Introduction
Attention-deficit/hyperactivity disorder (ADHD) has a prevalence of > 5% in children and adolescents (Thomas, Sanders, Doust, Beller, & Glasziou, Reference Thomas, Sanders, Doust, Beller and Glasziou2015). It is characterized by age-inappropriate symptoms of inattention, hyperactivity, and impulsivity (American Psychiatric Association, 2013). Moreover, ADHD is associated with an increased risk of psychiatric comorbidities such as depression and conduct disorders (Feldman & Reiff, Reference Feldman and Reiff2014). Although the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) provides diagnostic criteria for ADHD based on symptoms, significant neurobiological and cognitive heterogeneity remains. Psychoradiology applies medical imaging technologies to psychiatry and promises not only to improve insight into structural and functional brain abnormalities in patients with psychiatric disorders but also to have potential clinical utility (Luo, You, DelBello, Gong, & Li, Reference Luo, You, DelBello, Gong and Li2022). Previous studies using magnetic resonance imaging (MRI) and cognitive function assessment have not always yielded consistent results, perhaps because of the heterogeneity, although they have tried to clarify the underlying ADHD pathology.
Previous studies revealed smaller gray matter volumes (GMVs) in the frontal lobe and basal ganglia in patients with ADHD than in healthy individuals (Bonath, Tegelbeckers, Wilke, Flechtner, & Krauel, Reference Bonath, Tegelbeckers, Wilke, Flechtner and Krauel2018; Klein et al., Reference Klein, Souza-Duran, Menezes, Alves, Busatto and Louzã2021; Moreno-Alcázar et al., Reference Moreno-Alcázar, Ramos-Quiroga, Radua, Salavert, Palomar, Bosch and Clotet2016; Villemonteix et al., Reference Villemonteix, De Brito, Kavec, Balériaux, Metens, Slama and Massat2015). Nonetheless, other studies found larger GMVs in the prefrontal-temporal-parietal areas in patients with ADHD than in healthy individuals (Seidman et al., Reference Seidman, Biederman, Liang, Valera, Monuteaux, Brown and Makris2011; Semrud-Clikeman, Pliszka, Bledsoe, & Lancaster, Reference Semrud-Clikeman, Pliszka, Bledsoe and Lancaster2014; Wu et al., Reference Wu, Llera, Hoogman, Cao, Zwiers, Bralten and Wang2019). Moreover, larger ADHD studies on brain structure generally reported small effect sizes (Nakao, Radua, Rubia, & Mataix-Cols, Reference Nakao, Radua, Rubia and Mataix-Cols2011; Postema et al., Reference Postema, Hoogman, Ambrosino, Asherson, Banaschewski, Bandeira and Francks2021). This discrepancy between previous studies might reflect the diversity of the disorders themselves; ADHD is not a single pathology but an aggregate of heterogeneous pathologies.
Based on Barkley's theory, a growing body of evidence suggests various deficits in cognitive function, including executive function, in ADHD (Barkley, Reference Barkley1997). These studies have reported that patients with ADHD show impairments of response inhibition (Breitling-Ziegler, Tegelbeckers, Flechtner, & Krauel, Reference Breitling-Ziegler, Tegelbeckers, Flechtner and Krauel2020; Castellanos, Sonuga-Barke, Milham, & Tannock, Reference Castellanos, Sonuga-Barke, Milham and Tannock2006), non-verbal and verbal working memory (Anker, Ogrim, & Heir, Reference Anker, Ogrim and Heir2022; Blomberg, Johansson Capusan, Signoret, Danielsson, & Rönnberg, Reference Blomberg, Johansson Capusan, Signoret, Danielsson and Rönnberg2021), processing speed (Castellanos et al., Reference Castellanos, Sonuga-Barke, Milham and Tannock2006; Mohamed et al., Reference Mohamed, Butzbach, Fuermaier, Weisbrod, Aschenbrenner, Tucha and Tucha2021), and speech production (Blomberg, Danielsson, Rudner, Söderlund, & Rönnberg, Reference Blomberg, Danielsson, Rudner, Söderlund and Rönnberg2019). Although various executive function factors have been associated with ADHD, the evidence is not always consistent (Lacerda et al., Reference Lacerda, Martínez, Franz, Moreira-Maia, Silveira, Procianoy and Wagner2020; Salari, Bohlin, Rydell, & Thorell, Reference Salari, Bohlin, Rydell and Thorell2017; Willcutt, Doyle, Nigg, Faraone, & Pennington, Reference Willcutt, Doyle, Nigg, Faraone and Pennington2005). One possible reason is the difference in executive function domains, such as updating, shifting, and inhibition (or common executive function), according to some models (Friedman et al., Reference Friedman, Miyake, Young, DeFries, Corley and Hewitt2008; Miyake et al., Reference Miyake, Friedman, Emerson, Witzki, Howerter and Wager2000). Another possibility is the diversity of the executive function patterns. One study reported an association of inhibitory function with inattention and hyperactivity/impulsivity in ADHD (Cai et al., Reference Cai, Warren, Duberg, Yu, Hinshaw and Menon2023); however, another study only observed such symptom association with working memory deficit (Salari et al., Reference Salari, Bohlin, Rydell and Thorell2017). These findings suggest that ADHD results from underlying deficits in some executive function patterns, and such domains may help to clarify the diversity and pathogenesis of ADHD. Elucidating the neural structural system of cognitive function, including executive function, subtypes of ADHD may contribute to our understanding of inconsistent findings. However, investigations of the brain structure of ADHD subtypes have been limited to clinical symptom-related subtypes (Qureshi, Min, Jo, & Lee, Reference Qureshi, Min, Jo and Lee2016; Saad, Griffiths, & Korgaonkar, Reference Saad, Griffiths and Korgaonkar2020; Serrallach, Groß, Christiner, Wildermuth, & Schneider, Reference Serrallach, Groß, Christiner, Wildermuth and Schneider2022), and little is known about ADHD subtypes classified by cognitive function domains.
This study aimed to clarify this gap in the literature by investigating whether ADHD subtypes based on cognitive function domains, including executive function, can have different effects on the brain structure of affected individuals. The present findings may shed new light on ADHD heterogeneity and its pathogenesis. A better understanding of such heterogeneity in ADHD may be helpful for considering outcomes/prognoses or treatment planning.
