Introduction
Major depressive disorder (MDD) was identified as one of the leading causes of nonfatal health loss in the 2019 Global Burden of Disease Study, affecting more than 400 million people globally (‘Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019,’, 2020). Given the potential pathophysiological mechanism behind the dysregulation of the brain-gut axis, the relationship between MDD and gastrointestinal disorders (GID) has attracted significant research during the past decade (Zhu, Tu, & Chen, Reference Zhu, Tu and Chen2022). Clinical observations have long identified the coexistence and interaction between MDD and numerous GID, with both illnesses frequently coexisting, responding to comparable therapies, aggravating each other, and sharing common molecular causes (Mayer, Craske, & Naliboff, Reference Mayer, Craske and Naliboff2001). Some studies have also suggested that MDD and GID share several risk factors, including but not limited to obesity (Emerenziani et al., Reference Emerenziani, Guarino, Trillo Asensio, Altomare, Ribolsi, Balestrieri and Cicala2019), diabetes (Verne & Sninsky, Reference Verne and Sninsky1998) and insomnia (Ali, Choe, Awab, Wagener, & Orr, Reference Ali, Choe, Awab, Wagener and Orr2013). To comprehend the interplay between depression and GID and to propose clinical treatment strategies to improve the co-morbid population with MDD and GID, it is necessary to clarify the potential causal role between MDD and GID.
The findings of substantial observational studies support the concept that MDD may increase the risks of GID (Fang et al., Reference Fang, Yang, Liu, Zhang, Xu and Chen2019; Kim et al., Reference Kim, Yoo, Li, Tighe, Cholankeril, Harrison and Ahmed2019; Martín-Merino, Ruigómez, García Rodríguez, Wallander, & Johansson, Reference Martín-Merino, Ruigómez, García Rodríguez, Wallander and Johansson2010). Despite the plausible positive association between MDD and GID, there is also partial evidence to support the claim is non-existent (Kim et al., Reference Kim, Kim, Seo, Lee, Kim, Kim and Jung2013; Lee, Otgonsuren, Younoszai, Mir, & Younossi, Reference Lee, Otgonsuren, Younoszai, Mir and Younossi2013; Shaheen, Kaplan, Sharkey, Lethebe, & Swain, Reference Shaheen, Kaplan, Sharkey, Lethebe and Swain2021). Observational studies yield inconsistent results, and the real direction of causal association cannot be ascertained owing to measurement error, confounding, and reverse causality bias (Fewell, Davey Smith, & Sterne, Reference Fewell, Davey Smith and Sterne2007).
Mendelian randomization (MR), a method for inferring causality between a modifiable exposure and an outcome that utilizes genetic variants as instrumental variables, is less likely to be affected by residual confounding and reverse causation than traditional observational approaches (Smith & Ebrahim, Reference Smith and Ebrahim2003). In the absence of randomized controlled trials (RCTs), MR is an effective way to strengthen causal inferences. A recent genome-wide association study (GWAS) meta-analysis has revealed 44 genetic loci associated with MDD that may be used within an MR framework (Wray et al., Reference Wray, Ripke, Mattheisen, Trzaskowski, Byrne, Abdellaoui and Sullivan2018). Yeda et al. had used the MR framework to explore the genetically predicted effects of MDD on gastroesophageal reflux disease (GERD), irritable bowel syndrome (IBS) and peptic ulcer disease (PUD) (Wu et al., Reference Wu, Murray, Byrne, Sidorenko, Visscher and Wray2021b). Here, we extend this analysis to include non-alcoholic fatty liver disease (NAFLD) and use additional methods and datasets to verify the robustness of our findings.
The aim of this two-sample MR study was to test the genetically predicted effects of MDD on multiple GID (i.e. GERD, IBS, PUD and NAFLD) using summary genetic association data from (1) Wray et al. (N cases = 135 458; N controls = 344 901) (Wray et al., Reference Wray, Ripke, Mattheisen, Trzaskowski, Byrne, Abdellaoui and Sullivan2018), (2) the UK Biobank and several international genetic consortia (N cases = 4761–71 522; N controls = 261 079– 439 661) (An et al., Reference An, Gharahkhani, Law, Ong, Han, Olsen and MacGregor2019; Eijsbouts et al., Reference Eijsbouts, Zheng, Kennedy, Bonfiglio, Anderson, Moutsianas and Parkes2021; Fairfield et al., Reference Fairfield, Drake, Pius, Bretherick, Campbell, Clark and Spiliopoulou2022; Wu et al., Reference Wu, Murray, Byrne, Sidorenko, Visscher and Wray2021b) and (3) FinnGen (N cases = 894– 13 141; N controls = 182 423– 217 898).
Methods
Reporting guidelines and study design
This study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization (STROBE-MR) guideline (STROBE-MR Checklist) (Skrivankova et al., Reference Skrivankova, Richmond, Woolf, Yarmolinsky, Davies, Swanson and Richards2021). The overall study design is illustrated in Fig. 1. Three assumptions are deemed necessary for the MR study to guarantee the validity of results (described in Fig. 1) (Didelez & Sheehan, Reference Didelez and Sheehan2007).
