Diseases of the gastrointestinal tract are associated with an imbalance in the cellular redox system leading to an increased level of reactive oxygen species (ROS). To protect cells against oxidative damage by oxidants produced during the oxygen metabolism, an antioxidant system has presumably evolved in aerobic organisms(Reference Ceballos-Picot, Nicole and Clement1). GSH and its oxidised form GSSG (glutathione disulphide) constitute the major thiol redox system in cells, including enterocytes(Reference Aw2). It is responsible for the maintenance of an intracellular redox balance and detoxification of electrophilic xenobiotics and ROS(Reference Cnubben, Rietjens and Wortelboer3) being the redox state of GSH:GSSG ratio of crucial importance for cellular function(Reference Brigelius and Sies4). In addition, under physiological conditions, the primary defence against superoxide anion (O2∙−) and hydrogen peroxide (H2O2) in mitochondria is performed by the concerted action of the Mn-superoxide dismutase (SOD) and glutathione peroxidase (GPx). However, despite the activity of these enzymes, significant amounts of H2O2 can diffuse out from mitochondria to cytosol, where detoxification appears occurring through cytoplasmic Cu,ZnSOD and GPx or by peroxisomal catalase (CAT)(Reference Salvi, Battaglia and Brunati5). It has been suggested that ROS comprise an important mediator of apoptosis since initiation and regulation of apoptosis appear to be intimately associated with modifications in the oxidative environment(Reference Mates and Sanchez-Jimenez6). Overproduction of ROS causes peroxidation of the mitochondrial phospholipids bilayer, with the consequent severe damage to the membrane integrity and the activation of the mitochondrial permeability transition pore(Reference Mather and Rottenberg7), which permits the free diffusion of pro-apoptotic proteins as cytochrome c between the matrix and the cytosol and the induction of apoptosis(Reference Zoratti and Szabo8).
Epidemiological studies have shown an inverse correlation between a high ingestion of secondary plant metabolites (e.g. polyphenols, PP) and colorectal cancer risk(Reference Donaldson9). The beneficial effects of grape consumption reducing the risk of cancer, especially the gastrointestinal tract tumours(Reference Arii, Miki and Hosoyama10), are, at least in part, due to their polyphenolic content. Suggested mechanisms of anticancer effects of grape PP include antioxidant, anti-inflammatory and anti-proliferative activities, as well as induction of cell cycle arrest and apoptosis(Reference Seeram, Adams and Henning11). PP may act as free radicals scavengers(Reference Sanchez-Moreno, Larrauri and Saura-Calixto12), metal chelators and enzyme agonist or inhibitors(Reference Garbisa, Sartor and Biggin13, Reference Russo, Acquaviva and Campisi14). In vitro and in vivo studies have reported that grape intake appears to modulate cellular redox status and antioxidant enzyme defence system: PP-rich grape skin extract(Reference Young, Dragsted and Daneshvar15) and grape pomace(Reference Bobek16) were found to increase GPx activity in human erythrocytes and liver, respectively, whereas the intake of grape juice seems to improve SOD and CAT activities in both plasma and liver of Wistar rats, inducing a hepatoprotective effect(Reference Dani, Oliboni and Pasquali17). Grape seed procyanidin extract improved the rat's hepatic oxidative metabolism in vitro (Reference Roig, Cascon and Arola18) and induced a transcriptional regulation of glutathione-related enzymes such as GPx and glutathione reductase (GR), which increased both mRNA levels and enzyme activity when tested in hepatocarcinoma cell line(Reference Puiggros, Llopiz and Ardevol19). Also, procyanidins may stimulate the activation of γ-glutamylcysteine synthetase thus stimulating de novo synthesis of GSH(Reference Myhrstad, Carlsen and Nordstrom20). On the other hand, protective(Reference Abrahamse, Pool-Zobel and Rechkemmer21) and non-protective effects(Reference Rosignoli, Fabiani and De Bartolomeo22) of butyrate, a by-product of the bacterial fermentation of dietary fibre, on DNA damage induced by hydrogen peroxide or bile acids in isolated colonocytes have been reported, suggesting the participation of this SCFA in the antioxidant defence of colon.