Methods
Participants
The Adolescent Brain Cognitive Development (ABCD) Study is the largest longitudinal study examining child brain development and mental health in the United States (Jernigan, Brown, & ABCD Consortium Coordinators, Reference Jernigan and Brown2018). Recruitment began in 2016 and ended in 2018; however, the study is ongoing and actively collecting longitudinal data. Full recruitment details of the ABCD Study have been published previously (Garavan et al., Reference Garavan, Bartsch, Conway, Decastro, Goldstein, Heeringa and Zahs2018). Briefly, the ABCD Study primarily recruited participants through elementary schools, both public (including charter schools) and private (Garavan et al., Reference Garavan, Bartsch, Conway, Decastro, Goldstein, Heeringa and Zahs2018). The dataset consisted of 11 878 adolescents aged 9 to 11 years (mean age, 9.91 years [min, 8.91; max, 11.08]) recruited from 21 data collection sites, namely Oregon Health and Science University, Stanford Research Institute, Children's Hospital Los Angeles, University of California at Los Angeles, University of California San Diego, University of Utah, University of Colorado Boulder, Laureate Institute for Brain Research, University of Minnesota, Washington University, University of Florida, Florida International University, University of Wisconsin-Milwaukee, University of Michigan, Medical University of South Carolina, University of Pittsburgh, Virginia Commonwealth University, University of Maryland at Baltimore, University of Rochester, Yale University, and University of Vermont, as previously reported (Cheng et al., Reference Cheng, Rolls, Gong, Du, Zhang, Zhang and Feng2021; Hiraoka, Makita, Hamatani, Tomoda, & Mizuno, Reference Hiraoka, Makita, Hamatani, Tomoda and Mizuno2023; Wiker et al., Reference Wiker, Norbom, Beck, Agartz, Andreassen, Alnæs and Tamnes2023; Zhang et al., Reference Zhang, Huang, Jiao, Yuan, Hu, Yang and Huang2022a). The present study mainly used data from the ABCD 2.0 release. All parents provided written informed consents, and all children provided assent. All procedures complied with the Declaration of Helsinki. The Research Ethics Committee of the University of Fukui approved the data analysis (Assurance No. FU-20210067).
The variables used in this study are shown in online Supplementary Table S1, and we used the baseline data, e.g., the initial data collected at the beginning of the ABCD Study, for analysis. First, the current ADHD/non-ADHD diagnoses were based on the computerized Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) from the parents. The current episode diagnoses were based on computer self-administered K-SADS which shows high concordance rates (percent agreement in diagnostic categories: 88–96%) with the clinician-administered paper-and-pencil version of the K-SADS (Kobak, Kratochvil, Stanger, & Kaufman, Reference Kobak, Kratochvil, Stanger and Kaufman2013). The diagnoses were extracted using the identifier marked with ‘1’ as an ADHD diagnosis and marked with ‘0’ as a non-ADHD diagnosis, as previously reported (Bernanke et al., Reference Bernanke, Luna, Chang, Bruno, Dworkin and Posner2022; Kim et al., Reference Kim, Kim, Pack, Lim, Cho and Lee2023). A total of 3372 participants with no data on diagnosis at baseline were excluded. Second, quality control of structural imaging data and FreeSurfer cortical surface reconstructions were performed manually by the ABCD team, and 188 participants who had no T1 quality checks or imaging data were excluded. Third, duplicate participants caused by the binding of all data tables were removed (n = 501). After the primary data-cleaning process (n = 7817), missing values were excluded from the current ADHD/non-ADHD diagnoses (n = 106). As reported in previous studies (Ma et al., Reference Ma, Wang, Rolls, Xiang, Li, Li and Li2022; Zhao et al., Reference Zhao, Voon, Zhang, Shen, Zhang and Feng2022), we excluded missing values from the National Institutes of Health (NIH) Toolbox Tasks (n = 147) because the imputation approach may have caused bias, such as cluster bias, in the results (van Rossum, da Silva, Wang, Kouwenhoven, & Hermens, Reference van Rossum, da Silva, Wang, Kouwenhoven and Hermens2023). Moreover, we excluded cases ineligible for the T1 quality check (n = 307), which were extracted using the identifier marked with ‘0’ as unacceptable imaging results by the ABCD team. The final analysis included 7257 participants (see online Supplementary Fig. S1 for a flowchart of the sampling procedure). Statistical analyses, including data cleaning (R package ‘dplyr’), were conducted using R (version 4.3.0; The R Foundation for Statistical Computing, Vienna, Austria).
Demographic variables and covariates
The following covariates were included as categorical variables and dummy-coded: sex, handedness, race/ethnicity (White, Black, Hispanic, Asian, and other), and medication use. Based on previous studies (Hamatani, Hiraoka, Makita, Tomoda, & Mizuno, Reference Hamatani, Hiraoka, Makita, Tomoda and Mizuno2022; Hiraoka et al., Reference Hiraoka, Makita, Hamatani, Tomoda and Mizuno2023; Paul et al., Reference Paul, Hatoum, Fine, Johnson, Hansen, Karcher and Bogdan2021), annual household income was treated as a five-level categorical variable (Table 1). The following covariates were included as continuous variables: age, parental education level, pubertal status, and total intracranial volume. Parental educational level was recorded as follows: 12th grade, high school, and general education, 12 years; college and associate degrees, 14 years; bachelor's degree, 16 years; master's degree, 18 years; and professional and doctoral degrees, 20 years. The pubertal development scale was used to assess pubertal status (Petersen, Crockett, Richards, & Boxer, Reference Petersen, Crockett, Richards and Boxer1988). It was completed by both a parent or guardian and the participant, and the results of the two scores were averaged. The abovementioned covariates were selected based on previous ABCD-based studies (Bernanke et al., Reference Bernanke, Luna, Chang, Bruno, Dworkin and Posner2022; Hamatani et al., Reference Hamatani, Hiraoka, Makita, Tomoda and Mizuno2022; Hiraoka et al., Reference Hiraoka, Makita, Hamatani, Tomoda and Mizuno2023; Owens et al., Reference Owens, Allgaier, Hahn, Yuan, Albaugh, Adise and Garavan2021).
Table 1. Baseline demographics of the ADHD and non-ADHD groups

ADHD, attention-deficit/hyperactivity disorder; CBCL, Child Behavior Checklist; s.d., standard deviation.