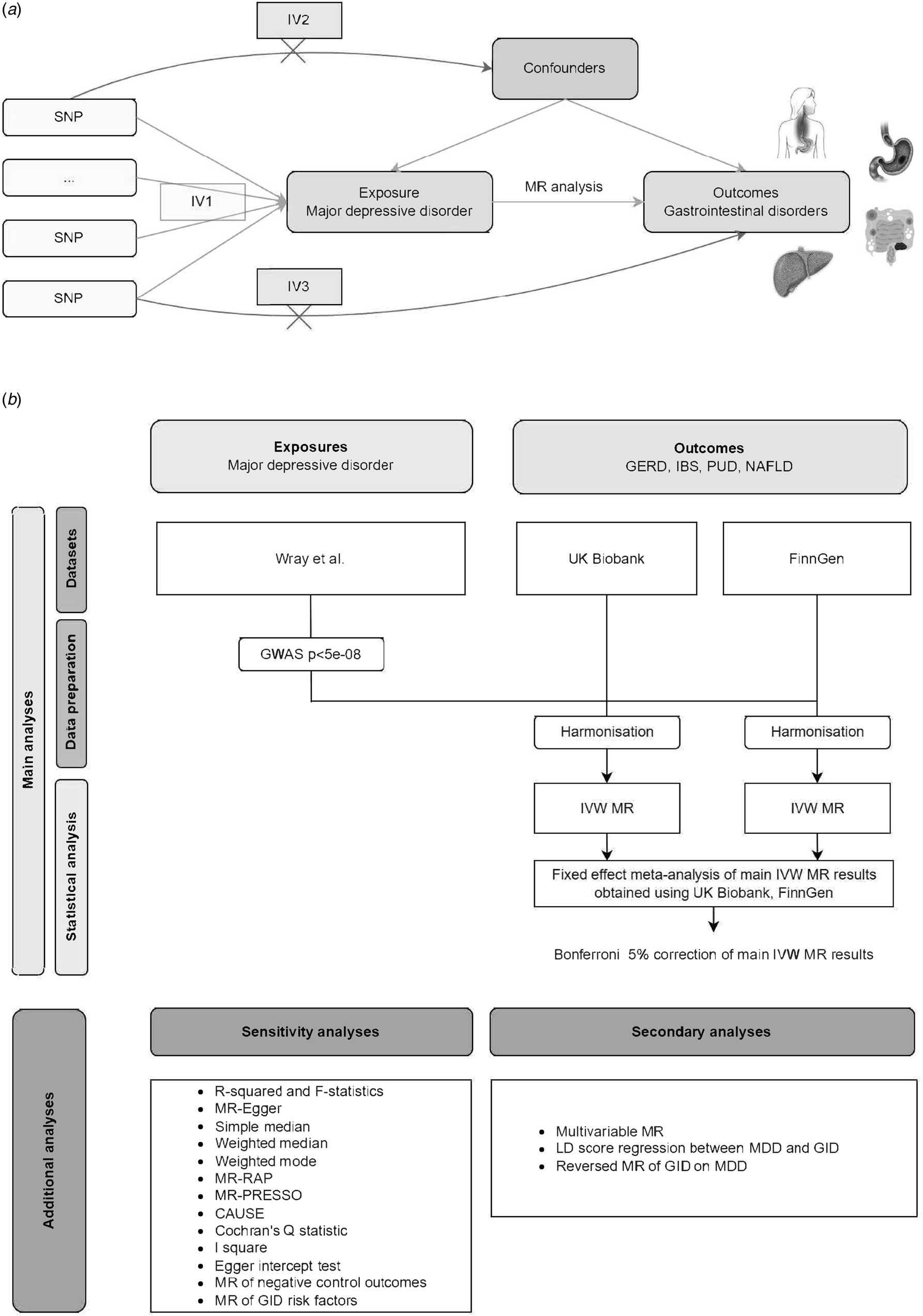
Figure 1. Diagrammatic overview of univariable and multivariable MR study design. (a) Three assumptions are deemed necessary for MR study to guarantee the validity of results, including (1) IVs are strongly related to the risk factor of interest (i.e. the relevance assumption); (2) IVs are independent of confounding factors (i.e. the independence assumption); (3) IVs affect outcomes only through exposure (i.e. the exclusion restriction assumption). (b) Flowchart summarizing study methods. Abbreviations: IVW, inverse variance weighted; MR, Mendelian randomization; GWAS, genome-wide association study; LD, Linkage disequilibrium; CAUSE, Causal Analysis Using Summary Effect estimates, SNP, single-nucleotide polymorphism; GID, gastrointestinal disorders; GERD, gastroesophageal reflux disease; PUD, Peptic ulcer disease; IBS, Irritable bowel syndrome; NAFLD, Non-alcoholic fatty liver disease.
Data source and sample overlap
Two-sample MR was performed using published summary-level data from GWAS of the traits of interest in European individuals. Details of the GWAS data sets utilized in this investigation are listed in Table 1. The ethical approval, participant informed consent, and participant eligibility criteria for each of the original GWAS can be found in their respective publications (An et al., Reference An, Gharahkhani, Law, Ong, Han, Olsen and MacGregor2019; Eijsbouts et al., Reference Eijsbouts, Zheng, Kennedy, Bonfiglio, Anderson, Moutsianas and Parkes2021; Fairfield et al., Reference Fairfield, Drake, Pius, Bretherick, Campbell, Clark and Spiliopoulou2022; Wray et al., Reference Wray, Ripke, Mattheisen, Trzaskowski, Byrne, Abdellaoui and Sullivan2018; Wu et al., Reference Wu, Murray, Byrne, Sidorenko, Visscher and Wray2021b).
Table 1. Detailed information of GWAS summary statistics for main exposure and outcome phenotypes

Abbreviations: GERD, gastroesophageal reflux disease; PUD, Peptic ulcer disease; IBS, Irritable bowel syndrome; NAFLD, Non-alcoholic fatty liver disease; MDD, major depressive disorder; ICD, International Classification of Diseases; OPCS, The OPCS Classification of Interventions and Procedures; DSM, The Diagnostic and Statistical Manual of Mental Disorders; UKB, UK biobank; PGC, Psychiatric Genomics Consortium; iPSYCH, The Lundbeck Foundation Initiative for Integrative Psychiatric Research; GenScot, Generation Scotland; GERA, Genetic Epidemiology Research on Adult Health and Aging.
Given the MDD GWAS includes a minor UK Biobank (UKB) sample (cases: 14 260, controls: 15 480), the dataset utilized for this investigation contains a partial sample overlap between MDD and GID. Sample overlap between exposure and outcome populations may skew two-sample MR estimates toward confounding relationships in the case of weak instruments (Burgess, Davies, & Thompson, Reference Burgess, Davies and Thompson2016). To formally assess the risk of bias resulting from sample overlap, we performed a calculation of bias and type I error rate using the method proposed by Burgess et al. (https://sb452.shinyapps.io/overlap) (Burgess et al., Reference Burgess, Davies and Thompson2016).