Being the interface between the organism and its luminal environment, the intestine is constantly challenged either by diet-derived or endogenous oxidants and ROS(Reference Giovannini, Matarrese and Scazzocchio23). Epithelial cells of intestinal mucosa are exposed to the highest concentrations of dietary fibre and PP. These compounds show affinity for cellular membranes and it has been reported that this feature is essential for their protective action against hydrogen peroxide cytotoxicity(Reference Nakayama, Niimi and Osawa24).
Grape antioxidant dietary fibre (GADF) is a natural product obtained from red grapes, the extracts of which show powerful antioxidant properties(Reference Saura-Calixto25). GADF intake enhanced the caecal antioxidant status(Reference Goñi and Serrano26) and induced hypocholesterolaemia in rats(Reference Martín-Carrón, Saura-Calixto and Goñi27). Supplementation of GADF to diet in human subjects significantly reduced plasma total cholesterol, LDL-cholesterol and blood pressure in hypercholesterolaemic subjects(Reference Pérez-Jiménez, Serrano and Tabernero28). In addition, we observed that in rats, GADF intake induced a decline in mucosal thickness, crypt depth and crypt density in colonic mucosa of Wistar rats, inducing epithelial hypoplasia(Reference López-Oliva, Agis-Torres and García-Palencia29). This result suggests a possible effect of GADF on the balance between proliferation and apoptosis of colonocytes. The antioxidant effect of GADF on colonic mucosa could modulate apoptosis by the modification of cellular redox balance.
The present study evaluates the ability of GADF intake to modify both glutathione redox status and mitochondrial and cytoplasmic antioxidant enzyme defence system of colonic mucosa, thus creating an environment capable of influencing apoptosis. Since mitochondrial and intracellular antioxidant enzyme system has separate functions protecting against oxidative stress, it may respond differently to dietary antioxidant. To tackle this aim, two important indicators of the glutathione redox status were used: the GSH:GSSG ratio and the redox state of the GSSG/2GSH couple defined by the half-cell reduction potential (E hc) in cells(Reference Schafer and Buettner30). Also, the antioxidant enzyme system (SOD (MnSOD and Cu,ZnSOD), GPx and CAT activities) as well as the lipid peroxidation (LPO) and DNA fragmentation levels in cytosol and/or mitochondria of proximal colonic mucosa in GADF-fed Wistar rats were measured.
Experimental methods
Dietary fibre
GADF is a natural product obtained from red grapes (Vitis vinifera var. Cencibel, La Mancha region, Spain). GADF combines the beneficial effects of dietary fibre and of antioxidants PP such as phenolic acid, anthocyanidin, proanthocyanidin, catechin and other flavonoids(Reference Saura-Calixto and Larrauri-García31, Reference Saura-Calixto and Goñi32). The proximate composition of GADF (% DM) was as follows: 73·48 (SD 0·79) % total indigestible fraction made of mainly insoluble (57·95 (SD 0·78) %) and soluble compounds (15·53 (sd 0·11) %), total PP (19·74 (sd 0·19) %; with the mayor fraction being proanthocyanidins, 14·81 (sd 0·19) %), protein (11·08 (sd 0·46) %), fat (7·69 (sd 0·49) %) and ash (5·25 (sd 0·19) %)(Reference Pérez-Jiménez, Serrano and Tabernero28). The antioxidant capacity of GADF was 124·4 (sd 0·3) μmol Trolox/g DM when measured by 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) method(Reference Re, Pellegrini and Proteggente33) and 214·2 (sd 38) μmol Trolox/g DM when using the oxygen radical absorbance capacity method(Reference Ou, Hampsch-Woodill and Prior34).
The dietary fibre was analysed following Prosky et al. (Reference Prosky, Asp and Schweizer35), introducing a separation of soluble and insoluble fibres through a dialysis system(Reference Mañas and Saura-Calixto36). To extract PP from GADF, an extraction with acidic methanol and later with a mixture of acetone–water was performed(Reference Saura-Calixto, Serrano and Goñi37). GADF PP were determined as gallic acid equivalents by the Folin–Ciocalteau procedure(Reference Montreau38) and by high-performance liquid chromatographic methods(Reference Lamuela-Raventos and Waterhouse39).
For analysis of crude protein of GADF, samples were combusted in a LECO FP-2000 analyzer (Analytical Instruments, LCC, Golden Valley, MN, USA). Fat content was extracted with diethyl ether at 40–60°C and gravimetrically determined (Soxtec System HT, Foss Tecator AB, Höganäs, Sweden). Ash was gravimetrically quantified using a muffle furnace for 16 h at 550°C.