Data are presented as the mean (s.d.) or n (%). p values for age, education, puberty, income, and CBCL scores are from t tests for group differences. p values for sex ratio and race/ethnicity ratio are from χ2 tests for group differences.
For additional demographic clinical variables, ADHD symptoms and psychiatric onset risk were assessed using the six DSM-5-oriented syndrome scales (symptoms of ADHD, depression, anxiety disorder, conduct disorder, oppositional defiant disorder, and somatic disorder) of the Child Behavior Checklist (CBCL) (Achenbach & Rescorla, Reference Achenbach and Rescorla2001). We used T scores calculated by the ABCD team, with higher scores representing greater behavioral problems.
Behavioral measurements and statistical analysis
NIH Toolbox Tasks, measured using an iPad-based program, were used to classify ADHD subtypes based on cognitive function domains, including executive function. The cognition battery comprised seven measures to assess five domains: executive function and attention (also known as cognitive control; using flanker inhibitory control and attention and dimensional change card sort), processing speed (using pattern comparison processing speed), working memory (using list sorting working memory), episodic memory (using picture sequence memory), and language (using picture vocabulary and oral reading recognition) (Fox, Manly, Slotkin, Devin Peipert, & Gershon, Reference Fox, Manly, Slotkin, Devin Peipert and Gershon2021; Nolin et al., Reference Nolin, Cowart, Merritt, McInerney, Bharadwaj, Franchetti and Visscher2023; Ott et al., Reference Ott, Schantell, Willett, Johnson, Eastman, Okelberry and May2022; see online Supplementary Methods S1 for the details of each task). Age-corrected scores were used, and the composite score of cognitive control was created by adding the flanker inhibitory control and attention and dimensional change card sort scores, whereas the composite score of language was created by adding the picture vocabulary and oral reading recognition scores, according to previous studies on the classification of the cognitive function domains of the NIH Toolbox (Nolin et al., Reference Nolin, Cowart, Merritt, McInerney, Bharadwaj, Franchetti and Visscher2023; Ott et al., Reference Ott, Schantell, Willett, Johnson, Eastman, Okelberry and May2022). Outliers were Winsorised at three standard deviations from the mean (R package ‘DescTools’).
Behavioral data were analyzed using unsupervised machine learning to classify the ADHD subtypes. This is based on previous studies that applied unsupervised machine learning to classify patient subtypes based on molecular and brain functions (Williams et al., Reference Williams, Bednarski, Pieszko, Miller, Kwiecinski, Shanbhag and Slomka2023; Yu, Wang, Ge, & Shi, Reference Yu, Wang, Ge and Shi2022; Zhang, Manza, & Volkow, Reference Zhang, Manza and Volkow2022b). Moreover, cluster analysis in this study was restricted to participants with ADHD as well as methods in studies (Scheerer et al., Reference Scheerer, Curcin, Stojanoski, Anagnostou, Nicolson, Kelley and Stevenson2021; Yu et al., Reference Yu, Wang, Ge and Shi2022). Thereafter, data were compared between each identified ADHD subtype and the non-ADHD group. To mitigate distance-associated issues in the cluster analysis, the behavioral scores were converted to z scores and hierarchically clustered to identify patterns of cognitive domains in this ADHD sample. The similarity between observations was calculated as the Euclidean distance and then clustered into distinct shapes followed by clustering based on magnitude using Ward's D2 method (R package ‘NbClust’). Based on the Ball-Hall index (Ball & Hall, Reference Ball and Hall1965), the number of clusters was automatically determined by the system. The support vector machine (SVM; R package ‘e1071’) supporting multi-class classifiers with one-v.-one approach was used to verify the reliability and reproducibility of the subtyping based on cognitive function domains. This simulation learning was repeated 1000 times for each training dataset (75% of the 656 participants with ADHD, n = 492) and test dataset (25% of the 656 participants with ADHD, n = 164). The uniform manifold approximation and projection (UMAP; R packages ‘umap’ and ‘ggplot2’) algorithm applying the Euclidean distance was used to visualize the cluster plot.
To assess cognitive features by comparing each ADHD subtype with the non-ADHD group, a linear mixed-effects model (R packages ‘lmerTest’, ‘MuMIn’, and ‘jtools’) was used with each cognitive function as the dependent variable and group (e.g. ADHD-A v. non-ADHD) as the independent variable. Based on previous studies (Hamatani et al., Reference Hamatani, Hiraoka, Makita, Tomoda and Mizuno2022; Hiraoka et al., Reference Hiraoka, Makita, Hamatani, Tomoda and Mizuno2023), family ID (used to denote sibling status), multiple data collection sites, and twin or triplet status were modeled as random effects. Covariates included the abovementioned variables. The statistical threshold was set at p < 0.05, false discovery rate (FDR)-corrected using the Benjamini–Hochberg method. Thereafter, corrections for the family-wise error (FWE, p < 0.05) rate were performed using the Bonferroni method for multiple group comparisons. For additional analyses using the linear mixed-effects model adjusted for comorbidities, see online Supplementary Methods S2.
Structural data and statistical analysis
Scanning was performed using three 3 T MR scanners (Siemens, General Electric 750, and Philips) to obtain high-resolution T1-weighted three-dimensional structural images (1 mm isotropic) with acquisition parameters as described previously (Casey et al., Reference Casey, Cannonier, Conley, Cohen, Barch and Heitzeg2018). Structural data were preprocessed by the ABCD data team using the standard morphometric pipeline (e.g. skull-stripping, white matter segmentation) in FreeSurfer software (version 5.3.0) (Hagler et al., Reference Hagler, Hatton, Cornejo, Makowski, Fair, Dick and Dale2019). We used 34 regions labeled with the Desikan atlas-based classification for cortical regional volume and six regions labeled with atlas-based segmentation for subcortical regional volume (68 and 12 regions in total, respectively). Outliers were Winsorised at three standard deviations from the mean.
