Case report
The patient was a 3-year-old male born at 38 weeks of gestation weighing 2.7 kg with a prenatal diagnosis of right atrium isomerism, large ventricular septal defect with small left ventricle, pulmonary atresia, mixed-type total anomalous pulmonary venous connection, and patent ductal artery. The right upper pulmonary vein was connected to the superior vena cava, and the other three pulmonary veins drained into a common chamber without an obstruction, although the common chamber was connected to the atrium with severe obstruction. The pulmonary venous pressure was not very high because of the abundant network of collateral veins between the right upper and lower pulmonary vein. The patient received a continuous injection of alprostadil to maintain patency of the ductal artery and underwent bidirectional Glenn surgery and repair of total anomalous pulmonary venous connection with a sutureless technique at 4 months of age. After the first operation, a left lower pulmonary venous stenosis developed. He underwent percutaneous transluminal balloon angioplasty of the left lower pulmonary venous stenosis at 5 and 7 months of age. In the second intervention, the pulmonary vein was almost occluded because of the intimal flap during the procedure. Two drug-eluting stents (Resolute Onyx®: 4 mm × 18 mm, 3 mm × 12 mm : Medtronic, CA, USA) were implanted in an overlapping manner to maintain the patency of the pulmonary vein. These stents were redilated to accommodate the patient’s growth at 11, 16, 23, and 29 months. At the final intervention, we used 18 YOROI® HC: 7 mm × 17 mm (KANEKA MEDIX, Osaka, Japan). Diagnostic catheter examinations were performed at the same time. The mean pulmonary artery pressure and pulmonary resistance index were 8 mmHg and 1.1 units × m2, respectively. The left lower pulmonary venous pressure and atrial pressure were 5 and 4 mmHg, respectively. The patient was regarded as a good candidate for the Fontan procedure. The pressure gradient between the left lower pulmonary vein and atrium was sufficiently low at that point, but as the patient grew older, the pulmonary venous stent diameter became inevitably inadequate. We planned to exchange the pulmonary venous stents with a larger stent during the Fontan procedure using a hybrid method. The Fontan procedure, with a fenestrated graft and stent exchange, was performed at 36 months of age. Removal of the left inferior pulmonary venous stents was initiated under direct vision in cardiac arrest. The proximal part of the stent was grasped with forceps and pulled anteriorly to slightly flip the pulmonary vein intima. The pulmonary vein intima was detached from the stent using an electrocautery scalpel (SHAPPER Dx: Senko Medical Instrument Mfg, Tokyo, Japan). Care was taken not to dislodge the intima inside the stent. This procedure was performed a little at a time. An electrocautery was used as usual at a power of 15 W. At the distal end of the stent, the continuity of the intima inside the stent and the pulmonary vein intima was smoothly cut. The stent was removed without difficulty or fracture. Stent removal took 10 minutes (Fig. 2). An Express Vascular® LD: 8 mm × 27 mm (Boston Scientific, MA, USA) was inserted into the left lower pulmonary vein without a sheath introducer under direct observation and expanded by a premounted balloon at 8 atm. He was extubated 11 hours after surgery, and the post-operative course was satisfactory. Catheter examination was performed 3 months after surgery. The left pulmonary arterial wedge and atrial pressure were 10 and 8 mmHg, respectively; therefore, the pressure gradient via the stent was estimated to be at most 2 mmHg. No in-stent stenosis was observed on angiography (Fig. 1b). Figure 2 shows the removed stent specimen. The stent lumen was widely patent without stenosis or thrombosis. The stent configuration was circular in shape. The neointimal thickness was approximately 500–1000 µm. The minimum inner diameter of the stent was 5 mm × 4 mm. The stent strut was completely covered with neointima containing collagen and proteoglycan (Fig. 2a). Mild inflammatory cell infiltration with neovessels was observed around the stent struts in the high-power image (Fig. 2b).

Figure 1. Surgical view of stent removal. The stents were removed using electrocautery to separate the stent and pulmonary vein at the intimal layer (a). Delayed phase of a left pulmonary arteriogram showing left inferior pulmonary vein. The stent implanted in the left inferior pulmonary vein was not stenotic (b ).
