Ketamine and its derivative S-ketamine improve symptoms of psychiatric disorders including substance use disorders (SUDs), major depressive disorder (MDD) and post-traumatic stress disorder (PTSD) over the short term.Reference Feder, Costi, Rutter, Collins, Govindarajulu and Jha1–Reference Jones, Mateus, Malcolm, Brady and Back3 A number of barriers limit the translation of these outcome studies to routine care, including: the predominant use of parenteral dosing, significant dissociative side-effects, and the need for repeated dosing with high rates of relapse following treatment cessation.Reference Corriger and Pickering4–Reference Short, Fong, Galvez, Shelker and Loo7 The majority of patients are treated for MDD relapse in the days following a single dose of ketamine.Reference Salloum, Fava, Hock, Freeman, Flynn and Hoeppner8 High relapse rates of depression have also been observed following a course of repeated ketamine doses. For example, Murrough et alReference Murrough, Perez, Pillemer, Stern, Parides and aan het Rot9 reported a median time to relapse of 18 days following a course of up to six ketamine infusions for treatment-resistant depression (TRD). Similar findings exist for the treatment of PTSD; Albott et alReference Albott, Lim, Forbes, Erbes, Tye and Grabowski10 reported a median time to relapse of 41 days after a course of six ketamine infusions.
The addition of psychotherapy to monoaminergic antidepressants improves outcomes and reduces relapse in MDDReference Karyotaki, Smit, Holdt Henningsen, Huibers, Robays and de Beurs11 and SUDs,Reference Ray, Meredith, Kiluk, Walthers, Carroll and Magill12 although the benefits of combination therapy are less clear for PTSD.Reference Hetrick, Purcell, Garner and Parslow13 It is therefore plausible that the addition of psychotherapy to ketamine could improve outcomes and delay relapse. This is of particular importance given the short-lived positive effects of ketamine treatment. The primary aim of this systematic review was therefore to identify and review studies that combined ketamine and psychotherapy for the treatment of psychiatric disorders. Our goal was to clarify the current evidence for the combined use of ketamine and psychotherapy for psychiatric disorders, highlight areas of importance in this literature and make recommendations for future research.
Method
This systematic review was guided by the PRISMA checklistReference Page, McKenzie, Bossuyt, Boutron, Hoffmann and Mulrow14 (see Supplementary Appendix A available at https://doi.org/10.1192/bjo.2023.53 for details of the checklist). The study protocol was prospectively registered with PROSPERO (registration number CRD42022318120) and can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID = 318120. No ethical approval or informed consent was sought because this was a systematic review of already published studies. The systematic review was conducted according to the prospectively registered protocol with the exception of quality assessment. The protocol proposed using the Cochrane risk of bias (RoB) 2Reference Sterne, Savović, Page, Elbers, Blencowe and Boutron15 and ROBINS-IReference Sterne, Hernán, Reeves, Savović, Berkman and Viswanathan16 tools. After trialling these tools in a sample of the included papers, we chose to complete RoB assessment using the Joanna Briggs critical appraisal tools.Reference Aromataris and Munn17 This decision was made because the RoB 2 and ROBINS-I tools were not well-suited to the range of study types identified by our review (including randomised control trials (RCTs), quasi-experimental studies, single-arm studies and case series). We considered other commonly used RoB tools but chose to use the Joanna Briggs suite of tools for consistency rather than apply multiple tools from different origins. Although this decision was made after included studies had been identified by the screening process, it occurred prior to detailed review of the studies. Supplementary Appendix B provides details of the RoB assessments. The study protocol included the option of meta-analysis. However, the highly heterogeneous nature of identified studies meant we chose not to do so.
Inclusion criteria
Studies were eligible for inclusion if they reported the use of ketamine or S-ketamine in conjunction with psychotherapy for the treatment of a psychiatric disorder. Psychotherapy was broadly defined and included any psychological treatment that uses regular communication between a patient and a psychologist, counsellor or other trained mental health professional for the purpose of improving well-being related to a psychiatric disorder. Psychiatric disorder was defined as any psychiatric disorder diagnosed using DSM or ICD diagnostic criteria. Clinical diagnoses made by expert clinicians outside of these classifications were also included. Eligible study designs included RCTs, cohort studies, and case series, open-label and feasibility studies. Single case reports and qualitative studies were not included in the review. Studies were required to compare measures of psychiatric disorder using validated rating scales pre- and post-ketamine and psychotherapy treatment.
Search and screening strategy
The search strategy combined a search for ketamine and its derivates and a search for psychotherapy using the Boolean classifier AND. The search was undertaken with assistance from a research librarian and was limited to English language and human trials. Supplementary Appendix C reports the search strategies used for each database. Potential studies were searched for in MEDLINE, Embase, PsycINFO, SCOPUS, the Cochrane library and Google Scholar. The study period was from database conception to May 2022. In addition, the reference lists of eligible papers were searched, and a forward citation search was undertaken.
Authors B.B. and B.M.K. independently screened titles and abstracts in accordance with the inclusion criteria using Covidence, the Cochrane Foundation's online platform for systematic review management.18 B.B. and B.M.K. also independently reviewed full-text articles for potential inclusion, and disagreements were resolved by discussion between the two authors at each step. B.M.K. conducted the data extraction using a data extraction sheet that was reviewed and adjusted after a trial period. B.B. independently completed data extraction, and any disagreements were resolved with B.M.K. through discussion.
