Chronic psychotic disorders (CPDs), such as schizophrenia and schizoaffective disorder, occur worldwide and cause significant burden, characterised by reduced quality of life, functional impairment and premature mortality owing to suicide and other causes. Although the standard of care for CPD includes antipsychotic drugs, poor medication adherence is pervasive globally and leads to negative outcomes that affect not just individuals with schizophrenia, but also families and communities.Reference Chidarikire, Cross, Skinner and Cleary1,Reference Velligan, Weiden, Sajatovic, Scott, Carpenter and Ross2 There are many barriers to medication-taking generally among people with schizophrenia, including erratic medication-taking, poor insight into illness, cognitive problems, stigma and limited access to care.Reference Chidarikire, Cross, Skinner and Cleary1,Reference Gilmer, Dolder, Lacro, Folsom, Lindamer and Garcia3–Reference Jenkins, Strauss, Carpenter, Miller, Floersch and Sajatovic5 A recent systematic literature review and meta-analysis of the global literature on medication non-adherence in major psychiatric disorder suggests that poor adherence is influenced by a variety of factors, such as patients’ individual behaviours; social or family support; clinical, illness and/or treatment factors, and overall healthcare system-related factors.Reference Semahegn, Torpey, Manu, Assefa, Tesfaye and Ankomah6
In sub-Saharan Africa, poor adherence is seen in approximately half of individuals with CPD; however, clinical correlates of poor adherence have not been well studied.Reference Adewuya, Owoeye, Erinfolami, Coker, Ogun and Okewole7–Reference Ho Odo, Agbonile and Esan11 A qualitative study from TanzaniaReference Sariah, Outwater and Malima10 suggested that both patients and caregivers perceived non-adherence to antipsychotic medication as a leading factor of relapse. A literature review on antipsychotic treatment for people with schizophrenia in sub-Saharan Africa, by Chidarikire et al,Reference Chidarikire, Cross, Skinner and Cleary1 noted that limited access to care, a poor understanding of illness and treatment, limited insight, lower levels of literacy, comorbid substance misuse, the presence of CPD symptoms and medication treatment side-effects can all negatively affect adherence.
To develop and deliver care approaches that may promote improved adherence, it is important to understand the clinical features and adherence barriers for people at greatest risk of poor adherence. This cross-sectional survey of patients with CPD in Tanzania characterised demographic and clinical features that appear to be associated with suboptimal medication adherence.
Method
Overview
Data from this analysis is derived from a larger 24-month project, funded by the USA's National Institute of Mental Health, to refine and preliminarily test an intervention intended to promote medication adherence in poorly adherent patients. Methods of the larger project have been described elsewhere.Reference Mbwambo, Kaaya, Lema, Blixen, Cassidy and Levin12 This analysis assessed clinical features and adherence correlates among 100 poorly adherent patients with CPD who were receiving care in the psychiatry department at a 70-bed national referral hospital located in urban Dar es Salaam, Tanzania. Demographic features, past resource use, substance use comorbidity and psychosocial support were evaluated with respect to their association with self-reported medication treatment adherence.
Study setting and recruitment
Adults aged ≥18 years, with a clinical diagnosis of schizophrenia or schizoaffective disorder, were recruited from the national psychiatric referral hospital and associated hospital ambulatory clinics. Patients at the hospital are referred from four catchment zones that includes three regional public and private hospitals. The out-patient clinic serves mainly discharged patients, for follow-up. Inclusion criteria were purposely broad to capture a representative sample of poorly adherent patients, and included a clinical diagnosis of schizophrenia or schizoaffective disorder, confirmed by a psychiatrist; self-reported poor medication adherence, defined as missing ≥20% of prescribed antipsychotic medication within the past week or past month;Reference Velligan, Weiden, Sajatovic, Scott, Carpenter and Ross2 and being able to participate in assessment procedures and be rated on standardised rating scales. Individuals at risk of harm to themselves or others, and those who could not provide written informed consent to study participation, were excluded. The study was approved by the local institutional review board and the Tanzanian National Institute for Medical Research (ethical approval registry number: DA.282/298/01.C; ClinicalTrials.gov Identifier: NCT04327843).
Assessments
Evaluations comprised demographic and clinical characteristics relevant to CPD relapse. Demographics included age, gender, marital status, children/number of children, educational status, employment and where the individual resided (with family, alone, etc.). Clinical variables included type and duration of CPD, family psychiatric history, personal history of physical or sexual abuse, past hospital admissions and antipsychotic medication treatments.