To assess brain structural characteristics by comparing each ADHD subtype with the non-ADHD group, a linear mixed-effects model was used with each regional brain volume as the dependent variable and group (e.g. ADHD-A v. non-ADHD) as the independent variable. In addition to multiple data collection sites and twin or triplet status, we included the family ID as a random effect nested inside a random effect of the MRI scanner to account for the large number of siblings and multiple data collection sites, as recommended by previous studies (Bernanke et al., Reference Bernanke, Luna, Chang, Bruno, Dworkin and Posner2022; Hagler et al., Reference Hagler, Hatton, Cornejo, Makowski, Fair, Dick and Dale2019; Heeringa & Berglund, Reference Heeringa and Berglund2020; Owens et al., Reference Owens, Allgaier, Hahn, Yuan, Albaugh, Adise and Garavan2021). The reason is the characteristics of the protocols in the ABCD Study: the data collection includes the recruitment of multiple students from schools, multiple children recruited from the same family, multiple children imaged on the same MRI scanner, and the use of multiple scanners at the same site (Heeringa & Berglund, Reference Heeringa and Berglund2020). Moreover, covariates included the abovementioned variables and total intracranial volume. The statistical threshold was set at p < 0.05, FDR-corrected using the Benjamini–Hochberg method. Thereafter, corrections for the FWE (p < 0.05) rate were performed using the Bonferroni method for multiple group comparisons. For additional analyses using the linear mixed-effects model adjusted for comorbidities, see online Supplementary Methods S2. Furthermore, based on differences in brain structural and cognitive functional results between groups (e.g. ADHD-C v. non-ADHD), we investigated associations between region-of-interest volumes and specific cognitive domains, using Pearson's correlation analyses (R package ‘psych’). The statistical threshold was set at p < 0.05, FDR-corrected using the Benjamini–Hochberg method.
Results
Demographics and cognitive function pattern clustering
Baseline demographic data of 7257 children (6601 non-ADHD and 656 with ADHD) were obtained (Table 1). Hierarchical cluster analysis identified three clusters based on the five cognitive domains (Fig. 1a). A scatterplot of the three clusters obtained using the UMAP algorithm is shown in Fig. 1b. The three subtypes were named ADHD-A (n = 212), ADHD-B (n = 190), and ADHD-C (n = 254). In subsequent SVM algorism, the result showed high prediction accuracy and recall in the test dataset (accuracy, 0.93 ± 0.02; precision, 0.92 ± 0.04; recall, 0.88 ± 0.05; F1 score, 0.90 ± 0.03), suggesting that the cognitive function model may accurately identify the true positive class. The demographic characteristics of each subtype are shown in online Supplementary Result 1 and Supplementary Table S2.

Figure 1. Cognitive function characteristics of ADHD subtypes based on the National Institutes of Health Toolbox Tasks. (a) Hierarchical cluster analysis identified three clusters based on NIH Toolbox Tasks. (b) Scatterplot performed by UMAP applying the Euclidean distance to visualize the three clusters. (c) Based on FDR-corrected and FWE-corrected thresholds (p < 0.05), the ADHD-A type showed better cognitive control, processing speed, working memory, and episodic memory than the non-ADHD group. The ADHD-B type showed poorer performances in cognitive control, processing speed, and episodic memory than the non-ADHD group. The ADHD-C type showed poorer performances in cognitive control, working memory, episodic memory, and language ability than the non-ADHD group. Parameters are indicated as the mean (s.d.). *** FDR-p < 0.001. ADHD, attention-deficit/hyperactivity disorder; FDR, false discovery rate; FWE, family-wise error; SD, standard deviation; UMAP, uniform manifold approximation and projection.
Cognitive features
As shown in Fig. 1c and Table 2, the main effect of group in the linear mixed-effects model showed that the ADHD-A type had significantly higher levels of cognitive control, processing speed, working memory, and episodic memory (FDR, p < 0.001 for each variable and FWE, p < 0.001 for each variable) than the non-ADHD group. Moreover, the main effect of group showed that the ADHD-B type had significantly lower levels of cognitive control, processing speed, and episodic memory (FDR, p < 0.001 for each variable and FWE, p < 0.001 for each variable) than the non-ADHD group. Furthermore, the main effect of group showed that the ADHD-C type had lower levels of cognitive control, working memory, episodic memory, and language (FDR, p < 0.001 for each variable and FWE, p < 0.001 for each variable) than the non-ADHD group. For significance comparing each ADHD subtype and the non-ADHD group adjusted for comorbidities, see online Supplementary Results 2, Supplementary Table S3, and Supplementary Fig. S2.
Table 2. Differences in behavioral results between each ADHD subtype and the non-ADHD group
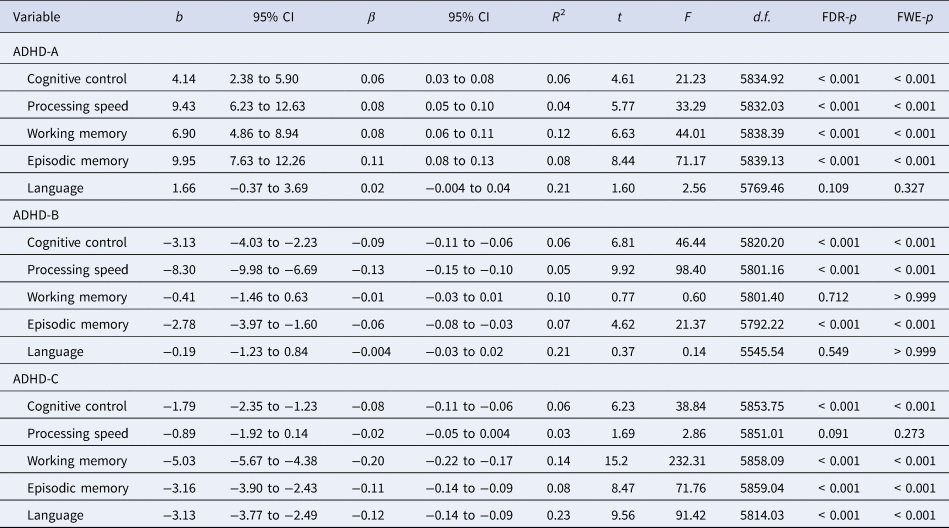
ADHD, attention-deficit/hyperactivity disorder; b, unstandardized coefficient; β, standardized coefficient; CI, confidence interval; d.f., degree of freedom; FDR, false discovery rate; FWE, family-wise error.
