In 2018, the five most common gastrointestinal (GI) cancers, oesophageal, gastric, pancreatic, liver and colorectal cancer, accounted for approximately 25 % of all new cancer cases and 36 % of all cancer deaths worldwide(Reference Ferlay, Ervik and Lam1).
Artificially sweetened soft drinks (ASSD) are drinks produced with high-intensity synthetic sweeteners with the purpose of reducing or eliminating calories in the products while imitating the sugary sweet taste(Reference Swithers2). The most popular artificial sweeteners used in beverage industries are aspartame (branded as NutraSweet, Sugar Twin or Equal), acesulfame (branded as Sunett or Sweet one), saccharin (branded as Sweet and Low, Sweet Twin, Sweet ‘N Low or Necta Sweet), sucralose (branded as Splenda) and neotame (branded as Newtame)(Reference Chattopadhyay, Raychaudhuri and Chakraborty3,Reference Chaudhary4) . The production and consumption of ASSD have increased worldwide, and this popularity has been further triggered by the growing epidemic of obesity in high-income countries and other calorie-related health concerns that arise from consumption of sugar-sweetened beverages(Reference Cuomo, Andreozzi and Zito5,Reference Sarfaraz, Bano and Fatima6) . The supplies and demands of these soft drinks have established their niches worldwide, including low- and middle-income countries at the peril of whole fruit consumption and home-made real fruit juice. The high sweetness intensity of artificial sweeteners seems also very attractive to ASSD producers.
Meanwhile, previous laboratory and animal studies have reported that low-dose exposure to aspartame had a significant multi-potential carcinogenicity in colon cell lines and increased the incidence of mammary cancer, lymphoma and leukemia in rats with a dose–response relationship(Reference van Eyk7,Reference Soffritti, Belpoggi and Tibaldi8) . The dose level used in the animal study was close to the acceptable daily intake of aspartame for humans(Reference Soffritti, Belpoggi and Tibaldi8,Reference Bessler and Djaldetti9) . This might indicate that lifespan exposure to aspartame in soft drinks could potentially increase the risk of an individual to cancer, especially GI cancer.
Also, previous observational epidemiological studies have reported inconsistent findings on whether consumption of ASSD increases the risk of GI cancer(Reference Lagergren, Viklund and Jansson10–Reference Mullee, Romaguera and Pearson-Stuttard30). Ten observational epidemiological studies (four case–control studies and six cohort studies) reported a significant association between the consumption of ASSD and the risk of GI cancer(Reference Chan, Wang and Holly15,Reference Mahfouz, Sadek and Abdel-Latief17,Reference Stepien, Duarte-Salles and Fedirko18,Reference Khan, Goto and Kobayashi21,Reference Larsson, Bergkvist and Wolk22,Reference Navarrete-Munoz, Wark and Romaguera24,Reference Bassett, Milne and English26) , while twenty-eight studies (fifteen case–control studies and thirteen cohort studies) reported no association between them(Reference Lagergren, Viklund and Jansson10–Reference Bosetti, Gallus and Talamini14,Reference Gallus, Turati and Tavani16,Reference Li, Petrick and Steck19,Reference Li, Petrick and Steck20,Reference Bao, Stolzenberg-Solomon and Jiao23,Reference Hodge, Bassett and Milne25,Reference Chazelas, Bernard and Elisa27–Reference Mullee, Romaguera and Pearson-Stuttard30) . However, no meta-analysis on this topic has been published so far.
Thus, we investigated whether the consumption of ASSD increases the risk of GI cancers by using a meta-analysis of observational epidemiological studies such as case–control studies and cohort studies.
Materials and methods
Literature search strategy
We conducted a literature search in both PubMed and Excerpta Medica dataBASE (EMBASE) databases up to May 2020. We used a combination of the National Library of Medicine Medical Subject Headings (MeSH) terms with a wide range of free-text terms as search terms in order to identify as many relevant articles as possible. A PICO framework was used to determine search terms related with the topic of the current study as follows: P for population is ‘general population’; I for intervention (exposure in the current study) is ‘consumption of ASSD’; C for comparison is ‘little or no consumption of ASSD’ and O for outcome is ‘incidence of GI cancers’. Additionally, we restricted a study design to case–control study and cohort study for the current study. Thus, by using Boolean operators for all the determined MeSH and free-text terms, we created a combination of search terms as follows: (artificially sweetened beverages or sweetened beverages or carbonated drinks or soft drinks or diet drinks or fizzy drinks or cola or soda or non-alcoholic beverages or non-alcoholic drinks) and (gastrointestinal neoplasms or oesophageal neoplasms or stomach neoplasms or liver neoplasms or pancreatic neoplasms or colorectal neoplasms) and (cohort study or case–control study). Appendix 1 shows the final search strategy for the PubMed example. We further reviewed the reference lists from the identified articles to find relevant studies not identified through this search strategy.