Diets
The experimental diets, control and GADF, were manufactured by Dyets Inc. (Bethlehem, PA, USA) having identical fibre content (50 g/kg diet) but varying in type of fibre (Table 1). Thus, the control diet contained cellulose and the GADF diet contained GADF. The GADF diet was prepared by modifying American Institute of Nutrition-93M(Reference Reeves, Nielsen and Fahey40) -purified rodent diet with GADF replacing cellulose. Both diets were formulated to be isoenergetic. The diets were analysed following the methods of the Association of Official Analytical Chemists(41).
Table 1 Composition of experimental diets*

AIN, American Institute of Nutrition; GADF, grape antioxidant dietary fibre.
* Experimental diets were supplied by Dyets Inc. (Bethlehem, PA, USA).
Experimental design
Male Wistar rats with an average body weight of 215 (sem 2) g were used (Harlan Ibérica, Spain). All animals were housed in individual metabolic cages and maintained in a room at 22 ± 1°C, 60 % humidity and with a 12 h light–dark cycle. Two treatment groups were utilised (n 10 each) and were fed either the control diet (control group) or the GADF diet (GADF group) for an experimental period of 4 weeks. Food and water were freely available. Directives of 86/609 EEC of European Community were followed for the use of experimental animals. Appropriate committee of the Universidad Complutense de Madrid approved the experiments.
At the end of the study, the rats were anaesthetised via intra peritonially with ketamine (100 g/l) and xylazine (20 g/l). The peritoneal cavity was opened by a midline incision, and the colon was stripped of mesenteric and vascular connections and removed from caecum to rectal ampulla. After tissue extraction, rats were killed by exsanguination. The lumen of colon was flushed at 4°C with cold PBS (NaH2PO4, 1·9 mm; Na2HPO4, 8·4 mm; NaCl, 145·4 mm, pH 7·4) to clear the intestinal contents. The proximal segment was excised, opened longitudinally and the mucosa was obtained by scraping with a glass slide. The samples were frozen quickly in liquid nitrogen and then stored at − 80°C for biochemical assays.
Tissue preparation
The proximal colonic mucosa was homogenised in a glass homogeniser in 10 volume (w/v) of ice-cold PBS, pH 7·4, and centrifuged at 800 g at 4°C for 15 min to remove the nuclei and cell debris. The supernatants were filtered through two layers of cheesecloth, and then the mitochondria were pelleted by centrifugation at 14 000 g at 4°C for 25 min. The resulting supernatant, representing the mitochondria-free cytosolic fraction, was collected, aliquoted and stored at − 80°C for later biochemical analyses. The mitochondrial pellet was resuspended in homogenising buffer, and centrifuged at 14 000 g at 4°C for 25 min. The supernatant was decanted and the mitochondrial pellet resuspended in lysis buffer and stored at − 80°C. The protein content of the colonic mucosa fractions was quantified in duplicate using the Bradford method(Reference Bradford42). Bovine serum albumin in a concentration range of 0–50 μg/ml was used as a standard.
Analytical procedures
Measurement of GSH and GSSG
GSH and GSSG concentrations were measured by a commercial kit supplied by Cayman Chemical. This kit utilises an optimised enzymatic GR recycling method for quantification of GSH(Reference Baker, Cerniglia and Zaman43). Just after homogenisation of the colonic mucosa, 100 μl of the colon cytosolic fraction was added to an equal volume of 5 % (w/v) metaphosphoric acid to remove protein. After 30 min of incubation on ice, samples were centrifuged for 20 min at 2000 g at 4°C. Then, 50 μl of 4 m triethanolamine were added for each millilitre of homogenate to increase the pH. For total GSH assay, 50 μl sample was added to 150 μl of a reaction mixture containing 0·4 m 2-(N-morpholino) ethane sulphonic acid, 0·1 m phosphate (pH 6·0), 2 mm EDTA, 0·24 mm NADPH, 0·1 mm 5,5′-dithiobis-2-nitrobenzoic acid and 0·1 unit GR. The reaction was carried out at 37°C for 25 min, and then total glutathione was determined by absorbance at 405 nm using GSSG as standard. For the measurement of GSSG, GSH was removed from the reaction by adding 10 μl of 1 m 2-vinylpyridine per ml of homogenate. Then the remaining GSSG in the reaction was quantified as total GSH assay. The amount of reduced GSH was obtained by subtracting GSSG from total glutathione. Each sample was assessed in duplicate, and the levels of GSH and GSSG were expressed as nmol/mg protein. The ratio of GSH over GSSG was used to indicate redox status that inferences the detoxification capacity.
