Pantothenate kinase-associated neurodegeneration (PKAN), also known as neurodegeneration with brain iron accumulation (NBIA) type I, is a rare autosomal recessive disorder, characterized by iron accumulation in the brain, mainly in the globus pallidus (GP). It results from underlying mutations in the PANK2 gene, which encodes for a protein that plays a pivotal role in coenzyme A (CoA) biosynthesis. The classical form of PKAN is characterized by young age of onset, mostly before the age of six, with prominent movement disorders (mainly dystonia and Parkinsonism) and pyramidal features. The non-classical (atypical) form presents in teenagers/young adults with similar motor symptoms; however, they are less severe and progress more slowly.Reference Kurian and Hayflick1
Currently, treatment of PKAN is limited to often ineffective trials of medications directed at the primary symptoms; and in some patients, deep brain stimulation may be a consideration.Reference Hogarth, Kurian and Gregory2 Given the limited treatment options and the fact that physicians may not consider amantadine as a regular therapeutic option, we would like to report our positive experience with this medication for gait dysfunction, postural instability, and freezing of gait in three young adult patients with the atypical form of PKAN.
Patients’ demographics, clinical features, amantadine treatment details, and outcomes are summarized in Table 1. Amantadine was prescribed for all three patients (added to their current medications listed in the table) to address complaints of recurrent falls because of postural instability and gait dysfunction. Freezing of gait was the main indication for the trial of amantadine in patient 2. In all three patients, the benefit did not appear to be associated with a change in dystonia or other Parkinsonian features. Further evidence for the benefit was the experience of patient 2 who developed bothersome ankle edema, after which the medication was withdrawn, with a recurrence of his disabling freezing. Subsequently, he was rechallenged with a lower dose (100 mg once daily) with gradual escalation over 3 weeks, back to 100 mg three times a day, with the original benefit restored. He tolerated the medication for 3 months without a recurrence of the bothersome ankle edema. Patients 1 and 3 have been receiving the medication for 4 years, with persistent benefit and without any physical or psychiatric adverse events.
Table 1: Patients’ demographics, clinical features, and amantadine treatment details and outcomes
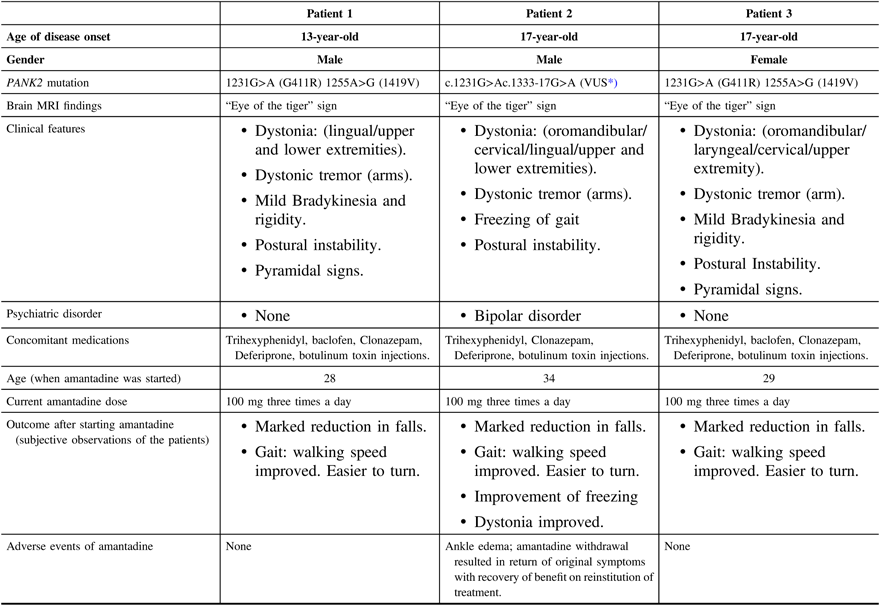
MRI = magnetic resonance imaging.
