, lymphoma or multiple myeloma [Reference Yazar13, Reference Gharavi, Roozbehani and Mandeh25]. Also, other cancer patients with solid tumours in the breast, ovary and lung, who were under chemotherapy, have been associated with toxoplasmosis [Reference Yazar13, Reference Kalantari26].
In relation to the socio-demographic features of the patients, the seroprevalence of T. gondii was only significantly associated with the level of education of the parents (P = 0.05), and there was no significant association between other socio-demographic data and toxoplasmosis. These findings are supported by the results obtained from a recent study where no significant relationship exist between Toxoplasma seroprevalence and demographic characteristics in cancer patients [Reference Wang16, Reference Mizani27]. However, our findings are in contrast with the documented data indicating that the seropositivity rate of T. gondii is related to age and place of residence [Reference Elsheikha28–Reference Nimir30]. Further studies with large sample sizes in different areas and along with molecular techniques should be developed to confirm this association and explore the potential molecular mechanisms. Regarding the behavioural characteristics of patients, the present study showed that soil exposure, animal contact, consumption of raw vegetables and raw/undercooked meat were not associated with the seroprevalence of T. gondii in both studied groups. These findings are not in line with some previous studies and indicated that consumption of raw/undercooked meat, contact with cats, contact with soil and consumption of raw vegetables are important risk factors for T. gondii infection in humans [Reference Elsheikha28, Reference Belluco31]. The possible explanation for these differences could be related to dietary habits of the population surveyed as they were under 18 years of age and tend to eat less raw meat or vegetables compared with adults.
Then again, in this study, there was a higher seroprevalence rate of anti-T. gondii IgG antibodies in patients with a history of blood transfusion (39% vs.26.3%), but this difference was not statistically significant in the patient group (P = 0.22). Nevertheless, with respect to blood transfusion history, the different prevalence of anti-T. gondii antibodies between the patient group and the control group was statistically significant (OR 4.74, 95% CI 2.432–9.240, P < 0.0001), suggesting that blood transfusion may be a major risk factor for T. gondii infection in childhood haematological malignancies. It has been reported that the seropositivity of T. gondii is related to the positive history of blood transfusion in cancer patients [Reference Nimir30, Reference Vogel and Lunde32].
In a study, by Ghasemian et al., it was reported that the seroprevalence of anti-T. gondii antibodies in haematologic cancer patients was much higher than individuals with different types of malignancies under 20 years of age in Ahvaz, southern Iran [Reference Ghasemian33]. This is associated with many factors, such as types of study patients, local customs and habits. Based on our best knowledge, there is a very little information about toxoplasmosis in childhood cancers, particularly haematological malignancies. However, in this study, physical examination of the cancer patients did not find any manifestations of acute Toxoplasma infection due to reactivation of the parasite or recent infection. Since clinical symptoms of toxoplasmosis are often non-specific, this infection is often overlooked in the process of clinical diagnosis and treatment [Reference Su34]. Nevertheless, in immunosuppressed patients, undetected T. gondii infection may result in severe toxoplasmosis, and therefore, cancer patients should be periodically evaluated for this infection to prevent the possibility of severe toxoplasmosis [Reference Abolghasemi35].
In conclusion, the present study demonstrated that T. gondii infection is prevalent in children and adolescents with haematological malignancies in the north of Iran. It also indicated that toxoplasmosis might have an association with an increased risk of paediatric blood cancers. Therefore, clinicians should take more care of immunosuppressed patients, particularly cancer patients, and the parasitological investigations of these patients should be regularly performed to prevent the possibility of severe toxoplasmosis. Furthermore, these results may be helpful in the research on blood neoplasia aetiology.
Acknowledgements
We are grateful to the personnel of the clinical laboratory of Amirkola Hospital, Amirkola, Iran, for the utmost assistance and Ms Taraneh Ghaffari for proofreading the manuscript.
Conflict of interest
None.