Nernst equation
The GSH redox state in tissues can be expressed in a more comprehensive manner as the reduction potential of the GSSG/2GSH redox couple (E hc). This reduction potential in cells can be estimated from the Nernst equation(Reference Schafer and Buettner30) using GSH and GSSG concentrations expressed in molarity and E 0 appropriate for the intracellular pH:
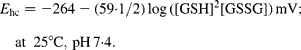
According to Wang et al. (Reference Wang, Wang and Andersson44), we assume a pH value of colonic mucosa of 7·3–7·4, and consider that it was unaffected by the treatment used. In these conditions, the value of E 0 is − 0·264(Reference Rost and Rapoport45).
Antioxidant enzyme activities
Cu,ZnSOD activity was determined in the colon cytosolic fraction by using a hypoxanthine–xanthine oxidase system to generate O2∙−. The capacity to scavenge superoxide radicals by suppression of the reduction in nitrotetrazolium was monitored at 550 nm(Reference Flohe and Otting46). Enzyme activity was expressed in units per milligram protein (1 unit of SOD is defined as the amount of enzyme required to inhibit the rate of nitrotetrazolium reduction by 50 %). MnSOD activity was determined in the mitochondrial fraction under the same conditions of cytosolic SOD assay, with the addition of 1 mm KCN to inhibit Cu,ZnSOD isoform. Each assay was performed in duplicate.
GPx was assayed by NADPH oxidation when GSSG is reduced back by GR with cumene hydroperoxide as a substrate(Reference Flohe and Gunzler47). The decrease in optical density due to the oxidation of NADPH at 340 nm was read at 60 s intervals for 6 min. The reaction rate was determined using the NADPH extinction coefficient of 0·00 622/μm cm. The GPx activities of colon cytosolic and mitochondrial fractions were expressed as nmol of NADPH oxidised to NADP+ per minute and per mg of protein at 25°C.
CAT activity was determined in the colon cytosolic fraction by the Aebi method(Reference Aebi48) by monitoring the decomposition of H2O2 at 240 nm. The mixture reaction (1 ml) contained 50 mm potassium phosphate (pH 7·0), 19 mm H2O2 and 20 μl sample. Each assay was performed in duplicate. The CAT activity was expressed as nmol of formaldehyde formed per minute and per mg of protein at 25°C.
Lipid peroxidation
The assay used is based on the reaction of a chromogenic reagent N-methyl-2-phenylindole in acetonitrile, with malonyldialdehyde (MDA) and 4-hydroxynonenal (4-HNE) at 45°C (Bioxytech LPO-586 kit, Oxis International, Portland, OR, USA). The samples containing 5 mm butylated hydroxytoluene were incubated with N-methyl-2-phenylindole and methane sulphonic acid reagent at 45°C for 60 min, and then centrifuged at 12 000 g for 15 min. The supernatant was then transferred to a ninety-six-well microplate, and the optical density was measured at 590 nm using a microplate reader. Mitochondrial and cytosolic MDA+4-HNE concentrations were expressed as nmol/mg protein.
Assessment of apoptosis
DNA fragmentation was quantified using a Cell Death Detection ELISA kit (Roche Applied Science, Barcelona, Spain). This assay is a photometric enzyme-linked immunoassay that quantitatively measures the internucleosomal degradation of DNA, which occurs during apoptosis. Briefly, the cytosolic fraction of colonic mucosa was used as an antigen source in a sandwich ELISA with a primary anti-histone mouse monoclonal antibody coated to the microtitre plate, and a second anti-DNA mouse monoclonal antibody coupled to peroxidase. The amount of peroxidase retained in the immunocomplex was determined photometrically by incubating with ABTS as a substrate for 10 min at 20°C. The change of colour was measured at a wavelength of 405 nm using a microplate reader. Measurements were performed in duplicate and the OD405 reading was then normalised to the milligrams of protein used in the assay.
