Introduction
Degradation of natural landscapes has led to habitat loss and fragmentation that can negatively affect the species that inhabit these areas (Fahrig Reference Fahrig1997, Reference Fahrig2001; Marzluff and Ewing Reference Marzluff and Ewing2001; Baker and Harris Reference Baker and Harris2007). Life history traits such as high specialisation, poor dispersal, patchy distributions, and/or an aggregated and seasonal appearance are linked to increased extinction risk when landscapes are degraded (McKinney Reference McKinney1997; Reinhardt et al. Reference Reinhardt, Köhler, Maas and Detzel2005). Thus, the ecology and distribution of a species form the foundation of its threat assessments – one example being the International Union for Conservation of Nature red list (International Union for Conservation of Nature 2012). In Sweden, 804 insect species are currently classified as threatened (vulnerable, endangered, or critically endangered) on the national red list, with the most common criteria for a taxon being included is that the distribution is small, largely fragmented, or only exist in few localities (Gärdenfors Reference Gärdenfors2010). The species that specialise in ephemeral habitats, prone to change because of natural successional processes, are vulnerable to local extinctions; especially if these species are poor dispersers (Warren Reference Warren1991; Ranius Reference Ranius2000).
One such species is the European beetle Apalus bimaculatus (Linnaeus) (Coleoptera: Meloidae) (Lundberg Reference Lundberg1995; Fauna Europaea 2011). Apalus bimaculatus inhabits open sandy areas (Lönnell and Edelsjö Reference Lönnell and Edelsjö2004), a habitat that has declined in Northern Europe following changes in land use where a reduction in grazing and forest fires has promoted re-vegetation of these areas (Emanuelsson Reference Emanuelsson2009). Unless active vegetation removal is carried out, this habitat usually becomes over-grown and unsuitable for sand-living beetles within a decade (Lönnell and Edelsjö Reference Lönnell and Edelsjö2004; Lönnberg and Jonsell Reference Lönnberg and Jonsell2012). Thus, the habitat specialisation of A. bimaculatus has resulted in this species having a highly fragmented distribution in central and southwest Sweden (Cederberg Reference Cederberg2003, Lönnell and Edelsjö Reference Lönnell and Edelsjö2004; Lönnell Reference Lönnell2005). Because of this, its current “near threatened” status on the Swedish red list is expected to be upgraded to vulnerable if the current habitat of <2000 km2 continues to degrade through succession (Gärdenfors Reference Gärdenfors2010). It is currently extinct in Finland (Lönnell Reference Lönnell2010) and Estonia (eBiodiversity 2012).
Apalus bimaculatus resides in the subterranean nests of its host species from the larval to the adult stage, where it feeds on food collected by the host (Notini Reference Notini1942). It is believed that A. bimaculatus in Sweden is dependent on the solitary bee species Colletes cunicularius (Linnaeus) (Hymenoptera: Colletidae) for its persistence (Cederberg Reference Cederberg2003). This bee also uses sandy habitats for its breeding chambers (Larsson and Tengö Reference Larsson and Tengö1989), which are aggregated in sub-populations and located several cm below the surface (Lönnell and Edelsjö Reference Lönnell and Edelsjö2004); however, it is not clear if the beetle also uses other sand-living bee species as hosts (Notini Reference Notini1942; Frycklund Reference Frycklund2006).
Because the adult beetle is a poor flyer (Notini Reference Notini1942; Lönnell and Edelsjö Reference Lönnell and Edelsjö2004), it has been suggested that A. bimaculatus, as with other species belonging to the family Meloidae, use phoresy – i.e., where one organism “hitches a ride” on another for the purpose of dispersal (Bologna and Pinto Reference Bologna and Pinto2001; Saul-Gershenz and Millar Reference Saul-Gershenz and Millar2006; Bologna et al. Reference Bologna, Oliverio, Pitzalis and Mariottini2008). Support for this comes from Notini (Reference Notini1942), who showed that A. bimaculatus behaviourally orient to substances secreted from C. cunicularius. The dispersal of A. bimaculatus may therefore be closely linked to the dispersal of C. cunicularius, further increasing the vulnerability of this species to the presence of a healthy host population. Thus, the distribution and dispersal ability of A. bimaculatus are likely to be linked to the distribution and dispersal behaviour of its host, suggesting that landscape variables influencing the distribution of C. cunicularius (or other sand-living bee species) are important for predicting the presence of A. bimaculatus.