* Variant of uncertain clinical significance.
PKAN is an inexorable progressive disorder that results in profound patient disability. No treatment has been shown to alter the underlying disease pathogenesis. The iron chelating agent deferiprone has not been associated with convincing benefit,Reference Hogarth, Kurian and Gregory2 while fosmetpantotenate (RE-024), which bypasses the biochemical defect in PKAN, leading to restoration of CoA levels, is actively being studied (NCT03041116).
Thus, current treatments remain symptomatic, targeting mainly dystonia, including anticholinergics, baclofen, benzodiazepines, and botulinum toxin injections. In cases, where medical treatment fails to control the dystonia, deep brain stimulation of GP interna is an option, particularly in the atypical form.Reference Hogarth, Kurian and Gregory2 Although amantadine is mentioned as a treatment option by Hogarth et al in their consensus clinical management guideline for PKAN,Reference Hogarth, Kurian and Gregory2 to our knowledge, no cases of such a response for this medication have been documented in the literature previously.
Amantadine use has been reported previously in some cases of “Hallervorden Spatz syndrome” as a trial in managing tremor in a tremor-dominant PKAN patient or an add-on therapy to levodopa for Parkinsonian symptomsReference Rohani, Shahidi and Alavi3, Reference Dooling, Schoene and Richardson4; in the latter patient when used with levodopa, swallowing improved and she continued to walk unassisted occasionally before deteriorating again, possibly reflecting an effect of amantadine on gait that was not explored further. The effect of amantadine in improving the postural instability in other neurodegenerative diseases such as Parkinson’s disease and progressive supranuclear palsy has been discussed in the literature.Reference Chan, Kukkle, Merello, Lim, Poon and Moro5, Reference Hiller, Murchison, Nichols and Quinn6 The data from these reports remain conflicting and inconsistent, and, as in our report the benefit was often more subjective than objective.
The mechanism(s) of action of amantadine on postural instability and gait dysfunction is uncertain. Dopaminergic and non-dopaminergic (i.e., N-methyl-D-aspartate and cholinergic antagonistic effects) mechanisms are possible.Reference Chan, Kukkle, Merello, Lim, Poon and Moro5 The latter compatible with the usual absence of striatal dopaminergic deficiency in PKAN cases.Reference Kwon, Lee, Kim, Kang and Koh7 The striking improvement in gait freezing with amantadine in one of our patients, to the best of our knowledge, has not been reported previously. Methylphenidate and trihexyphenidyl have been reported to reduce freezing in PKAN in two separate reports.Reference Kwon, Lee, Kim, Kang and Koh7, Reference Lyoo, Prokisch, Meitinger, Lee, Kim and Lee8 Interestingly, all three of our patients were taking trihexyphenidyl for dystonia, but none had noted an improvement in gait dysfunction or a reduction in falling rate as experienced after starting amantadine.
In conclusion, we acknowledge the main limitations of our report: the small number of patients, the open-label treatment, and the lack of any objective measures of clinical improvement. However, the consistent reports of substantial improvement in walking, postural stability, and quality of life in our patients and the confirmation of benefit in one patient who was temporarily withdrawn from amantadine due to ankle edema justify this brief report. The rarity of PKAN, its variable clinical manifestations, and the generic availability of amantadine make it unlikely that a higher level of evidence for this response will ever become available. Thus, we believe that a trial of amantadine should be considered in PKAN patients with gait disorders, including freezing and resultant postural instability.
Funding
None.
Statement of Authorship
DMAl-S: Writing of the first draft; AEL: review and critique.
Disclosures
DMAl-S has no disclosures to report. AEL reports personal fees (Consulting) from AbbVie, Acorda, Bristol-Myers Squib, Biogen, Merck, Sun Pharma, Corticobasal Solutions, Sunovion, Paladin, Medichem, Medtronic, outside the submitted work.