Statistics
Results are expressed as mean with their standard errors. Differences were assessed by unpaired Student's t test, and were considered statistically significant at the 5 % level on two-sided testing. Linear correlation analysis was used to explore the relationships between the studied continuous variables. Correlation coefficients (R) and P values were evaluated to judge the fit of the correlation; two-sided P < 0·01 and 0·001 values of correlations were considered significant and highly significant, respectively. The statistical analyses were conducted using SPSS 15.1 for Windows (SPSS, Chicago, IL, USA).
Results
Grape antioxidant dietary fibre-related changes in cytosolic GSH and GSSG contents of the rat proximal colonic mucosa
In order to evaluate glutathione redox state, reduced glutathione and glutathione disulphide levels were measured in the cytosolic fraction of the colonic mucosa (Table 2). GSH content was not modified (P>0·05) by GADF treatment. In contrast, GSSG content of GADF group exhibited a significant decline (0·41 (sem 0·04) nmol/mg protein for control group and 0·13 (sem 0·01) nmol/mg protein for GADF group), decreasing at 68 % of control. Thus, the GSH:GSSG ratio increased significantly (199 %) in GADF group (103·45 (sem 6·91) v. 34·60 (sem 2·17)) indicating that the GSH redox state of the mucosa was more reduced in GADF rats.
Table 2 Effect of grape antioxidant dietary fibre (GADF) on GSH, GSSG, GSH:GSSG ratio and the redox potential (E hc) associated with GSSG/2GSH couple in cytosol of the rat proximal colonic mucosa
(Means with their standard errors for ten animals per group)

a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05; two-sided unpaired t test, df 18).
Grape antioxidant dietary fibre-related changes in cytosolic glutathione redox potential
The GSSG/2GSH reduction potential (E hc) was derived from the Nernst equation to determine the effects of GADF intake. We found that E hc value was more negative, i.e. more pro-reducing ( − 247·34 (sem 1·36) mV) in GADF rats compared to control rats ( − 233·62 (sem 0·77); Table 2), showing the antioxidant effect of GADF in colonic mucosa.
Grape antioxidant dietary fibre-related changes in antioxidant enzyme system of rat proximal colonic mucosa
To determine whether GADF intake can induce a differential response between the cytosolic and mitochondrial antioxidant enzyme system to clear up cellular ROS, the activities of SOD, GPx and CAT in cytosolic and/or mitochondrial fractions were measured.
GADF induced a significant (P < 0·05) decrease (39 %) in cytosolic Cu,ZnSOD activity compared to control (6·87 (sem 0·21) U/mg protein for control group and 4·18 (sem 0·32) U/mg protein for GADF group), indicating a lower capacity for dismutation of O2∙− to H2O2 in colonic mucosa, whereas GPx and CAT activities in cytosol were unmodified (P>0·05) compared to control (Table 3). However, the Cu,ZnSOD:GPx and Cu,ZnSOD:CAT ratios that reflect a possible imbalance between these enzymes presented a significant (P < 0·05) decrease (31 and 44 %, respectively) in GADF rats compared to control rats. These results suggest that GADF intake enhances the hydrogen peroxide scavenging capacity of GPx and CAT.
Table 3 Effect of grape antioxidant dietary fibre (GADF) on cytosolic antioxidant enzyme activities of the rat proximal colonic mucosa
(Means with their standard errors for ten animals per group)

SOD, superoxide dismutase; GPx, glutathione peroxidase; CAT, peroxisomal catalase.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05; two-sided unpaired t test, df 18).
Antioxidant enzyme activities in colonic mitochondrial fraction showed a different pattern: whereas MnSOD activity was unmodified (P>0·05), the mitochondrial GPx activity increased significantly (65 %; P < 0·05) compared with control (14·86 (sem 0·89) nmol/min per mg protein for control group and 24·56 (sem 1·55) nmol/min per mg protein for GADF group (Table 4). Thus, the significant (P < 0·05) decrease in the MnSOD:GPx ratio (43 %) seems to indicate an enhanced capacity of mitochondria of GADF rats to reduce H2O2 or hydroperoxides.
Table 4 Effect of grape antioxidant dietary fibre (GADF) on mitochondrial antioxidant enzyme activities of the rat proximal colonic mucosa
(Means with their standard errors for ten animals per group)

SOD, superoxide dismutase; GPx, glutathione peroxidase.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05; two-sided unpaired t test, df 18).