To combat threats to A. bimaculatus populations in Sweden, the Swedish Environmental Protection Agency has established a species conservation management program (Lönnell Reference Lönnell2010). Since 2004, management has focused on habitat restoration by removing vegetation from sandy habitats in the distribution area of the beetle. The aim is to expose the sandy substrate and to reduce the shading to increase the available warm habitat that encourages establishment or persistence of the beetle. These actions are based on national expert knowledge, but little is actually known about the species ecology and behaviour, its dispersal and habitat requirements, as very few studies have been carried out in Northern Europe (but see Notini Reference Notini1942; Lönnell Reference Lönnell2010). The current management of the species therefore relies on a limited amount of information of the species requirements.
Thus, the objective of this study is to examine which landscape and habitat factors influence the distribution of A. bimaculatus in the core distribution area in Sweden. Environmental data were measured at two different scales to investigate both landscape/habitat and microhabitat variables. We used environmental variables that current management work is based on to evaluate the correlation of these variables to the presence of A. bimaculatus. Additionally, we gathered data on the presumed host bee C. cunicularius, related sand-living Hymenoptera and environmental variables potentially important for the bees. This was to investigate possible correlations with environmental variables important for the host and the presence of A. bimaculatus.
Methods
Species and environmental censuses
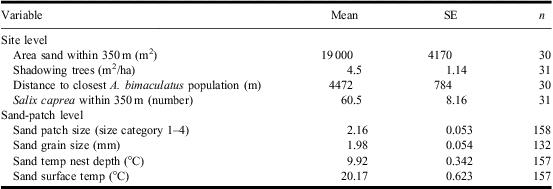
We gathered environmental and species presence data from surveys of 31 sites that had previously been surveyed for A. bimaculatus (in 2004, 2005, and 2006; Fig. 1A). All sites had areas of sand substrate that consisted of several discrete sand patches of potentially suitable habitat (Fig. 1B). Because the census work was labour-intensive and involved careful ground searching, we could not survey all sand patches when a site contained >15 sand patches. Thus, we selected between 5 and 15 (number in proportion to available patches) of the sand patches from within those available at each site. The selection of patches was done to maximise the variation of the environmental variables measured. This resulted in a total of 158 sand patches censused. The species census work was carried out at a constant time per unit area rate of ∼1 minute/m2, during which each selected sand patch was visually screened for beetle and bee individuals present at the sand surface or in the air. Sand patches covered by snow or water at the time of the census were not censused.

Fig. 1 Layout of the study areas: with (A) a map of Sweden and a closer map of the studied area showing the 31 study sites, and (B) a landscape view showing the distribution of the sand habitats and a close-up of one of the sites consisting of several patches of sand. Lines connecting the circles symbolise the measured distance between the study site and the nearest known Apalus bimaculatus population. The circles around the site (grey line representing 350 m radius) show the area used for measuring the amount of sand habitat and number of Salix caprea (black dots) outside the habitat.
Apalus bimaculatus adult activity occurs mostly between early March and mid-April (Lönnell and Edelsjö Reference Lönnell and Edelsjö2004), and C. cunicularius is one of the first bees to emerge during the Swedish spring (Peakall and Schiestl Reference Peakall and Schiestl2004) and is active mainly between March and May (Notini Reference Notini1942). In addition to C. cunicularius, there are at least 10 other Hymenoptera families (including members from all families within Apoidea and some taxa within Vespoidea, Chrysidoidea, and Specoidea) that are commonly encountered in the sand habitats during this period. All censuses for A. bimaculatus, C. cunicularius, and other sand-living Hymenoptera were carried out between 1 April and 30 April 2009 (Notini Reference Notini1942; Lönnell and Edelsjö, Reference Lönnell and Edelsjö2004). All census dates were noted and used in the analyses to help account for temporal relationship with species emergence. In all patches, the number, sex, and status (alive or dead) of all A. bimaculatus were noted. For C. cunicularius, we noted the number present in the patch. Other sand-living Hymenoptera were recorded only at the site scale, where we noted their presence or absence. All censuses were done between 0900 and 1800 hours. The study sites located furthest to the south (at slightly warmer latitudes) were censused first, to increase the likelihood of finding emerging beetles. To reduce the likelihood of false absences, we did not census during days with cold (<12 °C) or rainy weather, as the mobility of the beetles and bees are reduced at lower temperatures (Esch Reference Esch1988; Jian et al. Reference Jian, Jayas and White2003).