Inclusion criteria
We included observational epidemiological studies that met the following criteria: (1) a case–control study or a cohort study; (2) investigated the associations between the consumption of ASSD and any of the five major types of GI cancer (oesophageal, gastric, pancreatic, liver and colorectal cancer); (3) reported outcome measures with adjusted OR, relative risks (RR), or hazard ratios and 95 % CI. If data were reported in more than one study on the same cancer type, the study presenting the most comprehensive data was included. Studies that were not published in peer-reviewed journals or only presented in conferences were excluded.
Selection of relevant studies
Two authors (Jatho A and Cambia JM) independently selected all the studies retrieved from the databases. We extracted year of publication and first author’s name, type of study, country, year of the enrollment of participants, population (number of participants, gender and baseline age range), type of GI cancer, definition of ASSD intake (highest v. lowest category), adjusted OR/RR/HR with 95 % CI and adjusted variables for the general characteristics of the included studies.
Assessment of methodological quality
We evaluated the methodological quality of the included studies based on the Newcastle-Ottawa Scale for assessing the quality of case–control studies and cohort studies in the meta-analyses(Reference Wells, Shea and O’Connell31). The Newcastle-Ottawa Scale star system ranges from 0 to 9 representing the three subscales of the study quality dimensions: study selection, comparability and exposure assessment(Reference Wells, Shea and O’Connell31). Because there are no established cut-off criteria for high or low quality of a study, we classified a study with more than the mean score of each study type (case–control studies or cohort studies) into a high-quality study.
Main and subgroup analyses
We investigated the associations between the consumption of ASSD (highest v. lowest consumption or never consumed) and the risk of GI cancer for the main analysis. This was followed by subgroup meta-analysis by type of study design (case–control study or cohort study), type of GI cancer (oesophageal, gastric, pancreatic, liver or colorectal cancer), gender (female or male), continental region (Africa, America, Australia, Asia or Europe) and methodological quality of the included studies (high or low quality) in each study type. Also, we conducted subgroup meta-analysis by each factor (type of GI cancer, gender, region and study quality) under each type of study design.
Statistical analyses
We computed the pooled OR, RR or HR with its 95 % CI using the adjusted OR, RR or HR and its 95 % CI from each study reporting the association between the consumption of ASSD (highest v. lowest consumption or never consumed) and the risk of GI cancer. We further examined heterogeneity across the studies using Higgins I2(Reference Higgins and Thompson32), which measures the percentage of total variation across the studies(Reference Mayne, Risch and Dubrow11). I2 is calculated as follows:
where Q is Cochran’s heterogeneity statistic, and df indicates the df. Negative values of I2 were set at zero; I2 ranges from 0 % (no observed heterogeneity) to 100 % (maximal heterogeneity)(Reference Higgins and Thompson32). An I2 value greater than 50 % indicates substantial heterogeneity(Reference Higgins and Thompson32).
The pooled estimate was computed using the DerSimonian and Laird method(Reference DerSimonian and Kacker33). We used a random-effects model because the identified studies were conducted in a wide range of geographical settings and in different populations.
We also evaluated publication bias using the Begg’s funnel plot and Egger’s test(Reference Macaskill, Walter and Irwig34). Publication bias exists when the Begg’s funnel plot shows asymmetry or when the P-value of the Egger’s test is less than 0·05(Reference Macaskill, Walter and Irwig34) Further, we conducted sensitivity analyses to explore the influence of each study on the pooled estimate by omitting an investigation one by one and re-analysing. We used Stata se version 16.1 statistical software package (StataCorp) for all the meta-analyses.