Grape antioxidant dietary fibre-related changes in cytosolic and mitochondrial lipid peroxidation of rat proximal colonic mucosa
Cytosolic and mitochondrial MDA+4-HNE concentrations were determined to assess GADF-related oxidative changes in the colonic mucosa. Both of them are major decomposition products of lipid peroxides and are used as a reliable marker for LPO. The present results showed that MDA+4-HNE levels were unmodified in mitochondria (P>0·05), whereas they were significantly reduced (45 %) in GADF rats cytosol (0·662 (sem 0·036) nmol/mg protein v. 0·360 (sem 0·041) nmol/mg protein; Fig. 1). This suggests a higher antioxidant effect of GADF against the free radical attack in the cytosol than in the mitochondria.

Fig. 1 Effects of grape antioxidant dietary fibre (GADF) on lipid peroxidation in cytosolic (a) and mitochondrial (b) fractions of the rat proximal colonic mucosa. Lipid peroxidation (malonyldialdehyde (MDA) and 4-hydroxynonenal (4-HNE); nmol/mg protein) was determined using the Bioxytech LPO-586 kit. Values are means with their standard errors represented by vertical bars (n 10). * Significant difference between GADF and control groups (P < 0·05, two-sided unpaired t test, df 18).
Grape antioxidant dietary fibre-related changes in apoptosis of rat proximal colonic mucosa
The amount of histone-associated DNA fragments resulting from cleavage of double-stranded DNA decreased significantly (P < 0·05) about 34 % of control in GADF group (0·273 (sem 0·022) optical density/mg protein v. 0·180 (sem 0·015) optical density/mg protein; Fig. 2). This result indicates that GADF intake reduced cell death in the rat colonic mucosa.

Fig. 2 Effects of grape antioxidant dietary fibre (GADF) on apoptosis in the rat proximal colonic mucosa. Apoptosis (DNA fragmentation; optical density (OD)/mg protein) of cytosolic colon mucosa was determined using the Cell Death Detection ELISA Assay (Roche Applied Science). Values are means with their standard errors represented by vertical bars (n 10). * Significant difference between GADF and control groups (P < 0·05, two-sided unpaired t test, df 18).
Linear correlations
Linear correlations between MnSOD activity and GSSG content v. apoptosis of colonic mucosa of GADF rats are given in Fig. 3.

Fig. 3 Scatter plots of the relationships between mitochondrial Mn-containing superoxide dismutase (MnSOD) activity (U/mg protein) (a) and cytosolic GSSG concentration (nmol/mg protein) (b) with apoptosis (DNA fragmentation; optical density (OD)/mg protein) in the proximal colonic mucosa of GADF rats. Each linear regression analysis is represented by its correlation coefficient (R), P value and the fitted regression line. No significant correlation relationship was found in control group (P>0·01). (a) R − 0·870, P = 0·001; (b) R 0·891, P = 0·0005.
A negative correlation was found when mitochondrial MnSOD activity was related with apoptosis (R − 0·870; P = 0·001; Fig. 3(a)). This result indicates that apoptosis of colonic mucosa increases with the decrease in MnSOD activity. Thus, the cell death of colonic mucosa of GADF rats was dependent of O2∙− dismutation to H2O2 in mitochondria. No correlation was found in control rats.
A different trend was observed when GSSG content was related to apoptosis (Fig. 3(b)). The positive correlation (R 0·891, P < 0·0005) highlights the fact that apoptosis increases linearly with the increase in GSSG, whereas no correlation was found in control rats. This may indicate that the colonic apoptosis could be mediated by the glutathione redox system during the GADF treatment.
Discussion
The main findings of the present study were as follows: (1) the cytosolic GSH:GSSG ratio in colonic mucosa was increased by GADF intake primarily due to the decrease in the GSSG concentration; (2) the glutathione redox potential became more negative during GADF treatment reflecting a pro-reducing shift; (3) decreases in mitochondrial MnSOD:GPx ratio and in cytosolic Cu,ZnSOD:CAT and Cu,ZnSOD:GPx ratios appear leading to an enhancement in the capacity for reduction of H2O2 in the mitochondrial and cytosolic fractions of colonic mucosa of GADF rats; and (4) reduced apoptosis in GADF-treated colonic mucosa was related to changes in the cellular GSH/GSSG redox system.