Analysis
Substantial between-study heterogeneity prevented meta-analysis or the use of standardised metrics to report outcomes for the included studies. We therefore summarise findings according to categories that highlight key elements of the included studies to better allow consideration. The categories were as follows.
(a) Psychotherapy: the range of psychotherapies used in the included studies are described including any specific elements unique to ketamine treatment.
(b) Diagnosis: outcomes of included studies are reported according to diagnostic groupings. Results from RCTs then studies with larger sample sizes are reported first. Where possible, key statistics that highlight the magnitude of change pre–post treatment or between ketamine and ketamine psychotherapy arms are provided.
(c) Ketamine protocol: ketamine protocols for the included studies are described.
(d) Scheduling of ketamine and psychotherapy treatments: sequencing strategies for the use of ketamine and psychotherapy are reported.
(e) Study design and timing of outcome measurement: choice of comparators and timing of outcome measurement are reported to assist focus on whether psychotherapy improves outcomes in addition to ketamine treatment and inform the duration of any positive effects.
(f) Risk of bias: the RoB of included studies was assessed using the Joanna Briggs critical appraisal tools.Reference Aromataris and Munn17 This included using an adapted form for the single-arm studies in the review based on collating relevant items from other study types. The adaptation was undertaken to ensure the single-arm studies could be similarly assessed for bias in study inclusion, patient characteristics, intervention, outcome measurement, analysis and potential confounders. Three RoB categories (low, moderate and high) were created based on the distribution of positively endorsed items in the appropriate critical appraisal checklist for the study type. These categories were created prior to scoring of the RoB tools, to assist the reader. Studies with ≤30% of endorsed items were rated high RoB, studies with >30% to ≤70% endorsed items were rated moderate RoB and studies with >70% were rated low RoB. This approach is similar to that of other authors who regarded a score of 70% and ≥7 positively endorsed items on Joanna Briggs Institute scales as an indicator of quality.Reference Kisely, Yu, Maehashi and Siskind19,Reference Koh, Hegney and Drury20 Authors B.B. and B.M.K. independently completed the RoB assessments for each study, and a consensus rating for each study was reached through discussion.
Results
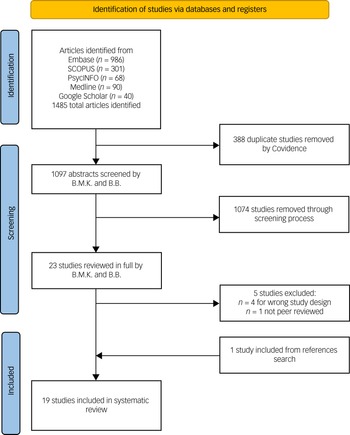
Figure 1 is the PRISMA flow diagram for the screening and inclusion process. Database searches were undertaken between 28 April 2022 and 2 May 2022. A total of 1485 abstracts were screened after removing duplicates, resulting in 23 papers for full-text review. After full-text screening, 19 studies were included in the systematic review. These studies comprised ten single-arm studies, eight RCTs and one non-RCT study. A total of 1008 patients were represented in the systematic review. The RCTs evaluated 337 patients receiving ketamine with psychotherapy compared with 252 control participants. A further 419 patients received ketamine and psychotherapy in the non-randomised studies. The sample sizes for included studies ranged from five to 235 participants. Tables 1 and 2 provide details of the included studies including diagnostic grouping, ketamine and psychotherapy treatments, key results and the outcome of the RoB assessments.

Fig. 1 PRISMA flow diagram for the search process.
Table 1 Controlled trials

AUD, alcohol use disorder; CD, cocaine dependence; HD, heroin dependence; PTSD, post-traumatic stress disorder; TRD, treatment-resistant depression; AE, alcohol education; CBT, cognitive–behavioural therapy; i.m., intramuscular; KPT, ketamine psychedelic therapy; MET, motivational enhancement therapy; MBRP, mindfulness-based relapse prevention; TAU, treatment as usual; TIMBER, trauma interventions using mindfulness-based extinction and reconsolidation; W, week; CAPS, Clinician Administered Scale for DSM; EOT, end of treatment; MADRS, Montgomery–Åsberg Depression Rating Scale; PCL, PTSD Checklist; QIDS, Quick Inventory of Depressive Symptomatology; TLFB, timeline follow back.
Table 2 Single-arm trials

CUD, cannabis use disorder; GAD, generalised anxiety disorder; OCD, obsessive–compulsive disorder; PTSD, post-traumatic stress disorder; TRD, treatment-resistant depression; BCT, body-centred therapy; CBT, cognitive–behavioural therapy; CoP, community of practice; i.m., intramuscular; KAP, ketamine-assisted psychotherapy; KEP, ketamine-enhanced psychotherapy; MBRP, mindfulness-based relapse prevention; MET, motivational enhancement therapy; PE, prolonged exposure therapy; SDS, Sheehan Disability Scale; TRIP, therapeutic reset of internal processes; W, week; BDI, Beck Depression Inventory; B-IPF, Brief Inventory of Psychosocial Functioning; PC-PTSD-5, Primary Care PTSD Screen for DSM-5; CAPS-5, Clinician-Administered PTSD Scale for DSM-5; EOT, end of treatment; GAD-2, Generalised Anxiety Disorder 2; HAM-A, Hamilton Rating Scale for Anxiety; MADRS, Montgomery–Åsberg Depression Rating Scale; PCL-5, PTSD Checklist for DSM-5; PHQ-2, Patient Health Questionnaire, TLFB, timeline follow back; YBOCS, Yale–Brown Obsessive Compulsive Scale; ED, eating disorder.