Habitat and landscape variables
We collected data on environmental variables that are considered important for the study species on two different scales, the sand patch and the site scale. In addition to amount and distribution of habitat, we measured other habitat features. It is believed that the beetle's activity is positively correlated to sand temperature, so factors that shade the ground (e.g., tree density around a habitat) may affect the habitat suitability for the species (Lönnell and Edelsjö Reference Lönnell and Edelsjö2004) and were therefore measured. As adult bees collect pollen and nectar primarily from Salix Linnaeus (Salicaceae) species within 350 m of their nest chamber (Wesserling 1996, cited in Gathmann and Tscharntke Reference Gathmann and Tscharntke2002), with Salix caprea Linnaeus suggested being the most important food resource for Swedish populations of C. cunicularius (Cederberg Reference Cederberg2003), information on this was gathered.
In the sand patches we collected the following data: (1) sand-patch size: we placed each patch into four categories based on visual estimates of the sand patch – i.e., small (<1 m2), medium (1–10 m2), large (10–100 m2), or very large (>100 m2); (2) sand grain size: the average size of surface sand particles in the surveyed sand patch was categorised using the Atterberg grain size scale (Wiklander Reference Wiklander1976) as reference: fine-grained (0.06–0.20 mm), medium-grained (0.2–0.6 mm), and coarse-grained (0.6–2.0 mm) sand; (3) sand temperature: sand temperature was measured at two depths with a digital thermometer (±0.1 °C) at the centre of each sand patch: (a) surface temperature (at bee and beetle emerging level), by placing the thermometer close to the surface of the sand without exposure to direct sun, and (b) breeding chamber level temperature, at a depth of 11.5 cm; and (4) the presence of the beetle, and the bee C. cunicularius.
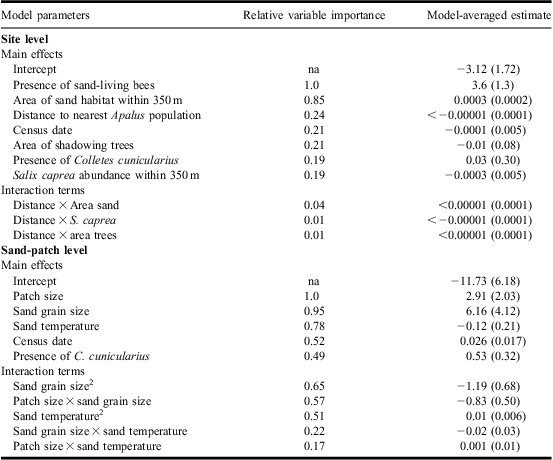
On the site-level scale we used five environmental variables: (1) area of bare sand (available habitat) within 350 m of the habitat center (estimated from orthophotos); (2) tree shade, to estimate tree shade of the habitat we used an angle gauge counting all trees within sight from a center point (Eid Reference Eid2001); this gives the basal area factor, which is an estimate of the local tree density; (3) Distance to nearest sandy habitat with known A. bimaculatus population. We calculated the amount of sandy habitat and the distances on the site scale using digital maps and orthophotos (with ArcGIS 9.2). One of the sites could not be detected on the orthophotos and this resulted in a reduced sample size of 30 for these variables; (4) Salix abundance: we counted the number of mature S. caprea (one of the major food resources for C. cunicularius) within the 350 m foraging distance of the centre of the surveyed sand patches between the 1 and 15 May, a period when the trees are easy to recognise; and (5) the presence of the beetle, C. cunicularius and other sand-living Hymenoptera. All variables and their measurements are in Table 1.
