Introduction
Mortality from cancer remains high, despite the advances in medical treatment. The biggest contributor of death in cancer is from metastatic cancer disease. The brain is a major site where metastasis arises from various solid tumours. The incidence of brain metastases is between 10 and 30% from all solid tumours.Reference Norden, Wen and Kesari1 Lung cancer, breast cancer, melanoma, renal cancer, colorectal cancer and lymphoma are among the most common primaries that metastasis to brain.Reference Lassman and DeAngelis2, Reference Bradley and Mehta3
Management of brain metastases has improved in recent decades, resulting in prolonged survival of those affected. There are various treatment options for brain metastasis such as whole brain radiotherapy, stereotactic radiosurgery, surgical removal, systemic therapy with cytotoxic chemotherapy, or the combination of those modalities. Radiotherapy, in particular stereotactic radiosurgery, is able to deliver a very highly conformal dose to lesions in the brain. This high dose radiation has potential to result in complete regression of the brain lesions after treatment.
Whole brain radiotherapy with the additional boost of stereotactic radiotherapy to brain metastatic lesions has been shown to provide good local control.Reference Khan, Lin and Liao4 However, good local control for brain metastases does not always translate into better overall survival.Reference Khan, Lin and Liao4 Although brain metastatic lesion is controlled, the overall survival can be reduced if the primary tumour or visceral metastases are not controlled.Reference Wick5 Due to that reason, there has been debate whether aggressively treating brain metastases is justifiable or if the best supportive care would be a better choice for those with predicted very short survival.Reference Ampil, Caldito, Mills, Marion, Balandin and Ponugupati6
When treating patients with perhaps none or minimal survival benefit, the costs of health care are increased by treating patients with brain metastases. Reference Peters, Bexelius, Munk and Leighl7 Higher costs are incurred if time-consuming treatments such as stereotactic radiosurgery are administered. Therefore, it is necessary to identify which patients would benefit the most from treatment and those who could potentially have a longer survival. Such patients with predictive longer survival could therefore benefit from aggressive and costly treatment, such as whole brain radiotherapy followed by stereotactic radiosurgery for brain metastases.
Various prognostic scoring systems for patients with metastatic disease are available for predicting patients’ survival.Reference Venur and Ahluwalia8 Several prognostic scoring systems that are most commonly utilised worldwide include recursive partitioning analysis (RPA),Reference Gaspar, Scott and Rotman9 graded prognostic assessment (GPA)Reference Sperduto, Erkey, Aspar, Ehta and Urran10 and basic score for brain metastases (BSBM).Reference Lorenzoni, Devriendt and Massafer11 These scoring systems have all been validated in multiple clinical settings worldwide with various accuracy.Reference Gaspar, Scott and Rotman9, Reference Sperduto, Erkey, Aspar, Ehta and Urran10, Reference Sperduto, Watanabe and Mullan12–Reference Gao, Huang and Du15
These commonly used prognostic scoring systems have been evaluated through clinical trials conducted mainly in developed countries, where patient characteristics, major tumour histology, treatment options available and patient’s preferences are different from developing countries such as Indonesia.Reference Redaniel, Laudico and Mirasol-Lumague16 Therefore, this study was conducted to assess the validity and determine which prognostic scoring system would be most predictive, in terms of survival rates, for an Indonesian cancer patient population.
Materials and Method
This study was a retrospective analysis of patients treated with radiotherapy for brain metastases. This study has received ethical approval from hospital human research and ethics committee. The sample was recruited from patients who received radiotherapy for brain metastasis at the Department of Radiotherapy, Cipto Mangunkusumo Hospital, Indonesia, from January 2012 until December 2014. All data regarding the patient’s medical history or subsequent death were gleaned from their medical records. To assess the current health and survival status of all assumed alive patients, patients were contacted by a telephone call, between June and July 2016. If patients were uncontactable, then a second attempt to call the patients was carried out within a 2-week interval from the first telephone call. For the purpose of this study, a total of three attempts were required before the patient was declared uncontactable.
A survival analysis was conducted with stratification based on the RPA, GPA and BSBM scoring systems used for each patient at diagnosis. Survival was calculated from the time of the last radiotherapy session until death. Uncontactable patients were excluded from the analysis. Patients that were still alive during the latest follow-up by phone call at July 2016 were censored. A comparison of median survival was undertaken between the results of this study and the published data of previous studies, comparing the three scoring systems.Reference Gaspar, Scott and Rotman9, Reference Lorenzoni, Devriendt and Massafer11, Reference Sperduto, Watanabe and Mullan12
Results
A total of 80 patients’ data were collected from patients treated with radiotherapy between January 2012 and December 2014. At follow-up telephone call, during June and July 2016, 18 patients were uncontactable, leaving 62 patients who were contactable, to be included in the analysis. Patient demographics, tumour primary and whole brain radiation doses for these patients can be viewed in Table 1. The majority of patients were female (58·1%). Median age was 51 years with an age range from the whole cohort from 8 years to 72 years. Median Karnofsky performance statusReference Agarwal, Chakraborty and Laskar17 was 70 with a range from 40 to 100. The most common primary tumour was lung cancer (35 cases; 56·5%) followed by breast cancer (17 cases; 27·4%). Twenty-four patients (38·7%) had the primary tumour site controlled. Majority (33 cases; 53·2%) had at least one more extracranial metastasis. Overall median survival of 62 patients was 9·2 months (Figure 1a), with 3 patients remained alive during the last follow-up by a telephone call on 29 July 2016.