Structural changes
As shown in Fig. 2a and Table 3, the main effect of group in the linear mixed-effects model showed that the ADHD-C type exhibited smaller volumes in the left inferior temporal gyrus (FDR, p = 0.002; FWE, p = 0.006) and right lateral orbitofrontal cortex (FDR, p = 0.004; FWE, p = 0.012) than the non-ADHD group. As for weak results, the main effect of group showed that the ADHD-C type had smaller volumes in the left entorhinal cortex (FDR, p = 0.046; FWE, p = 0.138), left lateral orbitofrontal cortex (FDR, p = 0.037; FWE, p = 0.111), right middle temporal gyrus (FDR, p = 0.036; FWE, p = 0.108), right precentral gyrus (FDR, p = 0.049; FWE, p = 0.147), right superior frontal gyrus (FDR, p = 0.037; FWE, p = 0.111), and right inferior temporal gyrus (FDR, p = 0.049; FWE, p = 0.147) than the non-ADHD group. However, the ADHD-A and ADHD-B types did not show such significant differences in regional brain volumes. For significance comparing each ADHD subtype and the non-ADHD group adjusted for comorbidities, see online Supplementary Results 3, Supplementary Table S4, and Supplementary Fig. S3.

Figure 2. (a) Brain regions in which the ADHD-C type showed smaller volumes than the non-ADHD group and (b) Associations of brain structures (left inferior temporal gyrus and right lateral orbitofrontal cortex) with cognitive functions in the ADHD-C and non-ADHD groups. Based on FDR-corrected and FWE-corrected thresholds (p < 0.05), the ADHD-C type showed lower volumes in the left inferior temporal gyrus and right lateral orbitofrontal cortex than the non-ADHD group. Based on the FDR-corrected threshold (p < 0.05), the ADHD-C type showed lower volumes in the left entorhinal cortex, left lateral orbitofrontal cortex, right middle temporal gyrus, right precentral gyrus, right superior frontal gyrus, and right inferior temporal gyrus than the non-ADHD group. Only associations based on FWE-corrected thresholds were considered, where the ADHD-C group had smaller volumes and lower cognitive performances than the non-ADHD group. The ADHD-C group showed a positive correlation between volumes in the right lateral orbitofrontal cortex and language performances. In the non-ADHD group, both regional volumes were positively correlated with cognitive control, working memory, episodic memory, and language performances. In Fig. 2a, parameters are indicated as the mean (s.d.). In Fig. 2b, parameters are indicated as the r value. * FDR-p < 0.05, ** FDR-p < 0.01, *** FDR-p < 0.001. ADHD, attention-deficit/hyperactivity disorder; CC, cognitive control; EC, entorhinal cortex; EM, episodic memory; FDR, false discovery rate; FWE, family-wise error; ITG, inferior temporal gyrus; L_ITG, left inferior temporal gyrus; LF, language function; LOFC, lateral orbitofrontal cortex; MTG, middle temporal gyrus; PreCG, precentral gyrus; R_LOFC, right lateral orbitofrontal cortex; SD, standard deviation; SFG, superior frontal gyrus; WM, working memory.
Table 3. Brain areas with significantly smaller volume in the ADHD-C type than in the non-ADHD group

ADHD, attention-deficit/hyperactivity disorder; b, unstandardized coefficient; β, standardized coefficient; CI, confidence interval; d.f., degree of freedom; FDR, false discovery rate; FWE, family-wise error.
Subsequently, we investigated correlations between the volumes of the left inferior temporal gyrus and right lateral orbitofrontal cortex and the scores of cognitive control, working memory, episodic memory, and language because these areas and cognitive functions significantly differed between the ADHD-C type and non-ADHD groups (Fig. 2b). In the ADHD-C type, the volume of the right lateral orbitofrontal cortex was positively correlated with language score (FDR, p = 0.005). In the non-ADHD group, the volumes of the left inferior temporal gyrus and right orbitofrontal cortex were both positively correlated with the scores of cognitive control, working memory, episodic memory, and language (FDR, ps < 0.001).
Discussion
This study aimed to identify ADHD subtypes based on cognitive function domains, including executive function, using unsupervised clustering algorithms and to find distinct structural characteristics in the neural system of these subtypes. Clustering revealed three distinct ADHD subtypes (Fig. 1): the ADHD-A type was characterized by high cognitive function scores, the ADHD-B type was characterized by low cognitive control, processing speed, and episodic memory scores, and the ADHD-C type was characterized by strikingly low cognitive control, working memory, episodic memory, and language scores. Moreover, the volumes of the lateral orbitofrontal cortex and inferior temporal gyrus were smaller in the ADHD-C group than in the non-ADHD group (Fig. 2a). Notably, the volume of the right lateral orbitofrontal cortex was positively correlated with language performance in the ADHD-C type (Fig. 2b). These findings specifically indicate anomalies in the right lateral orbitofrontal cortex associated with language deficit in the ADHD-C type. The strength of this study is that, compared to previous studies, we analyzed children with and without ADHD after taking into account various confounding factors. Using data from the ABCD Study, previous studies have successfully identified brain structural differences in children with predisposing factors while controlling for confounding factors such as socioeconomic and pubertal statuses (Bernanke et al., Reference Bernanke, Luna, Chang, Bruno, Dworkin and Posner2022; Hamatani et al., Reference Hamatani, Hiraoka, Makita, Tomoda and Mizuno2022; Hiraoka et al., Reference Hiraoka, Makita, Hamatani, Tomoda and Mizuno2023; Owens et al., Reference Owens, Allgaier, Hahn, Yuan, Albaugh, Adise and Garavan2021). However, there is a paucity of such confounding factors in other studies (e.g. Enhancing Neuroimaging Genetics through Meta-Analysis and ADHD-200). For the first time, we presented cognitive and structural brain characteristics of ADHD subtypes based on cognitive functioning heterogeneity while controlling for various confounding factors.
Cognitive functioning heterogeneity
We found that the ADHD-A type had higher levels of cognitive control, processing speed, working memory, and episodic memory than the non-ADHD group. Previous studies have reported that patients with ADHD had lower levels of cognitive control (Breitling-Ziegler et al., Reference Breitling-Ziegler, Tegelbeckers, Flechtner and Krauel2020; Castellanos et al., Reference Castellanos, Sonuga-Barke, Milham and Tannock2006; Kofler et al., Reference Kofler, Irwin, Soto, Groves, Harmon and Sarver2019), processing speed (Castellanos et al., Reference Castellanos, Sonuga-Barke, Milham and Tannock2006; Mohamed et al., Reference Mohamed, Butzbach, Fuermaier, Weisbrod, Aschenbrenner, Tucha and Tucha2021), and working memory (Anker et al., Reference Anker, Ogrim and Heir2022; Blomberg et al., Reference Blomberg, Johansson Capusan, Signoret, Danielsson and Rönnberg2021) than control participants, which is not supported by our results. Several studies have shown that cognitive function deficits in ADHD may be compensated by better functioning in other cognitive domains, including a high intelligence quotient (Milioni et al., Reference Milioni, Chaim, Cavallet, de Oliveira, Annes, Dos Santos and Cunha2017; Ward, Alarcón, Nigg, & Musser, Reference Ward, Alarcón, Nigg and Musser2015). We suggest that individuals with ADHD-A try to maintain higher cognitive function by employing unique intellectual approaches and cognitive compensatory strategies even in simple tasks.