Adherence with CPD medication in the past week and the past month (represented as proportion or percentage of days with a missed medication dose) was assessed with the Tablets Routine Questionnaire (TRQ).Reference Scott and Pope13,Reference Peet and Harvey14 The TRQ ranges from 0% (missed no medications) to 100% (missed all medications). The ten-item Drug Attitudes Inventory (DAI)Reference Awad15 assessed attitudes toward CPD medications, with scoring calibrated on a 0 (worse attitudes) to 10 (best attitudes) spectrum. CPD symptoms were assessed with the Brief Psychiatric Rating Scale (BPRS).Reference Overall and Gorham16 Global psychopathology was assessed with the Clinical Global Impressions scale (CGI).Reference Guy17 Life and work functional status was assessed with the Social and Occupational Functioning Scale (SOFAS).Reference Morosini, Magliano, Brambilla, Ugolini and Pioli18 Substance use or misuse was assessed with the Alcohol Use Disorders Identification Test (AUDIT)Reference Saunders, Aasland, Babor, Grant and Fuente19 and the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST).20 Although there is overlap between these two substance use instruments, the AUDIT specifically focuses on alcohol use patterns whereas the ASSIST evaluates a range of substances, including alcohol, tobacco, cannabis, cocaine, amphetamines, inhalants, sedatives and hallucinogens.
Medication adherence barriers
In preparation for a future phase of this research project involving pilot testing of an adherence promotion intervention,Reference Mbwambo, Kaaya, Lema, Blixen, Cassidy and Levin12 we assessed study participants on four main barriers to medication adherence: inadequate or incorrect understanding of CPD medication and consequences of missing medication, non-adherence related to substance use, limited communication with providers to address management of feared or experienced side-effects, and erratic medication-taking and/or lack of medication routines. The focus on these barriers and assessment process, drawn from iterative pilot work,Reference Jenkins, Strauss, Carpenter, Miller, Floersch and Sajatovic5,Reference Sajatovic, Levin, Ramirez, Hahn, Tatsuoka and Bialko21–Reference Sajatovic, Levin, Tatsuoka, Micula-Gondek, Fuentes-Casiano and Bialko23,Reference Edge, Degnan, Cotterill, Berry, Baker and Drake40 assessed an individual's specific attitudinal adherence barriers with selected items from the Rating of Medication Influences (ROMI)Reference Weiden, Rapkin, Mott, Zygmunt, Goldman and Horvitz-Lennon24 and the Attitudes Toward Mood Stabilizers Questionnaire (AMSQ).Reference Harvey25,Reference Adams and Scott26
Data analysis
We conducted descriptive statistics to characterise demographic and clinical variables, including the number of barriers to adherence. Because gender differences have been documented in treatment adherence and other variables among patients with CPD,Reference Abel, Drake and Goldstein27 we compared clinical characteristics of men and women in this sample, using chi-squared and two-tailed t-tests, reporting effect sizes. Correlational analyses with Spearman correlations (because of the non-normal distribution of lifetime hospital admissions) were conducted to evaluate the association between TRQ and demographic and clinical variables, as well as the relationship between lifetime number of hospital admissions for psychiatric illness and demographic and clinical variables. A multiple linear regression analysis was performed to assess the effects of in-patient versus out-patient status and DAI, BPRS, SOFAS and AUDIT scores on adherence over the past month, as measured by the TRQ.
Results
Sample description
Table 1 shows demographic and clinical variables in this poorly adherent sample. Notably, this was a relatively young sample (mean age 35.70 years, s.d. 8.80), with a slight preponderance of men (61%). The majority (80%) had schizophrenia, with an average age at onset of 22.38 (s.d. 7.64) years. The great majority of individuals lived with family (84%) and just over half (57%) had children. Most were in-patients (83%) at the time of assessment, and had an average of 4.72 (4.30) previous hospital admissions for psychiatric illness. Most (>80%) were prescribed first-generation antipsychotic drugs, with oral haloperidol being the most common medication therapy. Family history of mental illness and substance misuse were common, at rates of 53% and 43%, respectively. Compared with men, women with CPD were less likely to live with family (P < 0.01, w = 1.185), more likely to have a mood component to their CPD (schizoaffective diagnosis, P = 0.03, w = 0.706), had fewer lifetime hospital admissions for psychiatric illness (P = 0.02, d = 0.501) and had a greater likelihood of past sexual abuse (P = 0.01, w = 0.602).
Table 1 Demographic and clinical characteristics of poorly adherent individuals with CPD in Dar es Salaam, Tanzania

CPD, chronic psychiatric disorder.
a. Two-tailed t-test or chi-squared comparison between males and females.