Results
Identification of relevant studies
Figure 1 shows a flow diagram of how we selected the relevant studies for the current study. A total of 448 articles were identified by searching two electronic databases (PubMed and EMBASE) and by hand-search. We excluded ninety-two duplicate articles and additional 313 articles based on the predetermined selection criteria. We conducted the full-text review of the remaining forty-three articles. Among these, twenty-two articles were excluded for the following reasons: sugar-sweetened soft drinks (n 9); inclusion of sweets, snacks and desserts (n 4); undefined cancer sites (n 3); report of inflammatory scores or index (n 2); biliary track and gallbladder cancer (n 2) and irrelevant studies (n 2). The remaining twenty-one articles with eleven case–control studies(Reference Lagergren, Viklund and Jansson10–Reference Li, Petrick and Steck20) and eleven cohort studies(Reference Stepien, Duarte-Salles and Fedirko18,Reference Khan, Goto and Kobayashi21–Reference Mullee, Romaguera and Pearson-Stuttard30) involved individual twenty-one and seventeen studies, respectively, totaling to thirty-eight studies in the main and subgroup meta-analysis.
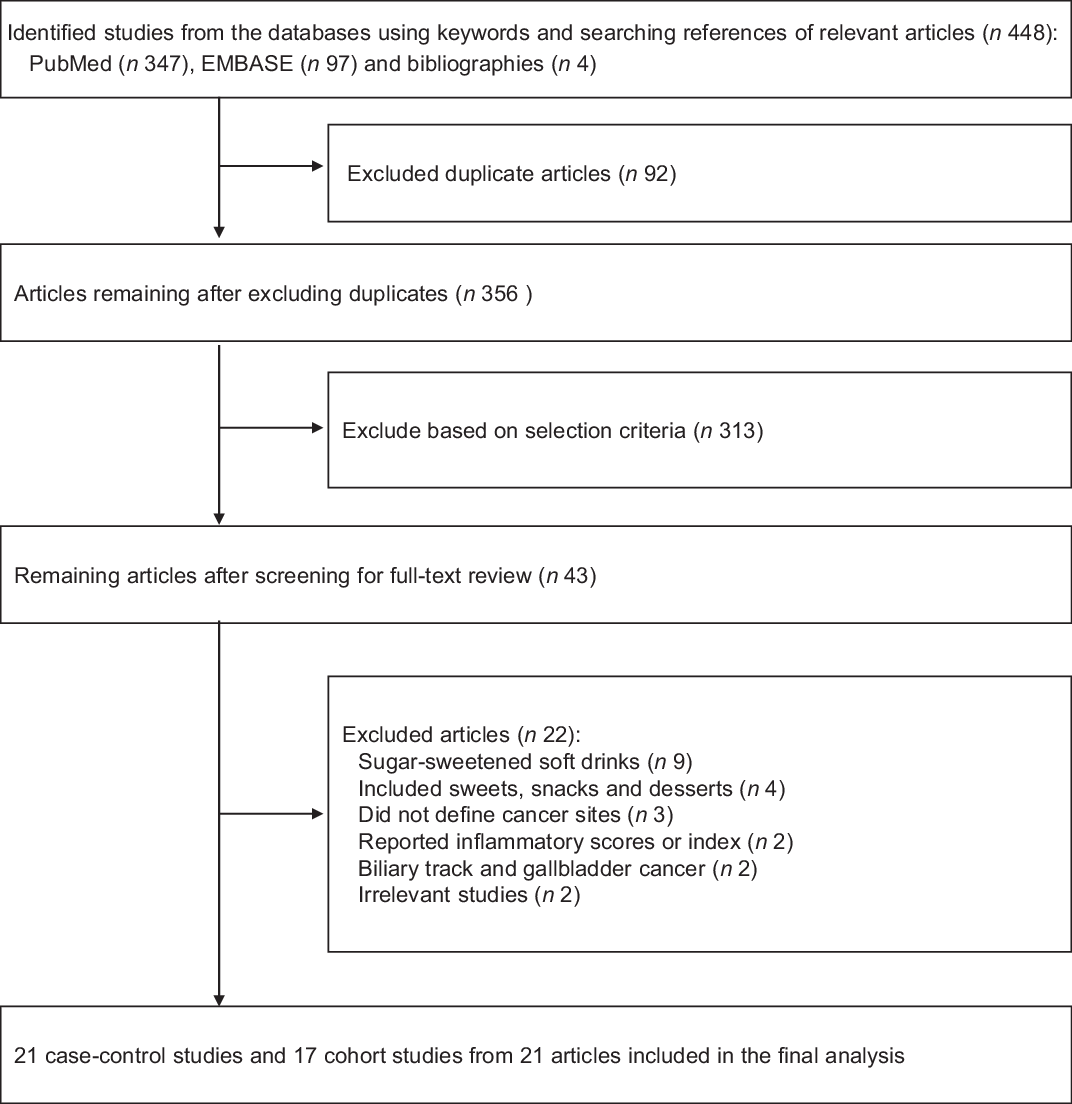
Fig. 1 PRISMA flow diagram for study selection
Characteristics of studies included in the final meta-analysis
We included thirty-eight studies in the final meta-analysis; twenty-one case–control and seventeen cohort studies from twenty-one articles that had 2 486 849 participants (12 397 cancer cases and 2 474 452 controls). The mean age of all the participants was 54 years (range, 18 to 97 years). Table 1 shows the general characteristics of the studies included in the final meta-analysis. The types of GI cancers were as follows: oesophageal(Reference Lagergren, Viklund and Jansson10–Reference Ibiebele, Hughes and O’Rourke13,Reference Li, Petrick and Steck19) , gastric(Reference Lagergren, Viklund and Jansson10,Reference Mayne, Risch and Dubrow11,Reference Bosetti, Gallus and Talamini14,Reference Li, Petrick and Steck20,Reference Khan, Goto and Kobayashi21,Reference Hodge, Bassett and Milne25,Reference Bassett, Milne and