The ADHD-B type displayed lesser cognitive control, processing speed, and episodic memory than the non-ADHD group, in line with findings of previous studies examining ADHD and cognitive function associations (Breitling-Ziegler et al., Reference Breitling-Ziegler, Tegelbeckers, Flechtner and Krauel2020; Castellanos et al., Reference Castellanos, Sonuga-Barke, Milham and Tannock2006; Kofler et al., Reference Kofler, Irwin, Soto, Groves, Harmon and Sarver2019; Mohamed et al., Reference Mohamed, Butzbach, Fuermaier, Weisbrod, Aschenbrenner, Tucha and Tucha2021). Notably, although deficits in working memory have been widely implicated as the core executive function impairment in ADHD (Anker et al., Reference Anker, Ogrim and Heir2022; Blomberg et al., Reference Blomberg, Johansson Capusan, Signoret, Danielsson and Rönnberg2021; Castellanos et al., Reference Castellanos, Sonuga-Barke, Milham and Tannock2006), the present study did not find such impairment in the ADHD-B type. Alternatively, the notable weakness in processing speed may be attributed to the core executive function deficit in the ADHD-B type. Anker et al. (Reference Anker, Ogrim and Heir2022) found a correlation between lower levels of processing speed and the attention-deficit in ADHD, suggesting that impaired processing speed is linked to inattentive behavior. As processing speed involves efficient cognitive resource allocation in task performance (Burge et al., Reference Burge, Ross, Amthor, Mitchell, Zotov and Visscher2013; Floyd, Keith, Taub, & McGrew, Reference Floyd, Keith, Taub and McGrew2007), individuals with ADHD-B may be particularly vulnerable to cognitive capacity for processing information and generating correct responses within constrained time limits.
Consistent with previous study results (Anker et al., Reference Anker, Ogrim and Heir2022; Blomberg et al., Reference Blomberg, Danielsson, Rudner, Söderlund and Rönnberg2019; Blomberg et al., Reference Blomberg, Johansson Capusan, Signoret, Danielsson and Rönnberg2021; Breitling-Ziegler et al., Reference Breitling-Ziegler, Tegelbeckers, Flechtner and Krauel2020; Castellanos et al., Reference Castellanos, Sonuga-Barke, Milham and Tannock2006; Kofler et al., Reference Kofler, Irwin, Soto, Groves, Harmon and Sarver2019), the ADHD-C type had poorer cognitive control, working memory, episodic memory, and language abilities than the non-ADHD group did. However, the ADHD-C type did not show any impairment in processing speed. Thus, executive function of the ADHD-C type may be vulnerable to working memory and cognitive control impairments. Working memory is involved in an active mental workspace (Fang et al., Reference Fang, Zhang, Zhou, Cheng, Li, Wang and Jiang2016) and plays important roles in basic memory maintenance and attention regulation to filter out irrelevant information (Emch, von Bastian, & Koch, Reference Emch, von Bastian and Koch2019). Several studies have shown that working memory capacity is associated with speech production and problem-solving (Emch et al., Reference Emch, von Bastian and Koch2019; Fang et al., Reference Fang, Zhang, Zhou, Cheng, Li, Wang and Jiang2016). Wiemers and Redick (Reference Wiemers and Redick2018) reported that during the continuous performance test, healthy participants with low working memory capacity had higher selective error and response time to specific stimuli and lower levels of proactive control with more time on the task than those with high working memory capacity, suggesting the involvement of working memory capacity in appropriate cognitive control shifting. We propose that individuals with ADHD-C may be vulnerable to impaired cognitive control and problem-solving abilities owing to their reduced working memory capacity, indicating that individuals with ADHD-C may have difficulties in refusing irrelevant information and cognitive shifting during task performance. These aspects of cognitive functioning heterogeneity may highlight the need for different approaches and considerations for the diagnosis and treatment of ADHD across the identified subtypes.
Structural characteristics
Structural analyses revealed significantly smaller volumes in the left inferior temporal gyrus and right lateral orbitofrontal cortex in the ADHD-C type than those in the non-ADHD group. Moreover, the ADHD-C type showed a positive correlation between the right lateral orbitofrontal cortex volume and language performance, whereas the volumes in both regions were in the non-ADHD group positively associated with cognitive control, working memory, episodic memory, and language performances. These findings suggest that specific structural anomalies in the right lateral orbitofrontal cortex may underlie language deficits in the ADHD-C type. In contrast, the non-ADHD group exhibited a more widespread association of the left inferior temporal gyrus, in addition to the right lateral orbitofrontal cortex, with various cognitive functions. However, both ADHD-A and ADHD-B types did not show such structural differences in comparison with the non-ADHD group. Therefore, the discussion on structural characteristics is restricted to the ADHD-C type.