Psychotherapy type
Mindfulness-based relapse prevention
Three of the 19 studies evaluated mindfulness-based relapse prevention (MBRP) and ketamine.Reference Azhari, Hu, O'Malley, Blocker, Levin and Dakwar21–Reference Grabski, McAndrew, Lawn, Marsh, Raymen and Stevens23 MBRP is a manualised treatment that combines components of cognitive–behavioural therapy (CBT), stress reduction and lessons to develop mindfulness skills.Reference Bowen, Chawla, Collins, Witkiewitz, Hsu and Grow24 MBRP aims to teach patients to recognise and address early warning signs of substance relapse.Reference Brewer, Sinha, Chen, Michalsen, Babuscio and Nich25
Cognitive–behavioural therapy
Three studies evaluated CBT and ketamine.Reference Rodriguez, Wheaton, Zwerling, Steinman, Sonnenfeld and Galfalvy26–Reference Wilkinson, Wright, Fasula, Fenton, Griepp and Ostroff28 CBT combines psychoeducation, cognitive restructuring and behavioural activation to address symptoms of psychiatric disorders.Reference Fenn and Byrne29
Ketamine psychedelic therapy
Three studies evaluated ketamine psychedelic therapy (KPT).Reference Krupitsky, Burakov, Dunaevsky, Romanova, Slavina and Grinenko30–Reference Krupitsky and Grinenko32 KPT is a three-stage ketamine and psychotherapy protocol developed to treat SUDs.Reference Krupitsky and Grinenko33 Stage 1 involves preliminary individual psychotherapy to prepare the patient for the ketamine treatment and explores factors relating to their substance misuse history. Stage 2 consists of ketamine dosing and psychotherapy while the patient is under the influence of ketamine. The psychotherapy in this stage revisits themes explored in stage 1 to integrate factors identified earlier and harness the potential for change. The final stage starts the day after the ketamine session and involves discussion, interpretation, and integration of the patient's experiences in stage 2.
Motivational enhancement therapy
Two studies evaluated motivational enhancement therapy (MET) and ketamine.Reference Azhari, Hu, O'Malley, Blocker, Levin and Dakwar21,Reference Dakwar, Levin, Hart, Basaraba, Choi and Pavlicova34 MET, also known as motivational interviewing, is a counselling technique used to enhance intrinsic motivation by addressing ambivalence towards behaviour change.Reference Smedslund, Berg, Hammerstrøm, Steiro, Leiknes and Dahl35
Trauma interventions using mindfulness-based extinction and reconsolidation
Two RCTs evaluated trauma interventions using mindfulness-based extinction and reconsolidation (TIMBER) and ketamine.Reference Pradhan, Wainer, Moaddel, Torjman, Goldberg and Sabia36,Reference Pradhan, Mitrev, Moaddell and Wainer37 TIMBER therapy incorporates components of CBT, yoga and mindfulness-based graded exposure to treat PTSD.
The remaining psychotherapies were only used in single studies. These were a mix of manualised and non-manualised therapies (see Tables 1 and 2 for details of the therapy type).
Diagnosis
Substance use disorders
Seven of the 19 studies evaluated treatment of SUDs. Three studies were for the treatment of alcohol use disorder (AUD), alcohol dependence or alcoholism.Reference Grabski, McAndrew, Lawn, Marsh, Raymen and Stevens23,Reference Krupitsky and Grinenko32,Reference Dakwar, Levin, Hart, Basaraba, Choi and Pavlicova34
Dakwar et alReference Dakwar, Levin, Hart, Basaraba, Choi and Pavlicova34 completed an RCT comparing MET and ketamine with MET and midazolam for patients with AUD (n = 40). Patients received six MET sessions within 5 weeks. At 12 days post-ketamine infusion, there was a significantly greater proportion of alcohol-abstinent patients in the ketamine group (52.9%) compared with the midazolam group (40.9%).
Grabski et alReference Grabski, McAndrew, Lawn, Marsh, Raymen and Stevens23 completed a four-arm RCT (n = 96) for AUD comparing combinations of ketamine or saline treatment with MBRP or alcohol education (an active therapy control). Patients allocated to receive MBRP received six sessions over 3–6 weeks. At 6 months after the end of treatment, higher rates of abstinence were reported for ketamine groups compared with saline groups when the results were pooled across therapy conditions (mean difference = 10.1, 95% CI 1.1–19.0). However, no significant difference in alcohol-abstinent days was present between the ketamine plus MBRP group and the ketamine plus alcohol education group.
Krupitsky and GrinenkoReference Krupitsky and Grinenko32 evaluated KPT for the treatment of alcoholism (n = 211). Patients received the KPT protocol at the end of a 3-month in-patient treatment plan that involved treatment of alcohol withdrawal syndrome and comorbid psychiatric and/or somatic disorders with individual and group psychotherapy. In this study, stage 3 of KPT was undertaken in groups with patients who had received ketamine the previous day. Outcomes were compared between those who had received KPT and a comparison group of in-patients that had not received KPT. At 1 year post-treatment, abstinence was reported in 65.8% of patients in the KPT group compared with 24.0% in the comparison group.