Table 1 Descriptive statistics for independent environmental variables used in analyses of site and sand-patch features affecting the presence of the beetle Apalus bimaculatus.
Statistical analyses
We used generalised linear mixed models (binomial GLMMs with logit link in R using the “lme4” package; R Core Development Team 2011) to evaluate the strength of support for the environmental variables on predicting the presence of A. bimaculatus at the sand-patch level (n = 158) and the site level (n = 31). Because of the large number of potential environmental predictors on beetle presence, and hence the very large number of potential combinations of these predictors, we used an information theoretic model-averaging approach to account for model uncertainty in model selection and parameter estimation (Burnham and Anderson Reference Burnham and Anderson2002). Because of the possibility that sand temperature and grain size might have an optimal value in the middle of measured extremes, we tested for nonlinear relationships between presence of the beetle and these variables by included quadratic terms in the model selection procedure. None of the predictor variables, included in the final models, were strongly correlated when tested using pairwise correlations, with the exception of the temperature measures in the sand-patch level analyses; thus, we only included the temperature data from the nest chamber after initial examination showed it was the better predictor of the two measures.
For model prediction at sand-patch level the site identity was included as a random factor, with fixed factors being combinations of (1) date of census, a Julian date starting on 1 April; (2) sand temperature in °C at a depth of 11.5 cm (approximate chamber depth where the beetles live); (3) presence of the host bee; (4) sand patch size (range 1–4, small–very large); and (5) sand grain size (range 1–5, fine-grained–coarse-grained). Both sand patch size and sand grain size were treated as continuous variables in the analysis. To consider interactions between environmental variables, we also included all two-way interactions between sand grain size, temperature and patch size. For model predictions at the site level, the following explanatory variables were examined: (1) presence of host bees, (2) area of bare sand (in m2) within 350 m radius of the center of the site, (3) presence of other sand-living Hymenoptera, (4) density of shadowing trees (m2/ha), (5) number of S. caprea trees within 350 m of the centre of the site, (6) distance (m) to the nearest known population of A. bimaculatus, and (7) the date of the census. To consider interactions between environmental variables, we also included interactions between the distance to nearest A. bimaculatus population and number of S. caprea, density of shadowing trees and area of bare sand. For both sets of analyses, we created a balanced candidate model set and ranked the models using Akaike's information criteria corrected for sample size (AICC), and used AIC differences (ΔAICC) and AICC weights (wi) to determine the strength of support for each model (Burnham and Anderson Reference Burnham and Anderson2002) using the R package “MuMIn” (Barton Reference Barton2012). In addition, we assessed the strength of support for explanatory variables by calculating their relative variable importance weights; for this the sum of AIC weights for each model that contains a particular variable was calculated, with one being the maximum value and indicating strong support. All models within ΔAICC 10 of the best model were used for generating model-averaged parameter estimates (using the zero method) and relative importance weights of variables (Burnham and Anderson Reference Burnham and Anderson2002). Means are presented with standard errors.
Results
Of the 31 sites surveyed, we found A. bimaculatus in 17, C. cunicularius in nine, and other sand-living Hymenoptera in 18 sites. The mean number of A. bimaculatus individuals found per site was 2.8 ± 0.53 (range 1–9), and for C. cunicularius, 4.2 ± 1.48 (range 1–15).
At the sand-patch scale, there was evidence that the presence of A. bimaculatus was correlated with higher sand temperature at the depth of the bee nests and also linked to areas with an intermediate size of sand grain (with some support for these relationships being quadratic rather than linear). Also, larger sand patches and the presence of sand-living Hymenoptera other than C. cunicularius were strong predictors for the presence of the beetle (Figs. 2, 3; Tables 2, 3). The negative interaction between sand grain size and patch size (Table 2; Fig. 2) strongly suggests that large grain sizes are poor habitat for the beetle regardless of patch size; while any aversion the beetle has to the smallest grain size is overcome in larger patches. Beetles were not found on any of the sand patches that belonged to the smallest size category (<1 m2). We found no support for a positive correlation between the presence of C. cunicularius and A. bimaculatus (Tables 2, 3).