Volume reductions in the ADHD-C type were observed in the right lateral orbital part of the inferior frontal gyrus, which is supported by the findings of previous studies on ADHD (Long et al., Reference Long, Pan, Ji, Qin, Chen, Zhang and Gong2022; Nickel et al., Reference Nickel, Tebartz van Elst, Manko, Unterrainer, Rauh, Klein and Maier2018). The lateral orbitofrontal cortex corresponds to cognitive control processes and emotion processing (Deng et al., Reference Deng, Rolls, Ji, Robbins, Banaschewski, Bokde and Feng2017; Rolls, Reference Rolls2019). Several studies have proposed some functional connections in the lateral orbitofrontal cortex, such that cognitive control is represented predominantly in the frontoparietal network (Lückmann, Jacobs, & Sack, Reference Lückmann, Jacobs and Sack2014; Tozzi, Goldstein-Piekarski, Korgaonkar, & Williams, Reference Tozzi, Goldstein-Piekarski, Korgaonkar and Williams2020), the detection of salient stimuli is associated with the ventral attention network (Corbetta, Patel, & Shulman, Reference Corbetta, Patel and Shulman2008; Seeley et al., Reference Seeley, Menon, Schatzberg, Keller, Glover, Kenna and Greicius2007), and emotional processing is implicated in the subcortical limbic circuit (Banks, Eddy, Angstadt, Nathan, & Phan, Reference Banks, Eddy, Angstadt, Nathan and Phan2007), suggesting that this cortex and its association with these networks underlies the functional architecture of cognitive control and emotional processing in ADHD. However, in our study, a significant association between the right lateral orbitofrontal cortex volume and cognitive control was not observed in the ADHD-C type. Instead, this type displayed an association between regional volumes and language abilities. A meta-analytic connectivity modelling study found coactivation of the lateral orbitofrontal cortex with language and auditory processing areas, such as the superior and middle temporal gyri, during tasks involving semantic processing (Zald et al., Reference Zald, McHugo, Ray, Glahn, Eickhoff and Laird2014), indicating the involvement of the lateral orbitofrontal cortex in the language network. This region is known to be involved in anatomical links with Broca's area via ventromedial connections (Miller, Collins, & Kent, Reference Miller, Collins and Kent2008). In our study, a volume reduction in the right lateral orbitofrontal cortex and its association with low language performance were specifically seen in the ADHD-C type. This volume reduction observed in individuals with ADHD-C may be associated with language impairment through the abovementioned anomalies in structural and functional connectivity.
Our work provides novel evidence that the ADHD-C type shows decreased left inferior temporal gyrus volume. The inferior temporal gyrus is involved in language processing (Hickok & Poeppel, Reference Hickok and Poeppel2004; Mani et al., Reference Mani, Diehl, Piao, Schuele, Lapresto, Liu and Lüders2008) and working memory (Ranganath, Cohen, Dam, & D'Esposito, Reference Ranganath, Cohen, Dam and D'Esposito2004). Notably, our uncorrected results (p < 0.05) indicated a positive correlation between the left inferior temporal gyrus volume and language performance in the ADHD-C type. Although these uncorrected results should be interpreted with caution, the ADHD-C type may induce a volume reduction in the inferior temporal gyrus, leading to impairments in language maintenance, in addition to the volume reduction in the lateral orbitofrontal cortex. In contrast to these structural results, Humphreys et al. (Reference Humphreys, Watts, Dennis, King, Thompson and Gotlib2019) reported that a large GMV of the inferior temporal gyrus is associated with severe ADHD symptoms. This discrepancy might be caused by differences in covariates such as handedness, medication use, household income, and parental education; these factors were not controlled for in their study, which may have weakened the influence of brain structure. Another possible reason is the age difference of the participants. The mean age of patients with the ADHD-C type in our study was 9.8 years. However, in the study by Humphreys et al. (Reference Humphreys, Watts, Dennis, King, Thompson and Gotlib2019), the mean age of the ADHD group was 11.4 years, indicating that the ADHD group may have included more participants who were older than 12 years; notably, developmental delays in several brain regions tend to change at this age (Shaw et al., Reference Shaw, Malek, Watson, Sharp, Evans and Greenstein2012).
In the non-ADHD group, we found positive correlations between the volumes of the left inferior temporal gyrus and right lateral orbitofrontal cortex and the levels of cognitive control, working memory, episodic memory, and language, indicating that in the non-ADHD group, both brain regions were more closely linked to these cognitive functions. This suggests that the roles of these two brain regions in cognitive function differ between the ADHD-C and non-ADHD groups, although these correlational results might be significant due to the larger sample size of the non-ADHD group compared to that of the ADHD-C group.
Our results, based on FDR-corrected thresholds (p < 0.05), also showed that the ADHD-C type had smaller volumes in the left entorhinal cortex, left lateral orbitofrontal cortex, right middle temporal gyrus, right precentral gyrus, right superior frontal gyrus, and right inferior temporal gyrus (Fig. 2 and Table 3). These regions, together with the inferior temporal gyrus and lateral orbitofrontal cortex, are involved in language processing (Behroozmand et al., Reference Behroozmand, Shebek, Hansen, Oya, Robin, Howard and Greenlee2015; He et al., Reference He, Xue, Chen, Chen, Lu and Dong2013; Hickok & Poeppel, Reference Hickok and Poeppel2004; Lee et al., Reference Lee, Raznahan, Wallace, Alexander-Bloch, Clasen, Lerch and Giedd2014; Shafritz, Marchione, Gore, Shaywitz, & Shaywitz, Reference Shafritz, Marchione, Gore, Shaywitz and Shaywitz2004), cognitive control (Duan et al., Reference Duan, Chen, Calhoun, Lin, Jiang, Franke and Liu2018; Lei et al., Reference Lei, Du, Wu, Chen, Huang, Du and Gong2015; Shafritz et al., Reference Shafritz, Marchione, Gore, Shaywitz and Shaywitz2004), and working memory (Buzsáki & Moser, Reference Buzsáki and Moser2013; Duan et al., Reference Duan, Chen, Calhoun, Lin, Jiang, Franke and Liu2018; Kopniczky et al., Reference Kopniczky, Dochnal, Mácsai, Pál, Kiss, Mihály and Szabo2006). Moreover, some studies have reported associations between these frontal-temporal regional volumes and cognitive functions such as language, cognitive control, and working memory (Behroozmand et al., Reference Behroozmand, Shebek, Hansen, Oya, Robin, Howard and Greenlee2015; Duan et al., Reference Duan, Chen, Calhoun, Lin, Jiang, Franke and Liu2018; John, Ritter, Wong, & Parks, Reference John, Ritter, Wong and Parks2022), suggesting that these structural changes may play an important role in the maintenance of verbal ability, cognitive control, and memory function. Although our FDR-corrected results should be interpreted with caution, they suggest that the ADHD-C type may lead to volume reductions in the entorhinal cortex, middle temporal gyrus, precentral gyrus, and superior frontal gyrus and related cognitive deficits, in addition to volume reductions of the lateral orbitofrontal cortex and inferior temporal gyrus.