Table 2 shows scores on standardised rating scales in the sample. The proportion of missed CPD medication in the past week/month was extensive, with patients missing an average of nearly two-thirds of prescribed medication in the past month. CPD symptom scores were relatively low. Approximately three-quarters of the sample were at low risk for having problems with substance misuse; however, one in ten had alcohol dependence, which was the most common substance of misuse. Substances other than alcohol and tobacco were not widely used. Based on the AMSQ and ROMI assessments, the most common adherence barrier domains were inadequate or incorrect understanding of the CPD and poor communication with providers. Approximately one in three had adherence barriers related to the use of substances.
Table 2 Standardised rating scale scores of poorly adherent Tanzanian patients with CPD
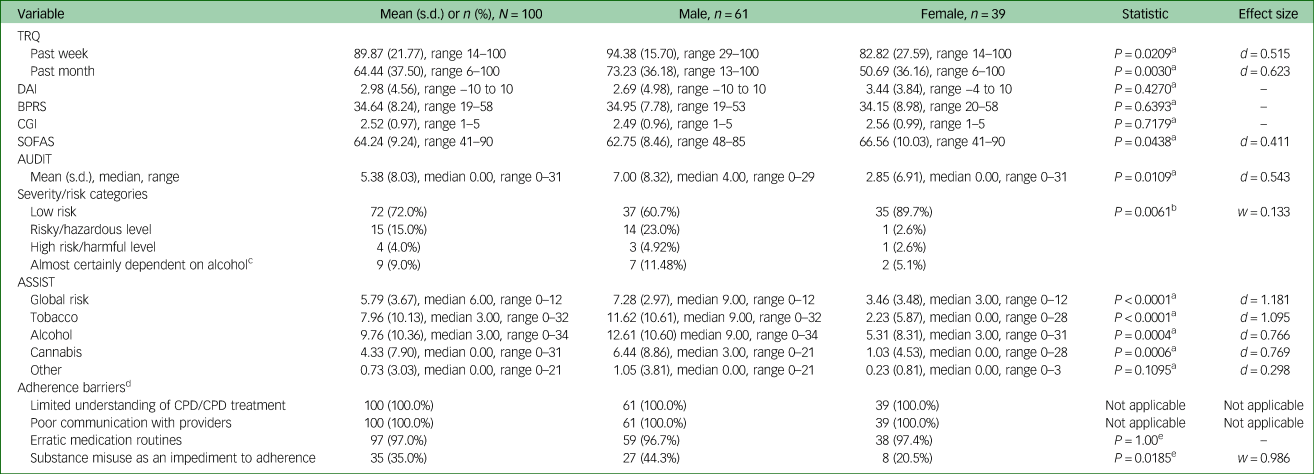
CPD, chronic psychotic disorder; TRQ, Tablets Routine Questionnaire; DAI, Drug Attitudes Inventory; BPRS, Brief Psychiatric Rating Scale; CGI, Clinical Global Impressions scale; SOFAS, Social and Occupational Functioning Scale; AUDIT, Alcohol Use Disorders Identification Test; ASSIST, Alcohol, Smoking and Substance Involvement Screening Test.
a. Independent samples t-test.
b. Fisher's exact test.
c. If AUDIT total score is ≥20 and the sum of questions 4–6 are ≥4, it is likely the person is almost certainly dependent on alcohol.
d. Adherence barriers (range 1–4) identified as a focus of treatment in the customised adherence enhancement (CAE curriculum).
e. Chi-squared.
Adherence correlates
Table 3 shows correlates of adherence over the past month, as measured by the TRQ. Demographic and most clinical variables were not significantly associated with TRQ levels; however, in-patients with CPD were more likely to have worse adherence (r = −0.26, P ≤ 0.01), as were individuals with worse drug attitudes (r = −0.28, P < 0.01), higher CPD symptom severity levels (r = 0.36, P < 0.001), worse functioning (r = −0.21, P < 0.05) and higher-risk use of alcohol (AUDIT score, r = 0.34, P < 0.001).
Table 3 Spearman correlations between adherence over the past month (measured by TRQ), demographics and standardised rating scales

TRQ, Tablets Routine Questionnaire; DAI, Drug Attitudes Inventory; BPRS, Brief Psychiatric Rating Scale; CGI, Clinical Global Impressions scale; SOFAS, Social and Occupational Functioning Scale; AUDIT, Alcohol Use Disorders Identification Test.
a. Marital status categories were collapsed such that ‘not married’ included those who were single/never married as well as those who were separated, divorced or widowed.
b. Ninety-nine participants completed the survey.
c. In-patient status is significantly correlated with a higher (worse) TRQ, more negative medication attitudes (lower DAI score) are associated with higher (worse) TRQ, more severe psychiatric symptoms are associated with higher (worse) TRQ, worse (lower) social functioning is associated with higher (worse) TRQ, more substance use problems are associated with higher (worse) TRQ.