Fig. 2 Estimated probability of finding Apalus bimaculatus within a sand patch (quadratic function from the highest ranked model prediction, Table 3) within the five categories of grain size (1 = finest to 3 = coarsest). Each line represent a simulation with the sand patch size set to 1 (minimum), 2.16 (average), or 4 (maximum), the three levels are included to show the dependence of sand patch area when considering effect of grain size. Observed data are presented as mean probability of occurrence (number of occurrences divided by total number of observations) ± SE for each sand grain size category.

Fig. 3 Estimated probability of finding Apalus bimaculatus within a sand patch (quadratic function from the highest ranked sand-patch model prediction, Table 3) within the sand temperature range of the study (1.5–21.0 °C). Grain size category has been set constant to the most common value of 2.5. Sand patch size category is set to 1 (minimum value), 2.16 (mean value), or 4 (maximum value). Observed data is presented as mean probability of occurrence (number of occurrences divided by total number of observations) ± SE for categories of 15 consecutive temperature observations, except for the last category containing only the seven remaining highest temperature observations.
At the site scale, there was evidence that the presence of the beetle was positively related to the amount of sandy habitat within a 350 m radius, and the presence of other sand-living Hymenoptera (Fig. 4; Tables 2, 4). Apalus bimaculatus was present in 83% of the 18 locations containing sand-living Hymenoptera other than C. cunicularius. As with the sand-patch scale analysis, we did not find any strong evidence for a link between the presence of the host bee and the presence of the beetle. Apalus bimaculatus was never detected at the sites where only C. cunicularius and no other Hymenoptera were noted, and was detected at 17% of the sites where no Hymenoptera at all were observed (in total 12 sites). At the site scale, tree cover, local S. caprea availability and distance to the nearest A. bimaculatus population had very little support as predictors of A. bimaculatus (Tables 2, 4).

Fig. 4 Estimated probability of finding the beetle Apalus bimaculatus at a site (quadratic function derived from the highest ranked model prediction, Table 4) with different sand area (0–90 000 m2) within the habitats. The lines represent simulations either with (—) or without (- - -) sand-living Hymenoptera present in the habitat. The raw data are included as habitats with (grey diamonds) or without (black triangles) sand-living Hymenoptera.
Table 2 AIC-weighted relative variable importance weights for predictor variables and model-averaged parameter estimates included in models (Tables 3, 4) when estimating the probability of finding Apalus bimaculatus at the site or sand-patch level.

Variable relative importance weights of main effects terms of >0.9 suggest strong support for the parameter, 0.5–0.9 moderate support and <0.5 weak or no support. Because interactions and quadratic terms are contained in fewer models than main effects terms, the relative importance strengths are lower and should only be compared with other interactions and not with main effects.
AIC, Akaike's information criteria.
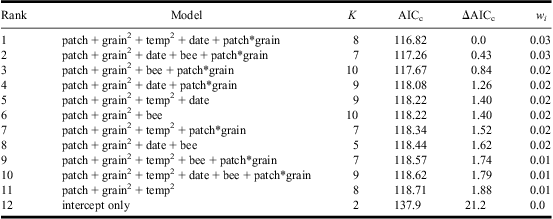
Table 3 AIC-ranked set of candidate models (ΔAICc < 2) for predicting Apalus bimaculatus presence at the local sand-patch level.

Variables included: sand temperature (temp), sand-patch size (patch), sand-grain size (grain), census date (date), presence of Colletes cunicularius within the sand patch (bee).
(+) indicates additive effects and (*) interactions.
Where quadratic terms are shown, the model includes the corresponding linear term (e.g., temp2 = temp + temp2).
Site identity was included as a random factor in all models.
K, number of parameters; AICc, Akaike's information criterion with sample size correction; ΔAICc, difference in AICc relative to the highest-ranked model; wi, Akaike weight (note that the sum of w i does not equal 1 because not all models are shown).
Table 4 AIC-ranked set of candidate models (ΔAICc < 5) for predicting Apalus bimaculatus presence at the site level.