Genetic instruments for major depressive disorder
Selection of instrumental variants
We obtained summary genetic association estimates for MDD from Wray et al.'s GWAS meta-analysis, which included 480 359 participants of European ancestry (Wray et al., Reference Wray, Ripke, Mattheisen, Trzaskowski, Byrne, Abdellaoui and Sullivan2018). For MDD, we initially extracted independent genome-wide significant (p < 5 × 10−8) SNPs reported in the corresponding source literature as instrumental variants (IVs). This resulted in 43 IVs for MDD. The complete list of instruments is summarized in online Supplementary Tables S1.
Assessing instrumental strength
We identified 43 independent SNPs for MDD (online Supplementary Table S1). The proportions of trait variance explained by genetic instruments (R 2) and instrument strength (F statistic) were calculated using the following formulae: R 2 = 2β 2 × MAF × (1-MAF) and F = [R 2 × (N-2))/(1-R 2] (where MAF = minor allele frequency, β = effect estimate of the SNP in the exposure GWAS, N = sample size) (Burgess et al., Reference Burgess, Davies and Thompson2016). The genetic instruments for MDD explained 1.84% of the trait variance. All the selected SNPs had F statistics greater than 10 (F statistics median 186 and range 167–436).
Genetic association data sources for gastrointestinal disorders outcomes
We obtained summary-level genetic association data for GID outcomes from the UK Biobank and FinnGen and several international genetic consortia: the QSkin health study, the Bellygenes cohorts and the Psychiatric Genomics Consortium (PGC) (Table 1). Further details of the studies and the data obtained are described in original publications (An et al., Reference An, Gharahkhani, Law, Ong, Han, Olsen and MacGregor2019; Eijsbouts et al., Reference Eijsbouts, Zheng, Kennedy, Bonfiglio, Anderson, Moutsianas and Parkes2021; Fairfield et al., Reference Fairfield, Drake, Pius, Bretherick, Campbell, Clark and Spiliopoulou2022; Wray et al., Reference Wray, Ripke, Mattheisen, Trzaskowski, Byrne, Abdellaoui and Sullivan2018; Wu et al., Reference Wu, Murray, Byrne, Sidorenko, Visscher and Wray2021b).
We extracted genetic association data for the selected SNPs from each GID GWAS (for GERD, IBS, PUD and NAFLD). LD proxies (r 2 > 0.8) were used when the SNPs of interest were missing from the GID GWAS dataset. The ‘LDlinkR’ R package (version 1.2.0) was used to find proxies for GID data. Finally, we used the TwoSampleMR R package (Hemani et al., Reference Hemani, Zheng, Elsworth, Wade, Haberland, Baird and Haycock2018) (version 0.5.6) to undertake a harmonization procedure for integrating IVs information between exposure and outcome (Hemani, Tilling, & Davey Smith, Reference Hemani, Tilling and Davey Smith2017). We also removed SNPs that were palindromic with intermediate allele frequencies. Then we applied Steiger filtering to ensure whether each IV explains more phenotypic variance in the exposure than the outcome and removes those genetic variants that do not satisfy this criterion (Hemani et al., Reference Hemani, Tilling and Davey Smith2017). The IVs with a ‘False’ Steiger direction will be excluded. online Supplementary Table S2–S3 contains further information on the harmonized datasets utilized in the current MR analysis.
Power calculation
We calculated the statistical power of this study based on the Burgess et al., method (https://sb452.shinyapps.io/power/) (Burgess, Reference Burgess2014). Calculations were performed separately for each MDD-GID combination. They were based on a type I error rate of 0.05, the proportion of phenotypic variance explained by genetic variants (R 2) for MDD, and the total sample size included in the meta-analysis for each GID. Across combinations of the MDD and four GID measures, we had 80% power to detect ORs as small as 1.09–2 (online Supplementary Table S4).
Statistical analyses
We estimated the genetically predicted effects of MDD on multiple GID using a two-sample MR framework (Fig. 1).
Main analyses
Main analyses were performed using multiplicative random effects inverse-variance weighted (IVW)-MR, a method that combines the genetically predicted effect of MDD on GID across genetic variants (Burgess, Butterworth, & Thompson, Reference Burgess, Butterworth and Thompson2013). We used fixed-effect meta-analysis to pool results across studies (i.e. UK Biobank and FinnGen). I 2 statistics and their corresponding confidence intervals (CI) were used to estimate heterogeneity across study estimates (von Hippel, Reference von Hippel2015). A Bonferroni corrected p value < 0.0125 (0.05/4 for 4 GID) was used to correct the pooled main IVW results for multiple testing. The methods for Sensitivity analyses and Secondary analyses are detailed in the online Supplementary methods section.
All MR analyses were performed using R software (version 3.6.1). Two sample MR analyses were conducted using the ‘TwoSampleMR’ (version 0.5.6), ‘MendelianRandomization’ (version 0.6.0), ‘mr.raps’ (version 0.3.1), ‘MRPRESSO’ (version 1.0) packages. Meta-analyses of IVW results were performed using the ‘meta’ package (version 4.14). CAUSE analyses were conducted using the ‘cause’ package (version 1.2.0). MVMR was performed using the ‘MVMR’ package (version 0.3). Forest plots were created using the ‘forestplot’ package (version 1.10). LD Scores were computed using the ‘ldsc’ command line tool (version 1.0.1). The code used in this study is available at: https://github.com/Rick-Chen-PKU/Depression_GI.
Results
Gastroesophageal reflux disease
Meta-analyses IVW MR findings suggested that genetically predicted MDD increased the risk of GERD [odds ratio (OR) = 1.293 per logOR change in MDD, 95% CI 1.204–1.389, p < 0.001] (Fig. 2, online Supplementary Table S8). These results survived multiple testing correction (Bonferroni p < 0.001) and there was little evidence of heterogeneity across UKB and FinnGen estimates (I 2 = 0%, 95% CI ‘NA’, p = 0.37). Additionally, the direction of the genetically predicted effect was consistent across other sensitivity MR analyses (i.e. MR-Egger, simple median, weighted median, weighted mode, MR-RAPS, MR-PRESSO) (Fig. 2, online Supplementary Tables S6–S8) and results were consistent in the main discovery cohort (IVW OR 1.318, 95% CI 1.214–1.431, p < 0.001) (Fig. 2, online Supplementary Table S6). This causal association did not change substantially in the sensitivity analysis after excluding the SNPs associated with potential confounders (IVW OR 1.321, 95% CI 1.196–1.459, p < 0.001; online Supplementary Table S9).