The glutathione redox status of a tissue is dependent upon two factors: the amount of GSH and the ratio between GSH and GSSG. The observed GADF-associated changes in the amounts of GSH, GSSG and GSH:GSSG ratio demonstrate that GADF intake is associated with a pro-reducing shift in the cytosol of rat colonic mucosa. The most proximate reason for this significant increase in the cytosolic GSH:GSSG ratio seems to be the decrease in GSSG concentration, since GSH concentration was unchanged in the GADF-treated colonic mucosa. It is consistent with the maintenance of the capacity to oxidise glutathione to GSSG as suggested by the unmodified cytosolic GPx activity. In contrast, it has been shown that flavonoids require the interaction with GPx for their intracellular antioxidative function, at least in the cells expressing the enzyme(Reference Nagata, Takekoshi and Takagi49). In this sense, grape seed procyanidin extract induced a dose-dependent significant increase in mRNA and the activity of GPx and GR in hepatocarcinoma cell line, indicating that grape seed procyanidin extract up-regulated the antioxidant enzyme defence system(Reference Puiggros, Llopiz and Ardevol19). In addition, the shift in the GSH:GSSG ratio seems to change the redox state of the colonic mucosa to a more negative potential. By removing GSSG, the denominator in the Nernst equation became smaller, thereby maintaining a more negative reduction potential(Reference Schafer and Buettner30). Thus, in contrast to the value of the reduction potential of the GSSG/2GSH redox couple showed by the colonic mucosa of cellulose-fed rats (E hc = − 233·62 (sem 0·77) mV), the half-cell reduction potential of GADF rats attained a value of − 247·34 (sem 1·36) mV, decreasing approximately 14 mV which is consistent with the GSH:GSSG ratio data. This suggests that the intake of GADF induced a more pro-reducing redox potential of the colonic mucosa possibly due to a higher GSSG efflux from cells, since a favourable cellular redox environment can be maintained through the efflux of GSSG(Reference Schafer and Buettner30). However, one must bear in mind that these estimates assume no change in pH. Since an increase in 0·2 pH units is equivalent to a 12 mV more negative E hc value(Reference Jonas, Estivariz and Jones50), direct measures of mucosal pH are needed to confirm the observed changes in GSH redox potential in the intestine.
Along with GPx, CAT activity was also unchanged in the cytosolic fraction of colonic mucosa of GADF rats. Since both CAT and GPx contribute to scavenge H2O2 excess, this suggests that smaller amounts of hydrogen peroxide are being generated. It is in agreement with the reduction in the Cu,ZnSOD activity found in GADF-treated colonic mucosa. This enzyme catalyses the dismutation of O2∙− producing hydrogen peroxide, and the reduction in its activity may result from a decrease in the superoxide production, as was also observed in liver of CD1 mice fed with pomegranate juice(Reference Faria, Monteiro and Mateus51). Interestingly, the divergence in SOD, CAT and GPx activities suggests a possible enzyme activity imbalance. Thus, diminished Cu,ZnSOD activity coupled to unmodified CAT and GPx activities (a decrease in SOD:CAT and SOD:GPx ratios) may yield a system that can eliminate H2O2 in a higher rate than it is formed. Indeed, dietary GADF seems to induce a better antioxidant protective status in a way that there would be no extra hydrogen peroxide to overcome Fenton reaction and to generate hydroxyl radicals in the cytosolic fraction of colonic mucosa. This is in agreement with Dani et al. (Reference Dani, Oliboni and Pasquali17) who reported reduced SOD:CAT ratio in liver of Wistar rats fed with purple grape juice indicating an enhancement of the antioxidant capacity.