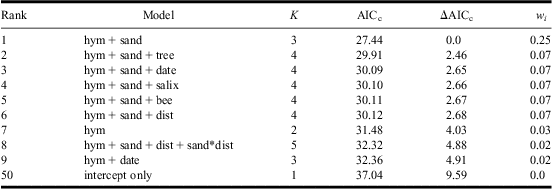
Variables included: presence of sand-living Hymenoptera other than Colletes cunicularius (hym), presence of C. cunicularius (bee), estimation of shadowing trees (tree), sand area within 350 m (sand), Salix caprea within 350 m (salix), distance to nearest known population of A. bimaculatus (dist), and Julian census date (date). Also included were interactions for salix, tree, and sand with dist.
(+) show additive effects and (*) show interactions.
K, number of estimated parameters; AICc, Akaike's information criterion with sample size correction; ΔAICc, difference in AICc relative to the highest-ranked model; w i, Akaike weight (note that the sum of w i does not equal 1 because not all models are shown).
Discussion
Several studies have emphasised the importance of considering spatial (Huxel and Hastings Reference Huxel and Hastings1999; Steffan-Dewenter et al. Reference Steffan-Dewenter, Münzenberg, Burger, Thies and Tscharntke2002; Morris Reference Morris2003) and temporal (Warren Reference Warren1991; Mac Nally Reference Mac Nally2008) dynamics in ecosystems for conservation planning. For species living in patchily distributed habitats, also the quality of existing patches are important (Warren Reference Warren1991; Gyllenberg and Hanski Reference Gyllenberg and Hanski1997). The current management activities for A. bimaculatus are aimed at modifying the vegetation in the habitat of this species to an early successional stage with plenty of bare sand (Lönnell Reference Lönnell2010). While our results support the current management practice of increasing the area of sand habitats, they also indicate that particular factors should be considered when undertaking this practice; namely grain size and the temperature of the sand. Our results suggest that the importance of environmental factors should be weighed depending on the scale at which habitat restoration is viewed (Tables 2–4).
Landscape factors correlated with species presence
The number of A. bimaculatus that we found in this study was low and never exceeded nine individuals in any sand patch and the number of C. cunicularius, ≤15 individuals/habitat, which responds to ∼0.08 individuals/100 m2, was lower than has been found in other studies (Larsson and Tengö Reference Larsson and Tengö1989, although this was within an extremely favourable habitat).
The presence of A. bimaculatus is believed to be positively correlated to fine-grained sand (Cederberg Reference Cederberg2003; Lönnell and Edelsjö Reference Lönnell and Edelsjö2004), but the exact grain fractions suitable for the species is not known (Lönnell Reference Lönnell2010). We found that contrary to this belief, the sand patches with the finest-grained sand were not the ones most likely to contain the beetle, instead fine-medium, medium, and medium–large-grained sand had the highest probability of containing A. bimaculatus (Fig. 2). One reason for A. bimaculatus preferring sand patches with intermediate grain fractions could be that this sand composition is preferred by Hymenoptera for their brooding chambers (Lönnell and Edelsjö Reference Lönnell and Edelsjö2004). The effect of grain size on beetle presence seemed to be dependent on the size of the sand patch, with small patches only being moderately suitable if also the optimal grain size was present. The largest grain size category was not correlated with the presence of the beetle even if the patch was large, indicating that this substrate size is not suitable for nesting sites (Fig. 2).
The size of the sandy habitat area appears important for A. bimaculatus (Tables 2–4, Figs. 2, 3). At both scales, the area of sandy habitat shows a strong positive correlation with the presence of the beetle. At the sites where A. bimaculatus was found, the area of sand cover was more than three times higher than in areas where the beetle was absent (27 500 ± 6560 m2 versus 7700 ± 2720 m2). A larger patch will likely offer a higher number of nests for A. bimaculatus to parasitise and a possibility to sustain larger populations (Cederberg Reference Cederberg2003). Larger areas of suitable sand habitat may be preferred by sand-living Hymenoptera, as large sand patches offer many nesting sites from the heterogeneity of the habitat (Larsson and Tengö Reference Larsson and Tengö1989). Overall, the larger habitats probably have both a higher probability of becoming colonised by individual beetles and Hymenoptera (Matter Reference Matter2009), and for established populations to survive (Hanski Reference Hanski1998). The reason that the effect of patch size is of a high importance for the existence of A. bimaculatus can be from the presence of a minimum habitat threshold, where a high-quality patch in terms of grain size composition cannot fully compensate for a too small area (but see Fleishman et al. Reference Fleishman, Ray, Sjögren-Gulve, Boggs and Murphy2002; Bauerfeind et al. Reference Bauerfeind, Theisen and Fischer2009; Heisswolf et al. Reference Heisswolf, Reichmann, Poethke, Schröder and Obermaier2009). For a species such as A. bimaculatus with assumed low dispersal ability (Notini Reference Notini1942; Cederberg Reference Cederberg2003), and high habitat specificity (Lönnell and Edelsjö Reference Lönnell and Edelsjö2004), the size of the habitat may be even more vital than for other species, as large habitats may both be able to hold larger populations and have a higher buffering capacity when environmental changes occur (Verboom et al. Reference Verboom, Schnippers, Cormont, Sterk, Vos and Opdam2010).