Figure 2. Associations of genetically predicted depression with risk of gastrointestinal disorders in discovery, replication, and combined datasets. OR, odds ratio; CI, confidence interval; IVW, inverse-variance weighted; GERD, Gastroesophageal reflux disease; PUD, Peptic ulcer disease; IBS, Irritable bowel syndrome; NAFLD, Non-alcoholic fatty liver disease.
In multivariable IVW MR analysis adjusting for BMI, the causal association between MDD and GERD was attenuated (OR 1.274; 95% CI 1.101–1.431). With adjustment for insomnia, the causal association was expanded (OR 1.469; 95% CI 1.353–1.595). There was little change in causal estimate after adjustment for T2D (Fig. 3).

Figure 3. Result of multivariable Mendelian Randomization analysis in evaluating the casual association between depression and numerous GID (based on UK biobank sample). OR, odds ratio; CI, confidence interval; GERD, gastroesophageal reflux disease; PUD, Peptic ulcer disease; IBS, Irritable bowel syndrome; NAFLD, Non-alcoholic fatty liver disease; BMI, body mass index; T2D, type 2 diabetes.
We did not find evidence of residual population stratification in MR analyses using negative control outcomes (online Supplementary Table S10), nor did we find evidence of horizontal pleiotropy via potential GID risk factors (online Supplementary Table S11). Reversed MR analysis revealed that genetically predicted GERD increased the risk of MDD (IVW OR 1.398, 95% CI 1.114–1.754, p = 0.0038; online Supplementary Table S12). Additionally, LDSC coefficients for MDD and GERD were also in the expected direction (rg = 0.53, p < 0.001; Table 2). MR-CAUSE sensitivity analysis revealed that the causal models of genetically predicted MDD on GERD outperformed the shared model and null model, indicating no bias due to correlated pleiotropy (Table 3). Formal assessment revealed a minimal risk of bias from sample overlap (<0.002, regardless of overlap proportion) (online Supplementary Table S13), with a minimum F statistic of 167.
Table 2. Genetic correlation of major depressive disorder (MDD) with gastrointestinal disorders (GID, based on UK biobank sample), estimated by linkage disequilibrium score regression

Note: Summary statistics for each trait were merged with Hapmap3 SNPs excluding the HLA region to estimate rg; GERD, gastroesophageal reflux disease; PUD, Peptic ulcer disease; IBS, Irritable bowel syndrome; NAFLD, Non-alcoholic fatty liver disease
Table 3. MR-CAUSE analysis, linking genetic predicted major depressive disorder (MDD) with gastrointestinal disorders (GID, based on UKB sample)

Abbreviations: CAUSE, causal analysis using summary effect; ELPD, expected log pointwise posterior density; MR, Mendelian randomization; GERD, gastroesophageal reflux disease; PUD, Peptic ulcer disease; IBS, Irritable bowel syndrome; NAFLD, Non-alcoholic fatty liver disease.
(MR-CAUSE parameter setting: r2_thresh = 0.01, pval_thresh = 1 × 10−3).
a Model 1 and Model 2 refer to the models being compared (null, sharing, or causal).
b Model fit is measured by Δ Expected Log Pointwise Posterior Density (Δ ELPD); Negative values indicate that model 2 is a better fit.
Irritable bowel syndrome
Meta-analyses IVW MR found evidence that genetically predicted MDD increased the risk of IBS (OR 1.484, 95% CI 1.358–1.621, p < 0.001) (Fig. 2, online Supplementary Table S8). These results survived multiple testing correction (Bonferroni p < 0.001) and there was little evidence of heterogeneity across UKB and FinnGen estimates (I 2 = 0%, 95% CI ‘NA’, p = 0.47). Additionally, the direction of the genetically predicted effect was consistent across other sensitivity MR analyses (i.e. MR-Egger, simple median, weighted median, weighted mode, MR-RAPS, MR-PRESSO) (Fig. 2, online Supplementary Tables S6–S8) and results were consistent in the main discovery cohort (IVW OR 1.464, 95% CI 1.329–1.612, p < 0.001) (Fig. 2, online Supplementary Table S6). This causal association did not change substantially in the sensitivity analysis after excluding the SNPs associated with potential confounders (IVW OR 1.447, 95% CI 1.305–1.605, p < 0.001; online Supplementary Table S9).
In multivariable IVW MR analysis adjusting for BMI, T2D or insomnia, the causal association between MDD and IBS were attenuated (BMI OR 1.338; 95% CI 1.174–1.525; T2D OR 1.362; 95% CI 1.225–1.513; Insomnia OR 1.388; 95% CI 1.209–1.592) (Fig. 3). Reversed MR analysis found evidence that genetically predicted IBS increased the risk of MDD (IVW OR 1.301, 95% CI 1.003–1.688, p = 0.047; online Supplementary Table S12). Additionally, LDSC coefficients for MDD and IBS were also in the expected direction (rg = 0.56, p < 0.001; Table 2). MR-CAUSE sensitivity analysis revealed that the causal models of genetically predicted MDD on IBS outperformed the shared model and null model, indicating no bias due to correlated pleiotropy (Table 3).
Peptic ulcer disease
Meta-analyses IVW MR found evidence that genetically predicted MDD increased the risk of PUD (OR 1.218, 95% CI 1.092–1.358, p < 0.001) (Fig. 2, online Supplementary Table S8). These results survived multiple testing correction (Bonferroni p = 0.0016) and there was little evidence of heterogeneity across UKB and FinnGen estimates (I 2 = 0%, 95% CI ‘NA’, p = 0.59). Additionally, the direction of the genetically predicted effect was consistent across other sensitivity MR analyses (i.e. MR-Egger, simple median, weighted median, weighted mode, MR-RAPS, MR-PRESSO) (Fig. 2, online Supplementary Tables S6–S8) and results were consistent in the main discovery cohort (IVW OR 1.237, 95% CI 1.093–1.401, p < 0.001) (Fig. 2, online Supplementary Table S6). This causal association did not change substantially in the sensitivity analysis after excluding the SNPs associated with potential confounders (IVW OR 1.217, 95% CI 1.055–1.405, p = 0.0072; online Supplementary Table S9).