The GADF-associated decrease of cytosolic GSSG content in colonic mucosa can also potentially arise from corresponding decreases in the rates of mitochondrial generation of O2∙−/H2O2. Mitochondria are the major source of superoxide anion production in cells and 1–2 % of oxygen is converted to H2O2 by MnSOD. However, MnSOD activity in mitochondria of the colonic mucosa of GADF rats remains unmodified, indicating no changes in the dismutation of O2∙− to H2O2. Moreover, the significant GADF-dependent increase in mitochondrial GPx activity can be responsible for an important decline in hydrogen peroxide inside mitochondria. Thus, the significantly decreased MnSOD:GPx ratio (43 % of control value) could indicate an enhanced capacity for reducing H2O2 enhancing the mitochondrial redox status and possibly decreasing the diffusion of H2O2 out of the mitochondrial matrix. In addition, since GPx is the primary antioxidant that inactivates lipid peroxides and thereby reduces their activities(Reference Wu, Fang and Yang52), the enhancement of mitochondrial GPx activity can contribute to maintain the unchanged mitochondrial LPO levels. Also, lower superoxide anion and mitochondrial ROS levels in dissociated brain cells of NMRI mice treated with Ginkgo biloba extract containing 24 % of flavonoids have been reported(Reference Abdel-Kader, Hauptmann and Keil53). However, the decrease of the LPO in the cytosol of the colonic mucosa of GADF rats, shown by the fall of the two aldehydic LPO products, MDA and 4-HNE, was possibly due to the cellular pro-reducing shift of redox balance elicited by GADF, since the glutathione redox cycle provides cellular protection against free radicals and ROS(Reference Gerard-Monnier and Chaudiere54). This effect could enhance the protection of phospholipids bilayer and thus the membrane integrity. Consequently, the possible stabilisation of the mitochondrial membrane with a lower activation of mitochondrial permeability transition pore, and a lower diffusion to cytosol of pro-apoptotic mediators, could explain the significant reduction in the colonic apoptosis elicited by GADF intake. It is known that the activation of the mitochondrial permeability transition pore creates an open channel across the mitochondrial inner and outer membranes, which permits the free diffusion of pro-apoptotic proteins to cytosol during oxidative stress(Reference Liu, Kim and Yang55). In the present study, the GADF-associated decrease in the mucosal apoptosis appears to be dependent of the mitochondrial MnSOD activity, as suggested by the strong inverse correlation found between MnSOD and apoptosis (Fig. 3(a)), indicating that an increase in the conversion of the O2∙− to H2O2 in the mitochondria led to a reduction of the colonic apoptosis in GADF rats. Accordingly, Du et al. (Reference Du, Guo and Lou56) reported that grape seed PP could protect against cardiac apoptosis via the induction of endogenous antioxidant enzymes. Conversely, chronic oxidative stress in mice with partial deficiency in MnSOD results in an increase of both mitochondrial ROS toxicity and sensitisation in the mitochondrial permeability transition pore, leading to premature induction of apoptosis(Reference Kokoszka, Coskun and Esposito57). In addition, a role for cellular GSH/GSSG redox balance in the reduction of apoptosis in the present study was suggested by the positive correlation found between apoptosis and the cytosolic GSSG levels (Fig. 3(b)) in colonic mucosa of GADF rats. Thus, the reduction in the apoptosis induced by GADF appears to be mediated by the low levels of glutathione disulphide and consequently by the maintained GSH concentration as well as by the pro-reducing shift of the GSSG/2GSH couple. In contrast, it has been established that an early loss of cellular and especially mitochondrial GSH:GSSG balance and GSH concentration can promote oxidative stress and induce colonocyte apoptosis(Reference Wang, Gotoh and Jennings58, Reference Circu, Rodriguez and Maloney59).
In conclusion, the present results suggest that GADF may potentially reduce apoptosis in colonic mucosa. The effect of GADF involves modulation of both mitochondrial and cytosolic antioxidant enzyme system together with an increase in cellular GSH:GSSG ratio, shifting the redox environment to a more reducing status. Constituents of GADF as dietary fibre, which enhance butyrate luminal production and PP as proanthocyanidins (which constitute a substantial proportion in the fibre), may work in concert for contribution to these antioxidant effects.
Acknowledgements
The present study was supported by the Projects CCG07-UCM/AGR 2721 of the Universidad Complutense de Madrid and AGL2008-01633 of the Spanish Ministerio de Ciencia e Innovación. M. E. L.-O. and A. A.-T. took care of the animals, performed the analytical determinations, analysed and participated in the discussion of the data. I. G. and E. M.-M. supervised and led all experiments and the analysis and discussion of all data. We thank Eduardo Sánchez-Mendoza for his assistance with manuscript preparation. The authors declare that there is no conflict of interest.