Figure 1. Survival curve of the study population, a) overall survival and survival stratified based on b) RPA, c) BSBM, and d) GPA scoring. Total number of subjects was 62.
Table 1 Patient demographics, tumour primary and whole brain radiation doses from the study samples

Abbreviation: SRS, stereotactic radiosurgery.
All patients completed the radiotherapy treatment as prescribed. The radiotherapy target for the entire study population was whole brain radiotherapy with six patients receiving additional stereotactic radiosurgery boost for the residual lesions. The majority of patients (58·1%) received whole brain radiotherapy with a dose of 30 Gy in 10 fractions, 20 patients (32·3%) received whole brain radiotherapy with a dose of 40 Gy in 20 fractions, while the remaining six patients received other radiation dosage regimens such as 30 Gy in 15 fractions or 20 Gy in 5 fractions.
Survival Stratification based on RPA Scoring
Based on the RPA scoring, age was categorised into <65 years and ≥65 years, while the Karnofsky performance status was categorised into <70 and ≥70. Following that cut-off value, the majority of patients (54 patients; 87·1%) were categorised into <65-year-old group, and also the majority (37 patients; 59 7%) were categorised into having Karnofsky performance status of ≥70.
Based on the RPA stratification, 50% of patients were categorised into RPA class II, 38·7% of patients were categorised into RPA class III and the rest 11·2% were categorised into RPA class I. Three patients that were still alive at the follow-up by telephone call were two patients in RPA class I and one patient in RPA class III. Median survivals stratified by RPA class were 16·3 months, 11·2 months and 4·7 months for RPA class I, RPA class II and RPA class III, respectively (Table 2, Figure 1b).
Table 2 Survival of study patients based on RPA, BSBM and GPA scoring system compared to referenced median survival
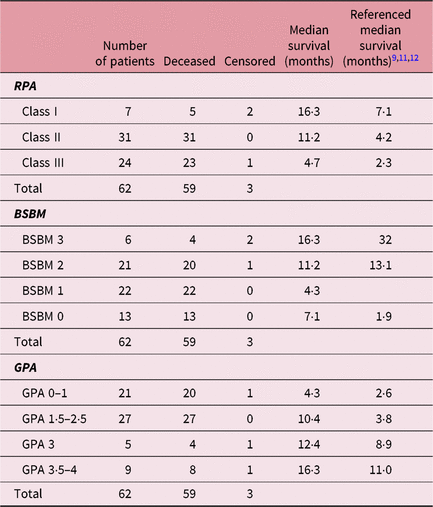
Abbreviations: RPA, recursive partitioning analysis; BSBM, basic score for brain metastases; GPA, graded prognostic assessment.
Survival Stratification based on BSBM Scoring
In the BSBM scoring system, there were four classes with scores range from 0 to 2. The score difference between the two adjacent classes was 0·5. Most of the patients were in BSBM class 1 (score 1) 33·8% of patients; BSBM class 2 (score 1·5) 35·4% of patients; and BSBM class 3 (score 2) 20·9% of patients. The minority of patients, 9·6% of patients, were in BSBM class 0 (score 0·5). Median survival rates were 16·3, 11·2, 4·3 and 7·1 months from BSBM classes 0–3, respectively (Table 2, Figure 1c). Two patients who remained alive at the time of the follow-up by telephone call were in BSBM class 0, and one patient who remained alive at the follow-up telephone call was in BSBM class 1.
Survival Stratification based on GPA Scoring
In the GPA scoring system, the number of lesions in the brain metastasis was crucial. In this analysis, 33 patients (53·2%) had brain lesions of more than three, 13 patients (21%) had brain lesions of two to three, while the remaining 16 patients (25·8%) had only one brain lesion. Most patients, 27 patients (43·5%) were categorised into GPA 1·5–2·5 and 21 patients (33·8%) were categorised into GPA 0–1.
Median survivals stratified based on GPA scoring were 4·3 months for GPA 0–1, 10·4 months for GPA 1·5–2·5, 12·4 months for GPA 3 and 16·3 months for GPA 3·5–4 (Table 2, Figure 1d). One patient each in GPA 0–1, GPA 3 and GPA 3·5–4 were alive at the follow-up by telephone call.