Two RCTs evaluated heroin dependence.Reference Krupitsky, Burakov, Dunaevsky, Romanova, Slavina and Grinenko30,Reference Krupitsky, Burakov, Romanova, Dunaevsky, Strassman and Grinenko31 In the study of Krupitsky et al,Reference Krupitsky, Burakov, Romanova, Dunaevsky, Strassman and Grinenko31 all patients (n = 70) received KPT, although stage 3 was provided individually. Patients were randomly assigned to receive high-dose ketamine or low-dose ketamine during stage 2 of KPT. The rate of heroin abstinence at 2 years post-intervention was approximately 17.5% in the high-dose ketamine group and approximately 2.5% in the low-dose ketamine group.
In a separate RCT, Krupitsky et alReference Krupitsky, Burakov, Dunaevsky, Romanova, Slavina and Grinenko30 treated heroin-dependent patients (n = 59) with KPT, with the third stage provided individually. Patients were then randomly assigned to receive two further addiction counselling sessions at monthly intervals or two additional stage 2 ketamine doses and addiction counselling at monthly intervals. Patients in the multiple KPT group also received an additional two psychotherapy sessions after each ketamine dosing to integrate their experiences. At 1-year follow-up, 50.0% heroin abstinence rates were reported in the multiple session group compared with 22.2% in the single session group.
One RCT evaluated cocaine dependence.Reference Dakwar, Nunes, Hart, Foltin, Mathew and Carpenter22 In this study, patients (n = 55) received 12 MBRP sessions over 5 weeks. At the end of treatment, abstinence from cocaine was observed in 48.2% of patients receiving ketamine and MBRP compared with 10.7% of patients receiving midazolam and MBRP. After controlling for route of use, the odds of abstinence in the ketamine group were 5.7 times those of the midazolam group (95% CI 1.3–25.1). One single-arm study evaluated cannabis use disorder.Reference Azhari, Hu, O'Malley, Blocker, Levin and Dakwar21 Patients (n = 8) received ketamine treatment combined with two sessions of MET, followed by six sessions of MBRP. At 12 days post-infusion, there was a significantly greater proportion of cannabis-abstinent patients in the ketamine group compared with the midazolam group.
Post-traumatic stress disorder
Five studies evaluated patients with PTSD, one of which also included patients with generalised anxiety disorder and MDD.Reference Davis, Mangini and Xin38 Two very small RCTs (n = 10–20)Reference Pradhan, Wainer, Moaddel, Torjman, Goldberg and Sabia36,Reference Pradhan, Mitrev, Moaddell and Wainer37 evaluated TIMBER therapy and ketamine for the treatment of PTSD. Neither study reported a significant difference in PTSD symptoms between groups at 24 h after treatment.Reference Pradhan, Wainer, Moaddel, Torjman, Goldberg and Sabia36,Reference Pradhan, Mitrev, Moaddell and Wainer37 However, Pradhan et alReference Pradhan, Mitrev, Moaddell and Wainer37 reported a longer treatment effect in the ketamine group (M = 34.44, s.d. = 19.12) compared with the comparator group (M = 16.50, s.d. = 11.39) following the intervention.
Two small (n = 10–11) open-label studies evaluated ketamine and psychotherapy for PTSD.Reference Keizer, Roache, Jones, Kalpinski, Porcerelli and Krystal39,Reference Shiroma, Thuras, Wels, Erbes, Kehle-Forbes and Polusny40 Both studies reported large effect size improvements in PTSD scores (d = 1.44, and d = 1.21) from pre-post treatment.37,38 A retrospective analysis of clinical records of patients receiving body-centred therapy for PTSD reported moderate effect-size improvements in PTSD scores (d = −0.48) that failed to reach statistical significance.36
Treatment-resistant depression
Three studies evaluated TRD.Reference Wilkinson, Rhee, Joormann, Webler, Ortiz Lopez and Kitay27,Reference Wilkinson, Wright, Fasula, Fenton, Griepp and Ostroff28,Reference Zydb and Hart41 . Wilkinson et alReference Wilkinson, Wright, Fasula, Fenton, Griepp and Ostroff28 conducted an open-label study of TRD patients (n = 16) involving 12 sessions of CBT over a 10-week period following ketamine treatment. Fifty per cent of the study population responded to the intervention, and 43.8% achieved remission. Wilkinson et alReference Wilkinson, Rhee, Joormann, Webler, Ortiz Lopez and Kitay27 then completed a follow-up RCT for patients with TRD (n = 28). Patients who responded to an initial ketamine dose were allocated to receive 15 sessions of CBT over 17 weeks or treatment as usual (TAU). TAU consisted of regular meetings with a study physician for concomitant medication management. Wilkinson et alReference Wilkinson, Rhee, Joormann, Webler, Ortiz Lopez and Kitay27 did not report significant between-group differences favouring the addition of psychotherapy to ketamine when measured by a clinician-rated depression scale (Montgomery–Åsberg Depression Rating Scale, MADRS), although the effect size was moderate (d = 0.65, 95% CI −0.55 to 1.82). However, a significant difference in favour of ketamine and psychotherapy treatment was observed when the Quick Inventory of Depression Symptomatology self-report depression scale was used (d = 0.71, 95% CI −0.30 to 1.70).