In multivariable IVW MR analysis adjusting for BMI, T2D or insomnia, we found little evidence of the causal association between MDD and PUD (Fig. 3). Reversed MR analysis found no evidence that genetically predicted PUD had a potential causal association with MDD (online Supplementary Table S12). Additionally, LDSC coefficients for MDD and PUD were in the expected direction (rg = 0.50, p < 0.001; Table 2). MR-CAUSE sensitivity analysis revealed that the causal models of genetically predicted MDD on PUD outperformed the shared model and null model, indicating no bias due to correlated pleiotropy (Table 3).
Non-alcoholic fatty liver disease
Meta-analyses IVW MR found evidence that genetically predicted MDD increased the risk of NAFLD (OR 1.310, 95% CI 1.084–1.583, p = 0.005) (Fig. 2, online Supplementary Table S8). These results survived multiple testing correction (Bonferroni p = 0.021) and there was little evidence of heterogeneity across UKB and FinnGen estimates (I 2 = 0%, 95% CI ‘NA’, p = 0.61). Additionally, the direction of the genetically predicted effect was consistent across other sensitivity MR analyses (i.e. MR-Egger, simple median, weighted median, weighted mode, MR-RAPS, MR-PRESSO) (Fig. 2, online Supplementary Tables S6–S8) and results were consistent in the main discovery cohort (IVW OR 1.284, 95% CI 1.047–1.576, p = 0.017) (Fig. 2, online Supplementary Table S6). This causal association did not change substantially in the sensitivity analysis after excluding the SNPs associated with potential confounders (IVW OR 1.378, 95% CI 1.084–1.751, p = 0.0088; online Supplementary Table S9).
In multivariable IVW MR analysis adjusting for BMI, T2D or insomnia, we found little evidence of the causal association between MDD and NAFLD (Fig. 3). Reversed MR analysis found no evidence that genetically predicted NAFLD had a potential causal association with MDD (online Supplementary Table S12). Additionally, LDSC coefficients for MDD and NAFLD were in the expected direction (rg = 0.40, p < 0.001; Table 2). MR-CAUSE sensitivity analysis revealed that the causal model of genetically predicted MDD on NAFLD was inferior to the shared model and null model (Table 3).
Discussion
In this comprehensive two-sample MR study of MDD and GID, we found evidence that genetically predicted MDD may increase the risk of GERD, IBS, PUD and NAFLD. Reverse MR found evidence of GERD or IBS may increase the risk of MDD. We also investigated if any potential confounder/mediator plays a role in the causal pathway from MDD to GID. LDSC analyses revealed that MDD and GID may have shared genetic etiology. Our findings are timely to draw attention to the co-morbid risk of MDD and GID and to provide more robust scientific evidence to guide treatment.
The novelty of this study is mainly reflected in the following aspects. First, it is the first time that a quasi-experimental approach based on a large sample has been used to explore the potential causal relationship between MDD and GID. There are currently no relevant RCTs in the field and the results of this study would be a strong addition to the evidence for a potential causal relationship in this area, given the available evidence. Furthermore, previous studies have reported higher statistical power for two-sample MR methods compared to classical epidemiological study design methods (Bowden et al., Reference Bowden, Del Greco, Minelli, Zhao, Lawlor, Sheehan and Davey Smith2019). Second, we pooled results from multiple sources using fixed effects meta-analysis to improve the precision of the estimates and adopted a thorough sensitivity analysis to validate the robustness of the results. Third, this study is the first to find an association between MDD and PUD in a population of European ancestry.
Causal association between major depressive disorder and gastroesophageal reflux disease
Using the MR study design, we found a positive relationship of genetically predicted MDD on GERD. We also found that genetically predicted GERD was associated with a higher risk of developing MDD. This finding was consistent with the result of a nested case-control study conducted in Korea by Kim et al. (OR 2.01, 95% CI 1.96–2.07, p < 0.001) (Kim et al., Reference Kim, Kim, Lim, Kong, Kim and Choi2018). However, our findings contrast with those highlighted in Zhi, et al., an observational cohort study that used Men Androgen Inflammation Lifestyle Environment and Stress data (On et al., Reference On, Grant, Shi, Taylor, Wittert, Tully and Martin2017). Nevertheless, it is possible that their analyses were underpowered, as their sample only included 221 GERD cases (13.7%). More importantly, the direction of the reported estimate is consistent with our findings and those previous observational study (Chen & Wang, Reference Chen and Wang2020; Choi et al., Reference Choi, Yang, Kang, Han, Lee, Lee and Kim2018; You et al., Reference You, Perng, Hu, Lu, Chen, Yang and Chen2015). MVMR analysis revealed that MDD had a direct causal effect on GERD independent of BMI, T2D and Insomnia, which was also found in these studies (Jansson et al., Reference Jansson, Nordenstedt, Wallander, Johansson, Johnsen, Hveem and Lagergren2007; On et al., Reference On, Grant, Shi, Taylor, Wittert, Tully and Martin2017).
There may be some biological mechanism to explain genetically predicted MDD increases the risk of GRED. Previous studies have shown a strong association between the brain and the gastrointestinal tract (Choi et al., Reference Choi, Yang, Kang, Han, Lee, Lee and Kim2018). Due to the gut-brain axis, psychological factors such as personal mood can influence gastrointestinal function and contribute to the progression of GID. Likewise, the state of the gastrointestinal tract may affect an individual's emotional state (Choi et al., Reference Choi, Yang, Kang, Han, Lee, Lee and Kim2018). MDD may precede GERD and increase the risk of GERD. First, depression can lead to hypochondria and create a dread of reflux symptoms, which may increase an individual's perception of reflux symptoms (Kamolz & Velanovich, Reference Kamolz and Velanovich2002). This may in turn reduce the body's sensory threshold and exaggerate the perception of reflux symptoms. Second, depression may cause physiological structural changes that increase reflux. MDD can decrease the pressure of the lower esophageal sphincter, alter esophageal motility, increase gastric acid secretion, and decrease acid clearance from the esophagus (Johnston, Reference Johnston2005; Kamolz & Velanovich, Reference Kamolz and Velanovich2002). This mechanism has also been demonstrated in animal study. Rats subjected to psychological stress show disruption of the tight junctions of the esophageal epithelium and subsequent weakening of the barrier function of the esophageal mucosa, thereby increasing its vulnerability to reflux (Farré et al., Reference Farré, De Vos, Geboes, Verbecke, Vanden Berghe, Depoortere and Sifrim2007). Third, antidepressant medication may worsen reflux (Martín-Merino et al., Reference Martín-Merino, Ruigómez, García Rodríguez, Wallander and Johansson2010). Antidepressants have been shown to delay gastric emptying and inhibit esophageal peristalsis (Brahm & Kelly-Rehm, Reference Brahm and Kelly-Rehm2011).