Discussion
All these three prognostic scoring systems utilise slightly different scoring components.Reference Venur and Ahluwalia8 The scoring components present in all scoring systems are the patients’ performance status and the status of the extracranial metastases. The BSBM does not require information of age in this scoring system. The GPA does not require information as to whether the primary tumour site is controlled or not, but the GPA is the only scoring system that requires information on the number of brain metastases lesions.
The RPA, BSBM and GPA have been tested for various treatment scenarios, and they have been found to be useful in providing prognostic value in various different settings.Reference Sperduto, Watanabe and Mullan12–Reference Gao, Huang and Du15, Reference Agboola, Beoit and Cross18 The RPA has also been shown to be of prognostic value in cases with resected brain metastatic lesions followed by whole brain radiotherapy.Reference Agboola, Beoit and Cross18 The RPA stratified survival curve provided a good stratification in our study population, although in the first 5 months, the curves of those patients with different RPA classes were found to overlap and then in subsequent months they diverged.
The BSBM and GPA did not stratify well among different scores and classes in our study. There were multiple overlaps in the BSBM and GPA stratified survival curves from our study population. The BSBM stratified survival curve in our study population only overlapped at class 1 and class 2, but class 0 and class 3 were well separated (Figure 1c). If the BSBM scoring criterion was modified to combine class 1 and class 2, then the BSBM would be considered prognostic by well-stratifying patients between the groups. Unlike BSBM, the GPA applied in our study population, for all four classes, overlapped at several time frames.
In our study, the RPA was found to be the most accurate prognostic scoring system, followed by the BSBM. In our study population, the BSBM class 2 was found to have lower median survival than the BSBM class 3, which was the other way round in the original BSBM study.Reference Lorenzoni, Devriendt and Massafer11 This finding further implies that the BSBM was not prognostic in our population. The finding in another study showed the GPA was less prognostic when compared to RPA, and this was consistent with our study.Reference Sperduto, Watanabe and Mullan12 The GPA is less prognostic than RPA probably because GPA does not incorporate whether the primary tumour was controlled or not. This factor is paramount and affects the overall survival in cancer patients.Reference Thorpe, Weiss, Goodman, Heyl and McGough19 Furthermore, the number of brain metastatic lesions, which is part of the GPA scoring system, did not confer any prognostic value in cases of multiple or large-size brain metastatic lesions.Reference Nishizaki, Saito and Jimi20
The median survival in all three-scoring systems tested with our study population showed a longer median survival across all stratification classes compared to their original prognostic studies.Reference Gaspar, Scott and Rotman9–Reference Lorenzoni, Devriendt and Massafer11 The longer median survival rates may be due to relatively small sample sizes in our study population, which did not truly represent the real general population with brain metastasis. Furthermore, there were 18 patients, a sizeable number of patients from the total of 80 patients treated during the study phase, excluded because they could not be contacted. Those 18 excluded patients might represent the lower survival group.
Other factors may also impact the overall survival of patients with brain metastases. Patients with brain metastases from various tumour histologies have been shown to respond differently following radiotherapy.Reference Sanghvi, Lischalk and Cai21 Generally, a radiosensitive tumour may respond better than a radioresistant tumour. Additional systemic treatment given after local brain metastases radiotherapy might also have impact on the overall survival.Reference Nieder, Marienhagen, Dalhaug, Aandahl, Haukland and Pawinski22 Furthermore, the more recent developments of new systemic treatments, with reduced side effects and improved cancer cell toxicity, might extend the survival of those patients with metastatic disease.Reference Tallet, Dhermain, Le Rhun, Noel and Kirova23, Reference Zhang, Tang, Liu and Chen24
Despite the limitations of this study, it is still reasonable to conclude that the RPA was found to offer a better prognostic indicator and deemed to be a valid prognostic scoring system for patients with brain metastases in Indonesian cancer patients. Furthermore, the RPA, which includes the age, performance status, extracranial metastasis and control of primary tumour, is a simple tool to use in daily clinical practice. The information required for RPA scoring does need excessive or costly procedures to obtain and no information regarding the number of brain metastases lesions is necessary, because this information may not be readily available. The number of brain lesions is usually best obtained by using magnetic resonance imaging (MRI) visualization, and MRI may not be readily available in developing countries. Thus RPA may be a good option to select and use in developing countries that may be resource constrained.
Conclusion
There is not a superior or most accurate prognostic scoring system for patients presenting with brain metastases. The treatment decision for all patients has to be viewed and tailored individually by their treating physicians. Social, economical and patient’s preference have to be taken into consideration when deciding which treatment to be administered to patient with brain metastases. However, as a guide and as a starting point, the RPA prognostic scoring system can be used to provide a rough prediction of prognosis. This study has shown a relatively good survival stratification in Indonesian patients with brain metastases.
Author ORCIDs
Soehartati A. Gondhowiardjo 0000-0002-9446-4361
Acknowledgements
None to be declared.