English26) , pancreatic(Reference Bosetti, Gallus and Talamini14–Reference Gallus, Turati and Tavani16,Reference Khan, Goto and Kobayashi21–Reference Navarrete-Munoz, Wark and Romaguera24) , liver(Reference Stepien, Duarte-Salles and Fedirko18,Reference Luo, Jing and Wanshui28) and colorectal cancer(Reference Gallus, Scotti and Negri12,Reference Mahfouz, Sadek and Abdel-Latief17,Reference Khan, Goto and Kobayashi21,Reference Hodge, Bassett and Milne25,Reference Chazelas, Bernard and Elisa27,Reference Malik, Li and Pan29,Reference Mullee, Romaguera and Pearson-Stuttard30) . Only six studies reported gender-disaggregated data(Reference Mayne, Risch and Dubrow11,Reference Gallus, Scotti and Negri12,Reference Bosetti, Gallus and Talamini14,Reference Chan, Wang and Holly15,Reference Khan, Goto and Kobayashi21,Reference Navarrete-Munoz, Wark and Romaguera24) . Studies were conducted in Europe(Reference Lagergren, Viklund and Jansson10,Reference Gallus, Scotti and Negri12,Reference Bosetti, Gallus and Talamini14,Reference Gallus, Turati and Tavani16,Reference Stepien, Duarte-Salles and Fedirko18,Reference Larsson, Bergkvist and Wolk22,Reference Navarrete-Munoz, Wark and Romaguera24,Reference Chazelas, Bernard and Elisa27,Reference Mullee, Romaguera and Pearson-Stuttard30) , America(Reference Mayne, Risch and Dubrow11,Reference Chan, Wang and Holly15,Reference Li, Petrick and Steck19,Reference Li, Petrick and Steck20,Reference Bao, Stolzenberg-Solomon and Jiao23,Reference Luo, Jing and Wanshui28,Reference Malik, Li and Pan29) , Australia, other Oceania(Reference Ibiebele, Hughes and O’Rourke13,Reference Hodge, Bassett and Milne25,Reference Bassett, Milne and English26) , Asia(Reference Khan, Goto and Kobayashi21) and Africa(Reference Mahfouz, Sadek and Abdel-Latief17).
Table 1 Characteristics of the studies included in the final meta-analysis of artificially sweetened soft drinks (ASSD) and the risk of gastrointestinal (GI) cancer (n 21)

AC, adenocarcinoma; SCC, squamous cell carcinoma; AEG, adenocarcinomas of oesophagogastric junction; GERD, gastroesophageal reflux disease; RR, relative ratio.
* Reported artificially sweetened drinks as diet drinks.