The remaining TRD study was an open-label study (n = 10) that reported significant improvements in self-reported depression symptoms at the end of ketamine and psychotherapy treatment.Reference Zydb and Hart41
Other diagnostic groups
The remaining four studies were single-arm studies that evaluated patients with eating disorders and comorbid generalised anxiety and depression symptoms,Reference Robison, Lafrance, Brendle, Smith, Moore and Ahuja42 obsessive–compulsive disorder (OCD)Reference Rodriguez, Wheaton, Zwerling, Steinman, Sonnenfeld and Galfalvy26 and mixed diagnostic samples.Reference Dames, Kryskow and Watler43,Reference Dore, Turnipseed, Dwyer, Turnipseed, Andries and Ascani44
Dore et alReference Dore, Turnipseed, Dwyer, Turnipseed, Andries and Ascani44 retrospectively analysed data from patients (n = 235) who had received ketamine and psychotherapy across three private psychiatric practices. Patients in this study had varying diagnoses including MDD, PTSD, complex PTSD, attention-deficit hyperactivity disorder, anxiety disorders and SUDs. The number of in-office ketamine and psychotherapy sessions ranged from 1–25. Some patients were also prescribed sublingual ketamine to take at home between in-office ketamine and psychotherapy sessions. Ketamine dose, route and frequency and psychotherapy frequency were individualised depending on the patients’ diagnosis and circumstances. Symptom rating scales were administered at intake and again at discharge from treatment. Significant decreases in the Beck Depression Inventory (M = 26.55 at baseline, M = 15.31 post treatment, P < 0.0001) and the Hamilton Rating Scale for Anxiety (M = 20.35 at baseline, M = 15.03 post treatment, P < 0.0001) were both reported at the end of treatment.
Dames et alReference Dames, Kryskow and Watler43 administered ketamine in a group setting at weekly intervals for 3 weeks to patients (n = 94) with treatment-resistant psychiatric disorders. Processing took place within the group after the effects of the drug had subsided (approximately 90 min after dosing). Processing included conversation about the patients’ respective ketamine experiences. This was in addition to psychotherapy leading up to and following the ketamine sessions. By treatment end, improvements were reported in 91% of patients with generalised anxiety disorder symptoms, and 76% of patients with depression symptoms. Eighty-six per cent of patients with a baseline PTSD diagnosis were reported to no longer meet diagnostic criteria.
One small (n = 10) open-label study examined the treatment of OCD.Reference Rodriguez, Wheaton, Zwerling, Steinman, Sonnenfeld and Galfalvy26 This study provided a single intravenous (i.v.) ketamine infusion followed by exposure-based CBT. The mean estimated Yale–Brown Obsessive Compulsive Scale score was significantly lower at week 2 (difference = −10.75 points, s.e. = 1.44, P < 0.0001) and at week 4 (difference = −6.88, s.e. = 2.61, P = 0.01). A wide variation in response among individuals was reported in this study. An eating disorders study (n = 5)Reference Robison, Lafrance, Brendle, Smith, Moore and Ahuja42 evaluated a course of ketamine alongside routine group and individual therapy. This study reported significant improvements in depression scores for four participants and significant improvements in anxiety scores for two participants following treatment with ketamine and psychotherapy.
Ketamine protocol
Intravenous
Eleven of the 19 studies examined psychotherapy and ketamine administered via i.v. infusion.Reference Azhari, Hu, O'Malley, Blocker, Levin and Dakwar21–Reference Grabski, McAndrew, Lawn, Marsh, Raymen and Stevens23,Reference Rodriguez, Wheaton, Zwerling, Steinman, Sonnenfeld and Galfalvy26–Reference Wilkinson, Wright, Fasula, Fenton, Griepp and Ostroff28,Reference Dakwar, Levin, Hart, Basaraba, Choi and Pavlicova34,Reference Pradhan, Wainer, Moaddel, Torjman, Goldberg and Sabia36,Reference Pradhan, Mitrev, Moaddell and Wainer37,Reference Keizer, Roache, Jones, Kalpinski, Porcerelli and Krystal39,Reference Shiroma, Thuras, Wels, Erbes, Kehle-Forbes and Polusny40 Doses ranged from 0.50 mg/kg to 1.41 mg/kg in the case of non-response. Treatment regimes include single and repeated infusions in combination with therapy.
Intramuscular
Six studies evaluated psychotherapy plus intramuscular (i.m.) ketamine injections.Reference Krupitsky, Burakov, Dunaevsky, Romanova, Slavina and Grinenko30–Reference Krupitsky and Grinenko32,Reference Robison, Lafrance, Brendle, Smith, Moore and Ahuja42–Reference Dore, Turnipseed, Dwyer, Turnipseed, Andries and Ascani44 . Treatment doses started at 25 mg in the ED populationReference Robison, Lafrance, Brendle, Smith, Moore and Ahuja42 and were typically in the 1–2 mg/kg range. Dosing frequency ranged from single ketamine dose protocolsReference Krupitsky and Grinenko32 to the case series reported by Dore et alReference Dore, Turnipseed, Dwyer, Turnipseed, Andries and Ascani44 with up to 25 ketamine treatment sessions.
Oral/sublingual
Two single-arm studies evaluated psychotherapy and sublingual ketamineReference Davis, Mangini and Xin38 or oral ketamine.Reference Zydb and Hart41 Zydb and HartReference Zydb and Hart41 examined oral doses of 75 mg administered once a week for 6 weeks. Davis et alReference Davis, Mangini and Xin38 examined sublingual doses between 100 and 200 mg administered three times over 6 weeks.