The probability of finding A. bimaculatus increased with the temperature of the sand at the depth of the bee nests (Fig. 3). The higher presence of the species in the warmer areas implies that nest temperature is an important variable for beetle populations and that patches may be of different quality in terms of temperature suitability (Sorvari et al. Reference Sorvari, Haatanen and Vesterlund2010). Ground temperature is highly dependent on sun exposure and the melting of snow cover early in the season and these factors are likely to vary between the patches depending on the direction and position of the slopes.
The support for a positive correlation between the presence of A. bimaculatus and other sand-living Hymenoptera was higher than that with C. cunicularius. This result could either be because we may not have timed the censuses equally well for the beetle and its host, or A. bimaculatus also uses other Hymenoptera as hosts.
The possibility for A. bimaculatus to use phoresy may mean that beetle populations are more connected by dispersal than they seem when the focus is solely on the beetle's active dispersal behaviour. If the beetle uses phoresy, the dispersal pattern of A. bimaculatus would be similar to the dispersal ability of their hosts (Schwarz and Koulianos Reference Schwarz and Koulianos1998; Krishnan et al. Reference Krishnan, Muralidharan, Sharma and Borges2010). Therefore, to fully understand the dispersal capacity of A. bimaculatus and their ability to reach and colonise new habitats, we need to understand more about both the interaction between the beetle and the species that it parasitises and the dispersal behaviour of the host.
Implications for conservation management
To decrease the threat to A. bimaculatus from a reduction of available habitat, we suggest the following should be considered in management of the species. When restoring sandy areas we recommend that the main aim should be to create a few sand patches, each with an area of at least >10 m2 and ensure that they have intermediate grained sand with high sun exposure. In addition to fulfilling the habitat demands of the species short term, large patches are likely to hold larger populations and increase the long-term survival of populations (Berggren et al. Reference Berggren, Carlson and Kindvall2001; Rosin et al. Reference Rosin, Skórka, Lenda, Moron, Sparks and Tryjanowski2011). Our study shows that for a successful management of this threatened species, it is important to acknowledge that sandy habitats are not equal in quality and restoration work is best focused in areas where physical properties are suitable for the species both on a small and large scale.
Additionally, this study shows that other sand-living Hymenoptera other than the host C. cunicularius might be used as indicator species for habitat suitable for A. bimaculatus. In more than 80% of the sites, the occurrence of this group of insects correctly predicted the presence of A. bimaculatus in the habitats searched. This indicates that there may be additional and unknown hosts among sand-living Hymenoptera that may be important for the beetle's persistence and that new knowledge in this area would be very useful. Also our findings show that using sand-living Hymenoptera as an indicator group can make the habitat suitability surveys for the beetle easier, since the beetle itself can be hard to detect due to its short timespan as an active adult.
Acknowledgements
This study was partly financed by the county administrative board in Uppsala, Sweden. The authors thank Niina Sallmén, Elisabet Odhult, and Kalle Mälson at the environment analysis department at the county administrative board in Uppsala for assisting them with information on their previous work. They also thank the landowners of the areas surveyed for letting us access the study areas. They are grateful to Niklas Lönnell, Ingemar Frycklund, Gillis Aronsson, and Jan-Olov Björklund that they shared their knowledge about the species and to Matthew Low for help with the statistics and comments on earlier versions of the manuscript.