Methodological quality of studies
We assessed the methodological quality of the included studies based on the Newcastle-Ottawa Scale. The quality scores ranged from 5 to 9; the average score was 7·4 for case–control studies (range 5–9) and 8·1 for cohort studies (range 6–9). Nine case–control studies and ten cohort studies are considered as high-quality studies (scores of 7 or higher in case–control studies and 8 or higher in cohort studies) (Table 2).
Table 2 Methodological quality of studies included in the final analysis based on the Newcastle-Ottawa scale* for assessing the quality of case–control studies and cohort studies (n 22)

* Each study can be awarded a maximum of one star for each numbered item within the selection and exposure categories, while a maximum of two stars can be given for the comparability category. 2016 Stepien et al.’s study consists of both a case–controls study and a cohort study.
Consumption of artificially sweetened soft drinks and risk of gastrointestinal cancer
As shown in Figure 2, the consumption of ASSD was not associated with the risk of GI cancer (OR/RR, 1·02; 95 % CI, 0·92, 1·14). In the subgroup meta-analyses by study design, no significant association between them was observed in both case–control studies (OR, 0·95; 95 % CI, 0·82, 1·11) and cohort studies (RR/HR, 1·14; 95 % CI, 0·97, 1·33).

Fig. 2 Consumption of artificially sweetened soft drinks and risk of gastrointestinal (GI) cancer in a random-effects meta-analysis of observational epidemiological studies. (a) All studies; (b) subgroup meta-analysis by type of study design; (c) subgroup meta-analysis by type of GI cancer. OR, OR; RR, relative risk; CI, CI; AC, adenocarcinoma; SCC, squamous cell carcinoma; AEG, adenocarcinomas of oesophagogastric junction, and HCC, hepatocellular carcinoma. *During data analysis using Stata se version 16.1 statistical software, the lower limit of the 95 % CIs of 0·0 that were observed in both men and women by Khan et al. (2004) study was rejected by the STATA software. We, therefore, chose 0·01 (the closest value to 0·0 that could be accepted by the software for the analysis to proceed)
In the subgroup meta-analyses by type of GI cancer, the consumption of ASSD was associated with a significantly increased risk of liver cancer (OR/RR, 1·28; 95 % CI, 1·03, 1·58; n 3), while no association was found in any other types of GI cancers (Fig. 2).
Consumption of artificially sweetened soft drinks and risk of gastrointestinal cancer by gender, region and methodological quality of study
Table 3 shows findings from the subgroup meta-analyses stratified by gender, region and methodological quality. In the meta-analysis of all the studies, overall, no significant association between the consumption of ASSD and the risk of GI cancer was observed except for liver cancer (OR/RR, 1·28; 95 % CI, 1·03, 1·58; n 3). The significantly increased risk of liver cancer by consumption of ASSD was found in cohort studies (RR/HR, 1·50; 95 % CI, 1·04, 2·16; n 2) as well as case–control studies (OR, 1·18; 95 % CI, 1·04, 1·34; n 1).
Table 3 Association between artificially sweetened soft drinks and risk of gastrointestinal cancers in subgroup meta-analyses using a random-effects model

Abbreviation: NA, not applicable.
Heterogeneity, publication bias and sensitivity analysis
Statistical heterogeneity was observed (I2 = 64·3 %) in the meta-analysis of all the included studies. In the subgroup meta-analysis by type of study, case–control studies showed substantial heterogeneity (I2 = 68·8 %), while cohort studies showed less heterogeneity (I2 = 48·3 %). Publication bias was not observed in both Funnel plot (Fig. 3) and Begg’s test (P = 0·33 for all the studies, 0·19 for case–control studies and 0·67 for cohort studies, respectively). Sensitivity analysis to discern the influence of each study did not show any substantial change in the pooled estimate of the effect size and statistical significance (data not shown in figure).

Fig. 3 Assessment of publication bias by Begg’s funnel plots with 95 % confidence limits on consumption of artificially sweetened soft drinks and risk of gastrointestinal cancer in a random-effects meta-analysis of observational epidemiological studies. (a) All studies (n 32); (b) case–control studies (21); (c) cohort studies (n 17)
Discussion
Summary of findings
In the current meta-analysis of observational epidemiological studies, we found that the consumption of ASSD was not associated with the risk of overall GI cancer. Also, there was no significant association between them in the subgroup meta-analysis by type of study design. In the subgroup meta-analysis by type of cancer, the consumption of ASSD was significantly associated with the increased risk of liver cancer, which association remained consistent in the subgroup meta-analysis of both case–control and cohort studies.