Using the five significant predictors at the bivariate level, we tested a multiple linear regression to determine which predictors contributed the most to adherence. In-patient status (standardised β = −0.219, unstandardised β = −21.872, s.e. 0.088, P < 0.05), worse symptom severity (standardised β = 0.211, unstandardised β = −1.529, s.e. 0.103, P < 0.05) and higher-risk use of alcohol (standardised β = 0.241, unstandardised β = 1.130, s.e. 0.092, P < 0.05) were found to be associated with higher TRQ scores for adherence over the past month. DAI and SOFAS scores were not significant. The model explained 26.1% of the variance in adherence.
Hospital admission correlates
Tables 4 and 5 show correlates of lifetime number of hospital admissions for demographic/clinical variables and standardised ratings, respectively. As seen in Table 4, males (P = 0.038), those with an earlier age at onset (P = 0.049), longer illness duration (P < 0.001) and those with children (P = 0.047) had significantly more hospital admissions for psychiatric illness. BPRS, CGI, AUDIT, DAI and TRQ scores were not associated with number of lifetime hospital admissions. Table 5 shows results of the multiple linear regression analysis assessing the effects of in-patient versus out-patient status and DAI, BPRS, SOFAS and AUDIT scores on adherence over the past month, as measured by the TRQ. In the linear regression analysis, there was a significant association between adherence and in-patient status, BPRS and AUDIT scores.
Table 4 Spearman correlations between demographic variables and number of lifetime hospital admissions for psychiatric illness (N = 100)

Men had significantly more hospital admissions compared with women. Those with children were more likely to have hospital admissions versus those without children. Those with earlier age at onset and longer duration of illness had significantly more hospital admissions for psychiatric illness.
a. Collapsed variable to dichotomous format.
Table 5 Associations from multiple linear regression models for past month medication adherence (TRQ)

TRQ, Tablets Routine Questionnaire; DAI, Drug Attitudes Inventory; BPRS, Brief Psychiatric Rating Scale; SOFAS, Social and Occupational Functioning Scale; AUDIT, Alcohol Use Disorders Identification Test.
Discussion
The cross-sectional assessment of poorly adherent patients with CPD characterised demographic and clinical variables associated with medication adherence. It must be noted that the sample was specifically enrolled because they were known to be poorly adherent, and findings may not generalise to the broader population of people with CPD in this setting, including those who are adherent with prescribed medications. For the most part, and aligned with reports from settings outside of sub-Saharan Africa,Reference Semahegn, Torpey, Manu, Assefa, Tesfaye and Ankomah6 a number of demographic and clinical variables were not significantly associated with adherence levels; however, those with the worst adherence were most likely to be in-patients with more severe CPD symptoms, have substance use comorbidity and endorse negative attitudes toward CPD medications. Most individuals appear to have multiple barriers to adherence, including attitudinal characteristic that may be a potential target of future interventions.
Reasons for poor adherence in Tanzania include low health literacy, lack of access to medicines and structural factors (e.g. long distances to clinics to collect medicines, clinic overcrowding and low proportion of staff to patients). Similar to our sample, a review by ChidarikireReference Chidarikire, Cross, Skinner and Cleary1 noted that the majority of people with schizophrenia were treated with first-generation antipsychotics that may cause motor and neurological side-effects, which are an impediment to adherence.Reference Esan28–Reference Van Rensburg and Oloruniu30 Although this analysis did not quantitatively assess for medication side-effects or medication preferences, recent qualitative work conducted by this team on a subset of patients in this sample found that burdensome side-effects with prescribed antipsychotic drugs included weight gain, weakness/tiredness and loss of libido,Reference Blixen, Lema, Mbwambo, Kaaya, Levin and Sajatovic31 all negatively affecting treatment engagement and adherence. Accommodating patient preferences for specific treatments may be particularly challenging in settings where fewer types of medication options might be practical and available.
The average rate of missed CPD medication in this Tanzanian sample was 64%, with an average of four or five past hospital admissions for psychiatric illness in just over a decade of having CPD. Other reports have documented the close association between poor adherence and poor outcomes, including relapse, leading to hospital admission and treatment-resistant illness.Reference Phan32,Reference Bozzatello, Bellino and Rocca33 The findings from this sample suggest that most poorly adherent patients experience barriers to adherence in multiple domains, and it seems likely that care approaches will need to target patient attitudes/beliefs about medication, communication with providers to address treatment expectations and discussions regarding side-effects, and managing medication in the context of lifestyle and routines.