Scheduling of psychotherapy and ketamine within the study design
Ketamine and psychotherapy were delivered in various sequences in the included studies. Nine studies involved the delivery of psychotherapy at the time of ketamine dosing (when participants were acutely affected by ketamine.Reference Krupitsky, Burakov, Dunaevsky, Romanova, Slavina and Grinenko30–Reference Krupitsky and Grinenko32,Reference Davis, Mangini and Xin38,Reference Keizer, Roache, Jones, Kalpinski, Porcerelli and Krystal39,Reference Zydb and Hart41–Reference Dore, Turnipseed, Dwyer, Turnipseed, Andries and Ascani44
One study (Grabski et alReference Grabski, McAndrew, Lawn, Marsh, Raymen and Stevens23) delivered ketamine and psychotherapy concurrently, whereby therapy sessions were timed so that they preceded ketamine administration in addition to occurring approximately 24 h later.
Nine studies delivered an initial package of ketamine treatment (sometimes combined with psychotherapy) followed by further psychotherapy.Reference Azhari, Hu, O'Malley, Blocker, Levin and Dakwar21,Reference Dakwar, Nunes, Hart, Foltin, Mathew and Carpenter22,Reference Rodriguez, Wheaton, Zwerling, Steinman, Sonnenfeld and Galfalvy26–Reference Wilkinson, Wright, Fasula, Fenton, Griepp and Ostroff28,Reference Dakwar, Levin, Hart, Basaraba, Choi and Pavlicova34,Reference Pradhan, Wainer, Moaddel, Torjman, Goldberg and Sabia36,Reference Pradhan, Mitrev, Moaddell and Wainer37,Reference Shiroma, Thuras, Wels, Erbes, Kehle-Forbes and Polusny40
Study design and timing of outcome measurement
The RCT by Wilkinson et al compared ketamine treatment plus CBT with ketamine treatment with TAU.Reference Wilkinson, Rhee, Joormann, Webler, Ortiz Lopez and Kitay27 As a consequence, this study was ideally situated to report on the additional benefits of providing psychotherapy with ketamine treatment. The comparison groups for RCTs by Dakwar et al,Reference Dakwar, Nunes, Hart, Foltin, Mathew and Carpenter22,Reference Dakwar, Levin, Hart, Basaraba, Choi and Pavlicova34 Grabski et al,Reference Grabski, McAndrew, Lawn, Marsh, Raymen and Stevens23 Pradhan et alReference Pradhan, Wainer, Moaddel, Torjman, Goldberg and Sabia36 and Krupitsky et alReference Krupitsky, Burakov, Dunaevsky, Romanova, Slavina and Grinenko30,Reference Krupitsky, Burakov, Romanova, Dunaevsky, Strassman and Grinenko31 included active medication controls, saline controls, high/low doses of ketamine and single/repeated ketamine doses. As a consequence, these studies were better suited to distinguishing between medication effects and the benefits of additional psychotherapy.
The single-arm studies evaluated packages of ketamine and psychotherapy, but, owing to the absence of control arms, these studies evaluated the combined treatments as opposed to the individual elements.Reference Azhari, Hu, O'Malley, Blocker, Levin and Dakwar21,Reference Rodriguez, Wheaton, Zwerling, Steinman, Sonnenfeld and Galfalvy26,Reference Wilkinson, Wright, Fasula, Fenton, Griepp and Ostroff28,Reference Davis, Mangini and Xin38–Reference Dore, Turnipseed, Dwyer, Turnipseed, Andries and Ascani44
Most studies completed outcome measurements at the end of the treatment package or shortly afterwards. The studies by Grabski et alReference Grabski, McAndrew, Lawn, Marsh, Raymen and Stevens23 and Krupitsky et alReference Krupitsky, Burakov, Dunaevsky, Romanova, Slavina and Grinenko30–Reference Krupitsky and Grinenko32 reported outcomes at time points distant from the end of psychotherapy and ketamine treatment and were therefore able to report relapse in patients with SUDs. They followed patients for 18 months after ketamine treatment and were therefore able to report maintenance treatment effects.
Risk of bias
Supplementary Appendix B reports the details of the RoB assessments. Seven of the 19 studies were rated as low RoB; six of these were controlled trials and two were single-arm studies.Reference Dakwar, Nunes, Hart, Foltin, Mathew and Carpenter22,Reference Grabski, McAndrew, Lawn, Marsh, Raymen and Stevens23,Reference Wilkinson, Wright, Fasula, Fenton, Griepp and Ostroff28,Reference Krupitsky, Burakov, Dunaevsky, Romanova, Slavina and Grinenko30,Reference Krupitsky, Burakov, Romanova, Dunaevsky, Strassman and Grinenko31,Reference Dakwar, Levin, Hart, Basaraba, Choi and Pavlicova34,Reference Pradhan, Mitrev, Moaddell and Wainer37,Reference Shiroma, Thuras, Wels, Erbes, Kehle-Forbes and Polusny40 Low RoB was characterised by adequate randomisation and blinding procedures (where applicable), sufficient detail in reporting study design, standardised disorder measurement and appropriate statistical analyses.