Possible biological mechanisms
Neoplastic induction by metabolites of artificial sweeteners
Even though we found that there was no significant association between ASSD and the risk of GI cancer, previous laboratory and animal studies have proposed possible biological mechanisms on the association. First, some laboratory and animal studies have showed that the ingredients of ASSD metabolise in the gut into their chemical constituents that could be harmful in long-term exposure(Reference Sarfaraz, Bano and Fatima6,Reference van Eyk7,Reference Soffritti, Belpoggi and Tibaldi8) . For example, aspartame is metabolised in the gastric tract into aspartic acid, phenylalanine and methanol(Reference van Eyk7). Low-dose exposure to aspartame in both laboratory and animal studies showed a significant multi-potential carcinogenicity in colon cell lines and increased the incidence of mammary cancer, lymphoma and leukaemia in rats with a dose–response relationship(Reference van Eyk7,Reference Soffritti, Belpoggi and Tibaldi8) . Moreover, these were conducted at a dose level similar to the recommended daily intake levels in humans(Reference van Eyk7,Reference Soffritti, Belpoggi and Tibaldi8) . Soffritti et al. also found a neoplastic induction by aspartame regarding carcinogenesis in the liver and lung in mice(Reference Soffritti, Manservigi and Tibaldi35), which is linked to the production of formaldehyde from the methanol constituent of aspartame. Liver and other body tissues metabolise methanol into formaldehyde(Reference Pietzke, Burgos-Barragan and Wit36–Reference Dorokhov, Shindyapina and Sheshukova38). Formaldehyde is genotoxic and damages the DNA due to the formation of formaldehyde adducts that increases the risk of chromosomal mutations due to DNA-protein cross-links formation(Reference Cuomo, Andreozzi and Zito5). Therefore, lifespan exposure to ASSD could increase the risk of liver cancer in human.
Effects of acidulants, colouring (4-methylimidazole) and flavouring agents in artificially sweetened soft drinks
Soft drinks in addition to artificial sweeteners contain acidulants and colouring and flavouring agents(Reference Sarfaraz, Bano and Fatima6). A colourant known as caramel (4-methylimidazole) is classified into Group 2B (possibly carcinogenic) by the international agency for research on cancer(Reference Morita and Uneyama39). Caramel is used as a colouring agent in the production of both the artificially sweetened and sugar-sweetened soft drinks in similar permissible level(Reference Tzatzarakis, Vakonaki and Moti40). Some artificial flavouring agents in ASSD are also chemically synthesised. However, their effects to promote neoplastic induction remain unclear.
Inflammation by artificial ingredients and proinflammatory markers
Systemic inflammation from the artificial ingredients in ASSD and proinflammatory markers such as C-reactive protein have also been implicated. C-reactive protein is a nonspecific acute phase protein primarily synthesised by the liver and used as a systemic inflammatory marker have been suggested to promote carcinogenesis(Reference Wang, Lee and Tworoger41). For example, C-reactive protein was found to increase the risk of breast cancer in a meta-analysis of cohort studies (RR, 1·26; 95 % CI, 1·07, 1·49)(Reference Wang, Lee and Tworoger41).
Also, altered GI track microbiota by ASSD might be related to inflammation. In both animal and human studies, Suez et al. (Reference Suez, Korem and Zeevi42) demonstrated that consumption of artificially sweetened products of saccharin, aspartame and sucralose triggers glucose intolerance due to the altered composition and functions of GI track microbiota. The altered microbiota decrease bacterial heterogeneity and the relative ratio of Bacteroidetes and Firmicutes(Reference Suez, Korem and Zeevi42,Reference Bokulich and Blaser43) . More importantly, lipopolysaccharide, a part of outer membrane of Gram-negative bacteria, is suggested to initiate obesity-related inflammation and insulin resistance. In the liver or adipose tissues, lipopolysaccharide triggers the innate immune response that increases proinflammatory cytokine expression(Reference Creely, McTernan and Kusminski44).