Conversely, GERD may also increase the risk of MDD. First, persistent reflux symptoms can be annoying and upsetting and may precipitate depression (Kamolz & Velanovich, Reference Kamolz and Velanovich2002). Previous studies have shown that those patients who do not respond well to medication are more likely to exhibit higher levels of depression, and this group is most common in the NERD group of GERD subtypes (Kimura et al., Reference Kimura, Kamiya, Senoo, Tsuchida, Hirano, Kojima and Joh2016). Second, the esophageal mucosa of GERD patients contains high levels of cytokines such as interleukin IL-6, IL-8, interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α) (Altomare, Guarino, Cocca, Emerenziani, & Cicala, Reference Altomare, Guarino, Cocca, Emerenziani and Cicala2013). Increased levels of these immune mediators may lead to chronic inflammation in the central nervous system (Lampa et al., Reference Lampa, Westman, Kadetoff, Agréus, Le Maître, Gillis-Haegerstrand and Svensson2012). This chronic inflammation plays a vital role in the pathophysiology of MDD and may lead to the development of depression (Berk et al., Reference Berk, Williams, Jacka, O'Neil, Pasco, Moylan and Maes2013; Goldstein, Kemp, Soczynska, & McIntyre, Reference Goldstein, Kemp, Soczynska and McIntyre2009). Again, the frequent arousal of GERD may affect sympathetic activation (Jansson et al., Reference Jansson, Nordenstedt, Wallander, Johansson, Johnsen, Hveem and Lagergren2009). Frequent reflux of gastric acid stimulates the vagus nerve and triggers bronchoconstriction (Demeter & Pap, Reference Demeter and Pap2004), which may lead to sleep disturbances and further affect MDD (Altena et al., Reference Altena, Micoulaud-Franchi, Geoffroy, Sanz-Arigita, Bioulac and Philip2016).
The relationship between MDD and GERD involves a complex interaction of various mechanisms. The results of bidirectional MR corroborate the potential pattern of co-morbidity between MDD and GRED. A recent study showed that treatment of GERD with a proton-pump inhibitor also slightly improved the symptoms of MDD (Wu, Chen, & Wen, Reference Wu, Chen and Wen2021a). Therefore, it is necessary to pay careful attention to the degree of depression in GERD patients or the symptoms of GERD in MDD patients to make the disease treatment more effective.
Causal association between major depressive disorder and irritable bowel syndrome
In the case of IBS, our study suggests that genetically predicted MDD increased the risk of IBS, and genetically predicted IBS increased the risk of MDD. This finding was consistent with those reported in Janssens, Zijlema, Joustra, and Rosmalen, Reference Janssens, Zijlema, Joustra and Rosmalen2015, a cohort study that used LifeLines data (OR 1.87, 95% CI 1.67–2.10) (Janssens et al., Reference Janssens, Zijlema, Joustra and Rosmalen2015). Additionally, evidence from numerous studies, including systematic reviews (Aziz, Kumar, Muhammad Nawawi, Raja Ali, & Mokhtar, Reference Aziz, Kumar, Muhammad Nawawi, Raja Ali and Mokhtar2021) and meta-analyses (Nikolova et al., Reference Nikolova, Pelton, Moulton, Zorzato, Cleare, Young and Stone2022; Sibelli et al., Reference Sibelli, Chalder, Everitt, Workman, Windgassen and Moss-Morris2016; Zamani, Alizadeh-Tabari, & Zamani, Reference Zamani, Alizadeh-Tabari and Zamani2019), case reports (Li et al., Reference Li, Xu, Guo, Chen, Li, Qi and Che2022), and animal study (Takajo et al., Reference Takajo, Tomita, Tsuchihashi, Enomoto, Tanichi, Toda and Hokari2019), suggest a co-morbid state of MDD and IBS. Additionally, MVMR analysis revealed that MDD had a direct causal association on IBD independent of BMI, T2D and Insomnia.
A potential mechanism might explain MDD may lead to higher IBS risk. Drossman et al., found by functional magnetic resonance imaging that depression can activate the cingulate region of the limbic system of the cerebral cortex (Drossman et al., Reference Drossman, Ringel, Vogt, Leserman, Lin, Smith and Whitehead2003); and the emotional activity center of the limbic system is in the same anatomical site as the vegetative center and the endocrine regulatory center, which govern the movement and secretion of the digestive tract; thus, the function of the vegetative center and the endocrine regulatory center is altered, and then the information is transmitted to the enteric nervous system through the brain-gut axis, causing changes in neurotransmitters and adrenocorticotropin-releasing factors, which affect visceral sensation, intestinal movement and endocrine function (Tan et al., Reference Tan, Pei, Xie, Wang, Liu, Cheng and Li2021). In addition, study has shown that depression can lead to increased levels of expression of cytokines such as IL-1β and TNF-α in the peripheral serum of patients (Miller, Maletic, & Raison, Reference Miller, Maletic and Raison2009). Elevated levels of IL-1β can stimulate inflammation, affect visceral sensitivity and ultimately lead to the development of IBS. TNF-α can contribute to the development of IBS by affecting the activation and expression of myosin light chain kinase, leading to impaired tight junction function and disruption of intestinal epithelial barrier function (Al-Sadi, Guo, Ye, Rawat, & Ma, Reference Al-Sadi, Guo, Ye, Rawat and Ma2016).