The in-patient setting may be an appropriate place to identify individuals at highest risk of poor adherence, and this might be an opportunity to provide education on the role of CPD medication in recovery. This may also be an opportunity to implement long-acting injectable antipsychotic medication for individuals who have a known history of poor adherence or those who miss medication as a result of forgetting. Recent studies have suggested that long-acting injectable antipsychotic medication can significantly reduce relapse rates among individuals with schizophrenia.Reference Tiihonen, Mittendorfer-Rutz and Majak34
Adherence promotion approaches in sub-Saharan Africa and elsewhere also need to consider comorbidity as an impediment to optimal medication adherence. At least one in ten poorly adherent individuals with CPD in our sample had alcohol dependence, and about one in three had clinically relevant problems with use/misuse of substances. Thus, adherence and recovery efforts ideally need to include treatment for substance use disorders as part of a comprehensive package of care.
Our analysis found gender differences in selected clinical variables, including health resource use, with men more likely to be admitted to hospital. Differences in premorbid or early illness progression, also reported in the literature, might explain the fact that men had more lifetime hospital admissions compared with women.Reference Abel, Drake and Goldstein35,Reference Leung and Chue36 It is possible that addressing treatment adherence soon after CPD diagnosis might help with reducing hospital admissions, a particularly important goal in low-resource settings.
An additional factor that needs to be considered is the incorporation of family challenges and support in helping patients to self-manage treatment and adherence. The overwhelming majority (84%) of individuals in this sample lived with family, and more than half had children of their own. Substance misuse and family history of both mental illness and substance misuse were common, and a pragmatic consideration would be to address the issue of mental health from an intergenerational perspective. Although our study did not find any correlation between living with family or living alone and treatment non-adherence, a Tanzanian qualitative study conducted by Sariah et alReference Sariah, Outwater and Malima10 suggested that family and peer support, employment and religion were perceived as ‘protective’ elements to minimise patient risk of relapse episodes. Family therapies, including psychoeducation, have been demonstrated as improving outcomes and family burden in CPD,Reference McFarlane37–Reference Edge, Degnan, Cotterill, Berry, Baker and Drake40 and culturally informed approaches could be helpful as an augmentation to adherence-promotion interventions.
This study has a number of methodological limitations that must be considered with respect to both interpretation and generalisability of findings. We did not survey an adherent group of patients, and findings on poorly adherent patients may not apply to those across the full range of adherence. Given the severe paucity of data on antipsychotic drug adherence in sub-Saharan African populations with CPD, this study was exploratory and not designed or intended to be powered to examine gender differences in CPD or the specific variables that might be associated with worse adherence, such as substance use or personal trauma history. Given this exploratory nature, we did not control for multiple comparisons, and the cross-sectional design does not permit for conclusion of causal inferences. The study methods also do not permit a clear assessment of structural barriers to adherence, which are noted in the review by Chidarikire et alReference Chidarikire, Cross, Skinner and Cleary1 to include poor access to care as a result of poverty or other factors, lack of education or poor health literacy and problems in patient–clinician communication. These factors need to be more specifically addressed in future investigations.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1192/bjo.2020.141.
Data availability
Scientists interested in accessing the data should reach out to the lead author (M.S.). Data access is subject to national USA and Ugandan data use agreement terms.
Author contributions
M.S., S.K., J.M., C.B., J.B.L., I.L. and K.A.C. contributed to either the initial design or refinement of the project design, as well as project implementation and completion. C.J.B., M.E.A., B.W. and G.N. contributed to project implementation, completion and data analysis. All authors have approved the manuscript, and the report is a reflection of team consensus.
Funding
This study was supported by the National Institute of Mental Health (NIMH) grant NIMH MH114700.
Declaration of interest
M.S. has received research grants from Otsuka, Alkermes, Janssen, Reuter Foundation, Woodruff Foundation, Reinberger Foundation, National Institutes of Health, Centers for Disease Control and Prevention and International Society of Bipolar Disorders, in the past 3 years; is a consultant for Bracket, Otsuka, Sunovion, Neurocrine, Supernus and Health Analytics; has received royalties from Springer Press, Johns Hopkins University Press, Oxford Press and UpToDate; and has participated in Continuing Medical Education activities with American Physician's Institute, MCM Education, CMEology, Potomac Center for Medical Education, Global Medical Education and Creative Educational Concepts. All other authors have nothing to disclose.
ICMJE forms are in the supplementary material, available online at https://doi.org/10.1192/bjo.2020.141.









eLetters
No eLetters have been published for this article.