Additionally, weight gain and elevated glycaemic index might be associated with promotion of inflammation, which could lead to the development of cancer. Fowler et al. (Reference Fowler, Resendez and Hunt45) in a cohort study on ‘obesity epidemic’ observed that participants who consumed ASSD showed a significant weight gain and obesity than those who did not consume ASSD. Similar findings were reported in two adolescent cohorts that examined effects of ASSD intake on BMI and fat percentage(Reference Laska, Murray and Lytle46). These findings suggest that artificial sweeteners stimulate food intake and weaken the validity of sweet taste by desensitising the natural ability of sweet taste to evoke physiological responses(Reference Swithers2,Reference Bokulich and Blaser43,Reference von Poser Toigo, Huffell and Mota47) . This could induce higher glycaemic index, hyperinsulinaemia and systemic inflammation that promote tumorigenesis(Reference Turati, Gandini and Augustin48). However, since ASSDa are generally viewed as healthier substitutes due to absence of sugar, individuals with underlying health disorders like obesity, diabetes and CVD might have experienced high consumption of ASSD in the past.
Possible explanations for no significant association between artificially sweetened soft drinks consumption and gastrointestinal cancer risk
We do not have clear explanations for no significant association between the consumption of ASSD and the risk of GI cancer. However, there are some possible ones for it. First, findings from preclinical studies such as laboratory studies or experimental animal studies are not always directly applied to humans. Laboratory studies and experimental animal studies are usually conducted in the limited and controlled settings and environments, and observational epidemiological studies are conducted in the different environmental settings. In addition,lifestyle factors could affect the disease outcomes in humans. Second, the lack of an apparent association in this meta-analysis might be due to confounders, which might affect the disease outcomes. For example, some studies did not adjust for the intake of fruits, fruit juice and vegetables, which contain various antioxidants that could attenuate the harmful effects of ASSD(Reference Ruxton, Gardner and Walker49). Third, the fundamental metabolic differences between humans and animals could also lead to different health status outcomes from a specific exposure such as ASSD. Besides the metabolic differences between laboratory animals and human, their anatomical, physiological and biochemical differences in particular in their GI tracts could influence the absorption and bioavailability values(Reference Kararli50) of ASSD and their ingredients. Fourth, the accuracy of the dietary survey to estimate the consumption of ASSD could also affect detecting the exact influence of ASSD on the development of GI cancer. Last, most of the observational studies included in the current meta-analysis used data from a single measurement of dietary intake at baseline. Therefore, such data might not reflect long-term dietary intake behaviour.
Strengths
This is the first most comprehensive meta-analysis of observational epidemiological studies such as case–control studies and cohort studies on this topic. Although a meta-analysis of observational epidemiological studies regarding this topic has been published in 2019, it only included pancreatic cancer and involved sweetened beverages including both sugar-sweetened soft drinks and ASSD(Reference Milajerdi, Larijani and Esmailzadeh51). Moreover, the authors only investigated the combined effects of both types of beverages.
Limitations
There are several limitations in our study. We included only observational epidemiological studies such as case–control and cohort studies in the current meta-analysis. In terms of evidence-based medicine, randomised controlled trials, which give us a higher level of evidence than observational studies, are warranted to confirm the association between the consumption of ASSD and the risk of GI cancer. However, no randomised controlled trials on this topic have been published so far, and it is not easy to conduct randomised controlled trials on this topic because of ethical concerns. Additionally, although we found that the consumption of ASSD increased the risk of liver cancer, the number of the included studies is too small to confirm the association between them. We included only three studies with a case–control study and two cohort studies for this association.
Conclusions
In this meta-analysis of observational epidemiological studies, we found that the consumption of ASSD is not associated with the risk of GI cancer. Further large prospective cohort studies are warranted to confirm its effect on the risk of liver cancer.
Acknowledgements
Acknowledgements: Alfred Jatho expresses his appreciation for the training support from the ‘International Cooperation & Education Program (NCCRI·NCCI 52210–52211, 2020)’ of the National Cancer Center, South Korea. Financial support: None. Conflict of interest: The authors have declared no conflict of interest. Authorship: AJ: Study concept and design, acquisition, statistical analysis, interpretation of data, drafting of the manuscript, revision of the manuscript and approval of the final article for submission. JMC: Acquisition, statistical analysis, interpretation of data and approval of the final article for submission. SKM: Study design, statistical analysis, interpretation of data, critical revision of the manuscript for important intellectual content and approval of the final article for submission. Prof. Seung-Kwon Myung had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Ethics of human subject participation: Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S136898002100104X