As for the causal association of genetically predicted IBS on MDD, a recent review by Mayer et al., showed that sensory input from the gut implicates the activity of a number of brain regions that are associated with various brain functions such as sensory, cognitive and emotional (Mayer, Ryu, & Bhatt, Reference Mayer, Ryu and Bhatt2023); Drossman et al., suggested that intestinal symptoms such as abdominal pain in IBS patients can induce activation of areas related to the limbic system of the cerebral cortex, which affects patients' depressive mood (Drossman, Reference Drossman2005). IBS may also worsen symptoms of depression by affecting behavior (Ballou et al., Reference Ballou, McMahon, Lee, Katon, Shin, Rangan and Iturrino2019). Concerns about bowel movements or IBS symptoms may lead patients to seek isolation and avoid social activities. These changes in behavior may cause psychological symptoms such as loneliness and helplessness, which can lead to feelings of depression.
Causal association between major depressive disorder and peptic ulcer disease
As for PUD, we found evidence that genetically predicted MDD increased the risk of PUD, while genetically predicted PUD had no causal association with MDD. Several previous observational studies were consistent with the present results but were based on East Asian populations (Fang et al., Reference Fang, Yang, Liu, Zhang, Xu and Chen2019; Hsu et al., Reference Hsu, Hsu, Chang, Lee, Chong, Lin and Kao2015; Kim, Min, Oh, & Choi, Reference Kim, Min, Oh and Choi2020; Lee et al., Reference Lee, Sung, Kim, Lee, Park and Shim2015). In contrast, the association between MDD and PUD has not been studied in European ancestry. Therefore, the present study partially fills this gap. Additionally, MVMR analysis showed no direct causal association of MDD on PUD after adjustment for BMI, T2D or insomnia, implying a potential mediating role for BMI, T2D or insomnia in this association.
Previous research has proposed many pathways involving different organ systems via which depression may elevated the development of PUD. A seemingly plausible explanation involves the function of the gut-brain axis. The gut-brain axis functions through mechanisms such as intestinal permeability, intestinal endocrine signaling and immune activation, which are important for regulating the GI and intestinal immune systems (Carabotti, Scirocco, Maselli, & Severi, Reference Carabotti, Scirocco, Maselli and Severi2015). However, chronic depression can interfere with the normal functioning of the gut-brain axis (Zhu et al., Reference Zhu, Han, Du, Liu, Jin and Yi2017), which may increase the susceptibility of the gastrointestinal tract to ulcer-causing agents such as H. pylori (Liu et al., Reference Liu, Xie, Lu, Cheng, Zeng, Zhou and Lu2018). Additionally, depression can affect sympathetic-adrenal stress response mechanisms, leading to dysregulation of the hypothalamic-pituitary-adrenal axis (Scott et al., Reference Scott, Alonso, de Jonge, Viana, Liu, O'Neill and Kessler2013). This neuroendocrinological abnormality may further affect gastroduodenal function by increasing cortisol levels and gastric acid secretion, potentially leading to an elevated risk of PUD (Lee et al., Reference Lee, Yu, Choi, Jeon, Kim, Kim and Chae2017). Moreover, the relationship between depression and PUD may involve the immune system (Lee et al., Reference Lee, Sung, Kim, Lee, Park and Shim2015). Depression-related stress may activate the release of certain pro-inflammatory cytokines, which may activate inflammatory cells in patients with H. pylori-induced ulcers (Sugimoto, Yamaoka, & Furuta, Reference Sugimoto, Yamaoka and Furuta2010).
Causal association between major depressive disorder and non-alcoholic fatty liver disease
We found evidence that genetically predicted MDD increased the risk of NAFLD, while genetically predicted NAFLD had no causal association with MDD. MVMR analysis showed no direct causal effect of MDD on NAFLD after adjustment for BMI, T2D or insomnia, implying that a large proportion of this association was mediated by BMI, T2D or insomnia. Previous studies have reported inconsistent results (Kim et al., Reference Kim, Yoo, Li, Tighe, Cholankeril, Harrison and Ahmed2019; Lee et al., Reference Lee, Otgonsuren, Younoszai, Mir and Younossi2013; Tomeno et al., Reference Tomeno, Kawashima, Yoneda, Saito, Ogawa, Honda and Nakajima2015; Youssef et al., Reference Youssef, Abdelmalek, Binks, Guy, Omenetti, Smith and Suzuki2013). Additionally, Labenz et al., found that NAFLD constituted an independent risk factor for emerging depression after controlling for confounding comorbidities, whereas the current MR analysis did not support this finding (Labenz et al., Reference Labenz, Huber, Michel, Nagel, Galle, Kostev and Schattenberg2020).
Several mechanisms support the association of genetically predicted MDD increased risk of NAFLD. First, systemic inflammation plays an important role in the pathogenesis of MDD and NAFLD. Increased overall body stress due to underlying obesity, diabetes, or metabolic syndrome leads to increased production of pro-inflammatory cytokines, cortisol, and epinephrine, which is further amplified by depression, increasing the propensity for NAFLD development (Chan, Cathomas, & Russo, Reference Chan, Cathomas and Russo2019; Huang, Liu, & Yu, Reference Huang, Liu and Yu2017). Pathophysiological factors associated with depression, such as increased monoamine oxidase-A enzyme activity, may enhance cellular oxidative stress and thus adversely affect NAFLD (Bhanji, Narayanan, Allen, Malhi, & Watt, Reference Bhanji, Narayanan, Allen, Malhi and Watt2017; Youssef et al., Reference Youssef, Abdelmalek, Binks, Guy, Omenetti, Smith and Suzuki2013). Another possible explanation is the association between NAFLD and obesity or diabetes, both of which are strongly associated with depression (Bica, Castelló, Toussaint, & Montesó-Curto, Reference Bica, Castelló, Toussaint and Montesó-Curto2017). After further adjustment for diabetes and obesity, the association was attenuated to null, suggesting that a large proportion of the association between depression and NAFLD is mediated by diabetes and obesity. New evidence for the involvement of insulin signaling in brain mechanisms associated with depression suggests that insulin resistance may be one of the major causal factors in NAFLD and that it may develop in the brains of depressed patients (Lyra et al., Reference Lyra, Lam, Soares, Munoz, Milev and De Felice2019).