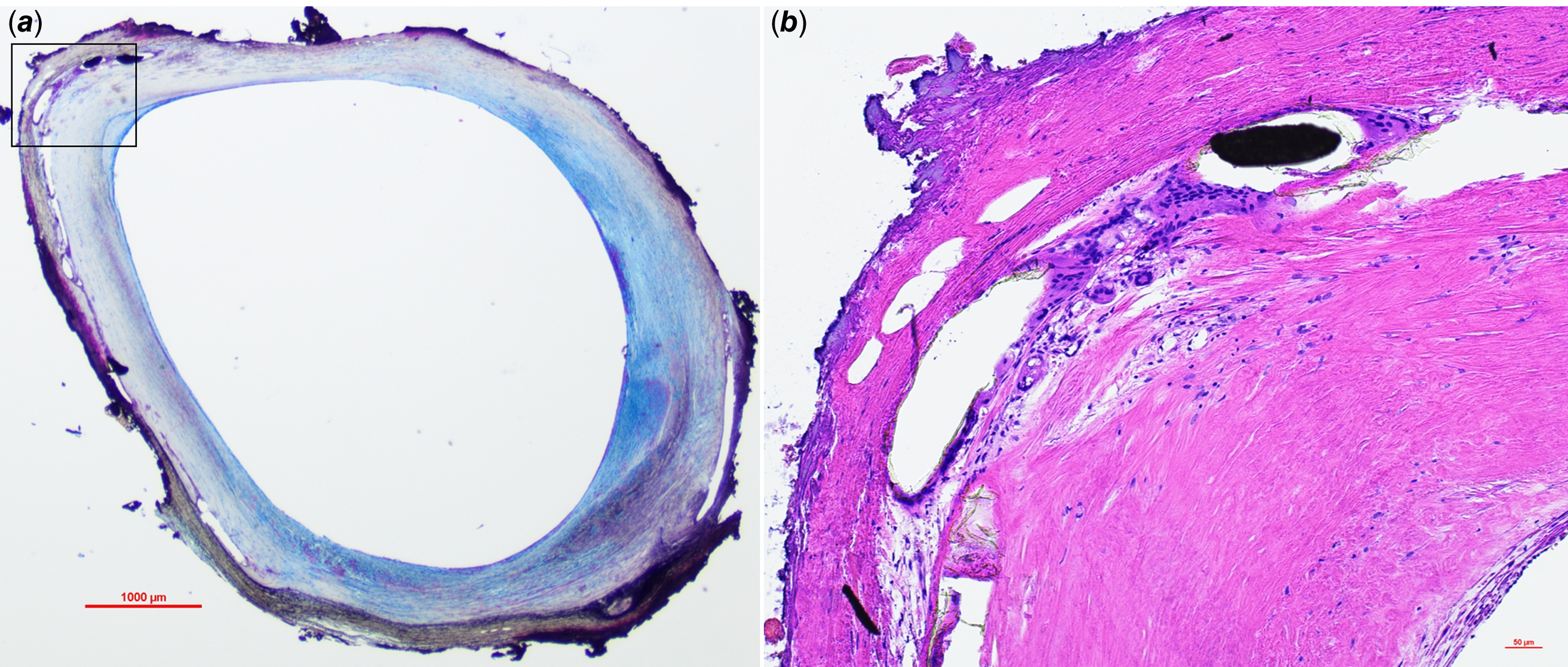
Figure 2. Pathology of drug-eluting stent in the pulmonary vein 855 days after implantation. A low-power image of the stented area (a. Movat pentachrome stain) demonstrates mild neointimal formation with collagen (light yellow-coloured area) and proteoglycan (green-coloured area). Mild inflammatory cell infiltration with neovessels was observed around the stent struts in the high-power image (b. Haematoxylin and eosin stain).
Discussion
Acquired pulmonary venous stenosis occurs following the repair of total anomalous pulmonary venous connections in approximately 10–15% of early survivors.Reference Seale, Uemura and Webber1–Reference Hancock Friesen, Zurakowski and Thiagarajan3 Post-operative pulmonary venous stenosis is not amenable to re-intervention and is sometimes fatal. In particular, single-ventricle physiologies complicated by pulmonary veinous stenosis have a significantly worse prognosis.Reference Spigel, Edmunds, Caldarone, Hickey, Binsalamah and Heinle4 The sutureless technique has the potential to improve outcomes,Reference Najm, Caldarone, Smallhorn and Coles5 but it has certain limitations. In such situations, stenting is a viable treatment option. However, stents implanted in the pulmonary veins are prone to in-stent stenosis owing to intimalisation. Therefore, stents with large diameters (≥7 mm) are preferred.Reference Balasubramanian, Marshall and Gauvreau6 However, small-diameter stents may be the preferred choice in some cases because of the small size of the patient, the complexity of the lesion, or the accidental situation, as in the present case. In such cases, one solution is to implant a drug-eluting stent, which is usually used for coronary lesions. Drug-eluting stents have reduced the risk of in-stent stenosis owing to intimal thickening; however, they inevitably become inadequate because of their small diameter as the patient grows.Reference Wong, Yoo and Lee7 Pulmonary venous stenting under open-heart surgery has already been performed in some institutions and has a good short- to medium-term prognosis.Reference Kurita, Baba and Kondo8 However, to the best of our knowledge, no published reports are available on the intraoperative removal of an already implanted pulmonary venous stent. In this case, we successfully removed the stent safely by carefully separating the stent and pulmonary vein at the intimal layer of the pulmonary vein using an electrocautery scalpel. Single-ventricle heart disease usually requires multistage surgeries. Planned intraoperative stent replacement is a new treatment strategy for pulmonary venous stenosis.
The stent specimens presented here provide additional valuable information. The pathology of drug-eluting stents implanted in the pulmonary vein for such long periods of time has not been reported. The stent was implanted in the pulmonary vein for 855 days. The patency of the stent was maintained, suggesting that the drug-eluting stent is effective even when implanted in the pulmonary vein, as long as no size mismatch exists because of patient growth.
Limitation
Our treatment strategy up to the first palliation may be considered uncommon by some readers. We prefer to repair the total anomalous pulmonary venous connection complicated by a single-ventricle physiology at the same time as the Glenn operation, because the haemodynamics after a Glenn operation is more stable than that after a Blalock-Taussig shunt. Therefore, if the patient’s status permits, we wait to perform the repair of total anomalous pulmonary venous connection at the time of the Glenn operation. This decision may reflect a unique Japanese situation of having to treat low-weight babies or just due to institutional policy.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951123003529.
Acknowledgments
We thank Dr Tsuyoshi Tachibana for conducting the Fontan surgery and stent replacement in this case. We also thank Dr Gaku Nakazawa from Kindai University Faculty of Medicine for preparing the stent specimens and for providing helpful insights.
Competing interests
None.
Patient consent statement
Written informed consent for publication of their details was obtained from the patient’s parents.