Nine studies were rated as moderate RoB, of which three were controlled trials and five were single-arm studies.Reference Azhari, Hu, O'Malley, Blocker, Levin and Dakwar21,Reference Rodriguez, Wheaton, Zwerling, Steinman, Sonnenfeld and Galfalvy26,Reference Wilkinson, Rhee, Joormann, Webler, Ortiz Lopez and Kitay27,Reference Krupitsky and Grinenko32,Reference Pradhan, Wainer, Moaddel, Torjman, Goldberg and Sabia36,Reference Davis, Mangini and Xin38,Reference Zydb and Hart41,Reference Robison, Lafrance, Brendle, Smith, Moore and Ahuja42 Moderate RoB indicated areas of potential weakness in study design, method, data collection or statistical analysis.
Three remaining studies were rated as high RoB, and all were single-arm studies.Reference Keizer, Roache, Jones, Kalpinski, Porcerelli and Krystal39,Reference Dames, Kryskow and Watler43,Reference Dore, Turnipseed, Dwyer, Turnipseed, Andries and Ascani44 High RoB was attributed to these studies owing to a lack of detail on inclusion and exclusion criteria, baseline characteristics and the intervention protocol. In the studies of Dames et alReference Dames, Kryskow and Watler43 and Dore et al,Reference Dore, Turnipseed, Dwyer, Turnipseed, Andries and Ascani44 treatment protocols differed between patients.
Discussion
We identified 19 studies treating 1008 patients with ketamine and psychotherapy across nine psychiatric disorders. The majority of the studies reported positive outcomes associated with ketamine and psychotherapy treatment. However, heterogeneity in diagnoses, psychotherapies and ketamine protocols meant that robust conclusions for clinical recommendations could not be made.
The largest studies evaluated the treatment of SUDs. For AUD, ketamine treatment performed better than a saline control (Grabski et al 2022Reference Grabski, McAndrew, Lawn, Marsh, Raymen and Stevens23), and KPT performed better than usual treatment (Krupitsky and GrinenkoReference Krupitsky and Grinenko32). For heroin dependence, patients allocated to higher-dose ketamine and repeated ketamine/psychotherapy groups had better outcomes than those in comparator groups.Reference Krupitsky, Burakov, Dunaevsky, Romanova, Slavina and Grinenko30,Reference Krupitsky, Burakov, Romanova, Dunaevsky, Strassman and Grinenko31
The diagnoses identified by this review were AUD/alcohol dependence/alcoholism, heroin dependence, cocaine dependence, cannabis use disorder, PTSD, TRD, eating disorders and OCD. There may be shared features across these disorders, such as the trait of neuroticism,Reference Kotov, Gamez, Schmidt and Watson45 which are targeted by ketamine and psychotherapy.Reference McNaughton and Glue46 It is also possible that non-specific treatment expectation effects mediate the benefits reported and that the specific benefits of combining ketamine and psychotherapy are at risk of being overestimated. Supporting this possibility is the finding that positive early-stage intervention studies are more likely to be reported and that publication bias exists in the early stages of researching new clinical interventions.Reference Hopewell, Loudon, Clarke, Oxman and Dickersin47 We note that ketamine is likely to be associated with significant expectation biases,Reference Muthukumaraswamy, Forsyth and Lumley48 and the addition of psychotherapy may amplify the already intense experience and possible placebo effect of ketamine.
The review reported a total of 12 psychotherapies. These included manualised and non-manualised psychotherapies, delivered individually or in group settings. This heterogeneity meant that it was unclear whether there is a preferred therapy protocol for combination with ketamine treatment. The manualised psychotherapies (MBRP, MET, CBT, TIMBER) are the most straightforward to replicate and better suited to research protocols. We therefore recommend their use, as well as the use of existing therapies with established indications for target disorders over unstructured psychotherapies and de novo therapies, in order to grow a more consistent research base in this area. The use of manualised therapies will also strengthen the translation of studies into routine clinical care by service providers.
A number of studies were not designed to delineate the individual effects of psychotherapy and ketamine. These included the single-arm studies evaluating the treatment combination and the RCTs that were controlled using placebos as opposed to a therapy control. Only the study by Wilkinson et al compared ketamine plus CBT with ketamine plus TAU to demonstrate the benefits of adding therapy to ketamine treatment. In order to further advance this area of research, we suggest that ketamine and psychotherapy studies consider the most appropriate comparator arms. A recent study published by Price et alReference Price, Spotts, Panny, Griffo, Degutis and Cruz49 is worth highlighting for design purposes. This study (n = 154) randomised patients with TRD to ketamine plus automated self-association training (ASAT), ketamine plus sham ASAT or saline plus ASAT. Although ASAT did not meet our therapy definition, the design of this study meant the separate effects of ketamine and ASAT could be distinguished. In addition, ASAT may point to a way forwards for the wider delivery of therapeutic interventions.
There was an extensive range of ketamine protocols applied by the studies. These included single-dose treatments, oral/i.m./i.v. administration and repeated dosing. The variation identified meant that it was difficult to conclude whether there is a preferred ketamine protocol for combination with psychotherapy. We therefore recommend that future researchers use standardised ketamine protocols that have been shown to be efficacious in the short-term treatment of psychiatric disorders. This will enable the additional benefits of psychotherapy to be delineated and will be easier to replicate. Although the evidence base for oral ketamine is at an earlier stage than that for parenteral ketamine,Reference Meshkat, Haikazian, Di Vincenzo, Fancy, Johnson and Chen-Li50 we suspect that the advantages of oral ketamine are significant when ketamine treatments are considered for more widespread use, and this factor should also be considered.