Summary of the above findings
The co-morbid mechanisms and development of MDD and GID have a mutually subtle character in that depression is characterized by somatic symptoms of gastrointestinal dysfunction, which in turn exacerbates depression with somatic visceral perception and other upstream symptoms of the central nervous system. The co-morbid neurobiological pathways may involve four major dimensions, including negative feedback impairment of the hypothalamic-pituitary-adrenal axis (HPA) (Dinan et al., Reference Dinan, Quigley, Ahmed, Scully, O'Brien, O'Mahony and Keeling2006), imbalance of gut microecology (Tillisch, Reference Tillisch2014) and dysregulation of inflammatory cytokine ratios (Dowlati et al., Reference Dowlati, Herrmann, Swardfager, Liu, Sham, Reim and Lanctôt2010). These potential neurobiological pathways will have important implications for the diagnosis and treatment of both disorders, improving the accuracy and sensitivity of therapeutic strategies adopted by clinicians and providing an entry point for research into the pathogenesis and pharmacological mechanisms of action of depression in combination with GID.
Strengths and limitations
The most significant highlight of this study is the pooling of results from multiple sources using fixed-effect meta-analysis to improve the precision of the effect size estimates. Thorough sensitivity analyses, such as MR of negative control outcomes, MR of GID risk factors, Steiger filtering and CAUSE analyses, were conducted to assess the validity of the MR assumptions. Moreover, we performed MVMR, reversed MR and LDSC as secondary analyses to validate the robustness of MR results. Additionally, our findings contribute to the identification of modifiable targets for future interventions aimed at MDD for the prevention of GID. The current study is well-powered (online Supplementary Table S4) and the relatively high F statistic (⩾167) of the genetic instruments involved in the main primary MR analyses of depression implied a lower chance of weak instrument bias.
This study also has limitations. First, there was partial sample overlap between MDD and GID. However, the relatively high F statistic (⩾167) of the genetic instruments involved in the main primary MR analyses of depression and MR-RAPS analysis results implied a lower chance of weak instrument bias. The relative bias from sample overlap is also very small (<0.002). Second, some of our sensitivity analyses, such as the MR-Egger intercept test used to detect uncorrelated horizontal pleiotropy, had low power, resulting in imprecise estimates. The weighted mode method may also be misleading in this context, as its use is limited in the presence of very few SNPs. Although these limitations potentially undermine the validity of our results, it is reassuring that point estimates for the genetically predicted effect of MDD on GID were consistent across MR methods. Third, partial cases of GID are derived from self-reporting, which may be affected by recall bias and response bias. Another limitation is that we did not have access to individual-level data. Therefore, we were unable to stratify the analyses by potential effect modifiers, such as sex, smoking, and sleep quality. Finally, to reduce bias due to population stratification, this study is limited to individual of European ancestry and cannot be generalized to other ancestral backgrounds. Furthermore, our MR of negative control outcomes suggests that our MR results are unlikely to be biased by residual population stratification. Despite this, confounding due to population stratification, dynastic effects and assortative mating cannot be ruled out completely.
Clinical implications
We highlight certain aspects here that may advance the field; however, we advise that due to numerous limitations, the current findings should not be overinterpreted.
From a public health perspective, our work provides potentially relevant findings. Observational and MR studies suggest that MDD may be influenced by some GID risk factors, such as obesity and smoking (Tyrrell et al., Reference Tyrrell, Mulugeta, Wood, Zhou, Beaumont, Tuke and Hyppönen2019; Yao et al., Reference Yao, Xu, Cai, Liu, Ma, Li and Li2021). If MDD is a causal mediator between these risk factors and GID, then MDD may be a manageable mediator when targeting the underlying risk factors is not feasible or too difficult to accomplish. However, we believe that it may be premature to make claims about the clinical utility of our findings. From a treatment perspective, the associations between MDD and GID comorbidities appear to be bidirectional. When treating patients with MDD, clinicians should focus on their GID symptoms; for patients with gastrointestinal symptoms, physicians should also focus on their emotional problems simultaneously, which may help in the choice of treatment measures or in reducing comorbidities. In addition, exploring drugs that can both treat MDD and improve GID will better advance clinical care; however, this study could not provide confirmatory evidence. Notably, a small clinical trial reported that Probiotics treatment of IBS resulted in improvement of MDD symptoms (Pinto-Sanchez et al., Reference Pinto-Sanchez, Hall, Ghajar, Nardelli, Bolino, Lau and Bercik2017).
Future directions
Although promising in terms of consistency and biological plausibility, further research is required to confirm our findings. For example, MVMR could be used to disentangle the causal effects of MDD on GID from other shared heritable factors (e.g. Gut microbiota, H. pylori infection, education level, neuroticism, dietary intake, physical activity, c-reactive protein, insulin resistant, etc.). Moreover, we did not have sex-specific instruments for MDD. Given the higher prevalence of MDD in women, the genetic structure of MDD may differ between the sexes. We also didn't have access to summary statistics of GID subtypes. The clinical features and genetic components differ between GID subtypes. Further studies should focus on sex-specific and GID subtypes based on individual data. Despite these suggestions, we acknowledge that it may be challenging to get access to suitable datasets for replication purposes in the short term.
Conclusions
In conclusion, genetically predicted MDD may increase the risk of GERD, IBS, PUD and NAFLD. Genetically predicted GERD or IBS may increase the risk of MDD. The findings may help elucidate the mechanisms underlying the co-morbidity of depression and GID and may have clinical implications. However, further clinical studies are required to replicate the findings and investigate the effects of comorbidity therapies. Further investigations are also warranted to elucidate the mechanism involved.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723000867
Acknowledgements
Not applicable.
Authors’ contributions
ZH, JJ and TH designed the study. DC performed the statistical analysis. DC and YZ wrote the manuscript. All authors helped interpret the data, reviewed, and edited the final paper, and approved the submission. The corresponding author had full access to all the data.
Financial support
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Ethical standards
Not applicable.
Data availability
The following publicly available datasets of summary statistics were used:

Code availability
Custom R scripts used to generate results in this study can be found in: https://github.com/Rick-Chen-PKU/Depression_GI.
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.