A range of psychotherapy sequencing protocols were also identified, with psychotherapy preceding, following and being provided at the same time as ketamine treatments. A number of rationales exist for the variety of sequencing. These include preparation of the patient for ketamine treatment, direct influence of psychotherapy on the patient's ketamine experience and taking advantage of opportunities to change following ketamine response. It is still unclear whether the dissociative experience of ketamine augments or hinders the psychotherapy component. At this stage, there is not sufficient evidence to support a particular sequencing protocol for future ketamine and psychotherapy studies, but this area is important to resolve. A systematic review by Joneborg et alReference Joneborg, Lee, Di Vincenzo, Ceban, Meshkat and Lui51 discussed possible mechanisms for the efficacy of combined use of ketamine and psychotherapy, although only a small number of studies were identified that had reported on this topic. This review also noted that despite possible synergistic effects, evidence for this possibility remained speculative.Reference Joneborg, Lee, Di Vincenzo, Ceban, Meshkat and Lui51 We therefore recommend that future research considers comparing different sequencing protocols and includes design elements that address mechanisms of effect.
The timing of outcome measurement varied substantially between studies. Given the established short-term efficacy of ketamine treatment, a key question is whether psychotherapy delays relapse or improves outcomes over the medium to longer-term. The studies by Grabski et al (2022)Reference Grabski, McAndrew, Lawn, Marsh, Raymen and Stevens23 and Krupitsky et al (1996, 2002, 2007)Reference Krupitsky, Burakov, Dunaevsky, Romanova, Slavina and Grinenko30–Reference Krupitsky and Grinenko32 suggest that the addition of psychotherapy to ketamine treatment improves long-term abstinence rates for the treatment of AUD and heroin dependence. Pradhan et al (2018)Reference Pradhan, Mitrev, Moaddell and Wainer37 reported longer term outcomes but did not include a therapy control arm. The other 14 studies did not include sufficient follow-up periods to clarify whether psychotherapy improves outcomes over the medium to longer term. We therefore recommend that future research in this area includes outcome measurement at time points distant to treatment end for medium- to long-term benefits to be clarified.
Limitations
This systematic review included all studies that evaluated ketamine treatment in combination with any psychotherapy for the treatment of any psychiatric disorder. It therefore provides a high-level overview of the extant literature. We believe this approach suited the current status of the field and recognise that comparisons between specific therapies for differing indications are not yet able to be made. Like other researchers, we were challenged by the lack of a gold-standard RoB assessment tool for quantitative studies.Reference Bero, Chartres, Diong, Fabbri, Ghersi and Lam52,Reference Losilla, Oliveras, Marin-Garcia and Vives53 We elected to use the Joanna Briggs critical appraisal tools (modified for the single-arm studies) for the RoB assessments as these appeared most suited to the diversity of studies included in the systematic review. We were required to modify the critical appraisal tool for the single-arm studies to ensure a comprehensive RoB assessment. Commonly used RoB tools such as the ROBINS-I or Newcastle–Ottawa Scale and the Joanna Briggs tools used here do not provide an easy means of quantifying their findings.Reference Sterne, Hernán, Reeves, Savović, Berkman and Viswanathan16 We therefore created cut-points for the RoB assessments to assist the reader; however, we recognise that this approach has not been validated and that the findings should be regarded with caution. The result of the RoB assessments was that the majority of studies had either low or moderate RoB. However, there were no obvious trends for the impact of the quality of included studies on outcome. The sample sizes of the included studies ranged from five to 235 participants. RoB and sample size are critical elements in determining the impact of individual studies. Owing to study heterogeneity, we did not complete a meta-analysis or test for publication bias but it is possible that among smaller studies, those with positive results are more likely to be put forward or accepted for publication.
In conclusion, the addition of psychotherapy to ketamine treatment for psychiatric disorders is associated with largely positive outcomes and appears to offer promise. The range of diagnoses, psychotherapies and treatment protocols identified by this review suggests that definitive recommendations for integrating psychotherapy into ketamine protocols cannot yet be made. We recommend that larger-scale RCTs are undertaken using manualised psychotherapies and consistent, reproduceable ketamine protocols to advance knowledge in this area. We also recommend that future studies are oriented to evaluate whether the addition of psychotherapy to ketamine treatment enhances short-term benefits and delays relapse.
Supplementary material
Supplementary material is available online at http://doi.org/10.1192/bjo.2023.53.
Data availability
All available data are contained within the submitted work.
Author contributions
B.B. conceived the study. B.B and B.M.K. prepared the study protocol and performed screening of studies and data extraction. B.M.K. wrote the first draft and revised further drafts with B.B. All authors contributed to the study protocol, reviewed drafts and contributed to the submitted study.
Funding
This research was completed without specific funding.
Declaration of interest
K.M.D. uses software provided free of charge by Scientific Brain Training Pro for Cognitive Remediation trials. Within the past 3 years, P.G. has had a research contract with Douglas Pharmaceuticals to develop oral formulations of ketamine and has attended an advisory board for Janssen Pharmaceuticals. R.J.P. has received the use of computer software at no cost for research, provided by SBT-pro, and received support for travel to educational meetings from Servier and Lundbeck. R.J.P. and K.M.D. are members of the BJPsych Open editorial board but had no role in the decisions for this paper.






eLetters
No eLetters have been published for this article.