Although structural and functional anomalies of the basal ganglia (e.g. reward-related striatum) have often been reported in patients with ADHD (Bonath et al., Reference Bonath, Tegelbeckers, Wilke, Flechtner and Krauel2018; Klein et al., Reference Klein, Souza-Duran, Menezes, Alves, Busatto and Louzã2021; Li et al., Reference Li, He, Li, Chen, Huang, Lui and Gong2014; Luo et al., Reference Luo, Chen, Wang, Li, He, Li and Li2023; Moreno-Alcázar et al., Reference Moreno-Alcázar, Ramos-Quiroga, Radua, Salavert, Palomar, Bosch and Clotet2016; Villemonteix et al., Reference Villemonteix, De Brito, Kavec, Balériaux, Metens, Slama and Massat2015), this study did not find group differences in these regional brain volumes between the ADHD subtypes and the non-ADHD group. One possible reason is the neurobiological difference in ADHD, as previous studies have considered a single ADHD pathology, whereas we distinguished ADHD subtypes based on cognitive function domains, including executive function. Another possible reason is the characteristics of the cognitive function used for clustering: the reward-related task was not included in our clustering method, which may not have identified the reward-related ADHD subtype in the present study. Another possibility is the difference in the sample size and data analysis plan. Previous studies controlled for basic confounders such as age and sex; however, we controlled for additional confounders (e.g. pubertal status, medication use, race/ethnicity, and family conditions).
Considering that a symptom-based diagnosis does not always lead to effective therapy, the National Institute of Mental Health in the United States launched the Research Domain Criteria projects to establish a novel framework for pathophysiological research (Insel & Cuthbert, Reference Insel and Cuthbert2015). Based on this model, the present study aimed to clarify ADHD heterogeneity based on cognitive function domains categorized by unsupervised machine learning and compared brain structural characteristics. The main findings of our study may help establish criteria for more accurate diagnoses based on the underlying ADHD pathophysiology.
Our study has some limitations. First, this study measured the brain volumes of 34 cortical regions labeled with the Desikan atlas and 12 subcortical regions; thus, regional volume measurements may be affected by combined influences of cortical morphology (e.g. close associations with the cortical surface areas). A whole-brain vertex-based analysis might better address the issue of atlas bias, as previously reported (You et al., Reference You, Li, Chen, He, Li, Long and Li2024). Furthermore, cortical thickness, which is used to evaluate cortical maturation abnormalities, may be relatively stable and distinct from heritable brain volume effects (You et al., Reference You, Li, Chen, He, Li, Long and Li2024). Therefore, further analyses as mentioned above are needed. Second, this study was cross-sectional; thus, future longitudinal studies are needed, as previous longitudinal studies have reported differences in atypical brain structures depending on the developmental stage (Hoogman et al., Reference Hoogman, Muetzel, Guimaraes, Shumskaya, Mennes, Zwiers and Franke2019). Third, this study used a restricted cognitive assessment battery measured using the NIH Toolbox. Previous meta-analyses have explored the associations between a broad spectrum of neuropsychological measures (i.e. domains of decision-making, verbal fluency, planning, and vigilance) and ADHD pathology (Frazier, Demaree, & Youngstrom, Reference Frazier, Demaree and Youngstrom2004; Pievsky & McGrath, Reference Pievsky and McGrath2018). It remains unclear how other cognitive domains could have influenced the results of our study. Finally, the ABCD Study data are still being updated and revised with each release, and the data, including sample size, differential diagnosis of psychiatric disorders, clinical measures, neuroimaging, and neuropsychological measures, might differ between previous release versions and the new release version. However, studies using previously released versions of the ABCD Study have built an accumulating body of recent evidence regarding neurobiological mechanisms in ADHD (Kim et al., Reference Kim, Kim, Pack, Lim, Cho and Lee2023; Norman et al., Reference Norman, Price, Ahn, Sudre, Sharp and Shaw2023; Wang, Zhou, Gui, Liu, & Lu, Reference Wang, Zhou, Gui, Liu and Lu2023). Thus, the findings obtained from the ABCD Study should be interpreted with caution, and they should be re-evaluated using revised data in the future.
Conclusions
To the best of our knowledge, this study is the first to find brain structural anomalies in ADHD subtypes based on cognitive functioning heterogeneity. Behaviorally, ADHD-A was characterized by high levels of cognitive function, ADHD-B by low levels of cognitive control, processing speed, and episodic memory, and ADHD-C showed low levels of cognitive control, working memory, episodic memory, and language abilities. Structurally, only the ADHD-C type exhibited volume reductions in the inferior temporal gyrus and lateral orbitofrontal cortex. Moreover, the volume of the right lateral orbitofrontal cortex was associated with language performance in the ADHD-C type. The present results demonstrate anomalies in the lateral orbitofrontal cortex associated with language deficits in individuals with ADHD-C. Our approach, based on cognitive functioning heterogeneity, may help explain the inconsistencies of previous study findings.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291724002368
Acknowledgements
Data used in the preparation of this article were obtained from the ABCD Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10 000 children aged 9–10 years and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
We are grateful to Dr Daiki Hiraoka of the University of Fukui for supporting us in the R analysis.
Author contributions
Conceptualization, MY, YM; Data Curation, MY; Formal Analysis, MY; Funding Acquisition, MY, YM; Investigation, MY; Methodology, MY, QS, YM; Project Administration, MY, YM; Resources, MY, YM; Software, MY; Supervision, YM; Validation, MY, QS; Visualization, MY; Writing – Original Draft Preparation, MY; Writing – Review & Editing, MY, QS, YM. All authors have read and approved the final manuscript.
Funding statement
This work was supported by the Japan Society for the Promotion of Science through Grants-in-Aid for Scientific Research (KAKENHI) (grant numbers: 21K02380 to YM and 23K12814 to MY), Kawano Masanori Memorial Public Interest Incorporated Foundation for Promotion of Pediatrics (AY 2022 to YM), Research Grants from the University of Fukui (AY 2022 to YM), and Taiju Life Social Welfare Foundation (AY 2023 to MY). The funding sources had no involvement in the study design or conduct; the collection, analysis, and interpretation of data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
Data can be accessed through registration with the ABCD Study at https://nda.nih.gov/abcd. The data used in this manuscript are available from the ABCD Study's Data Release 2.0 (https://doi.org/10.15154/1504041). Information on how to access ABCD data through the NDA is available on the ABCD Study data-sharing webpage: https://abcdstudy.org/scientists_data_sharing.html. Instructions on how to create an NDA study are available at https://nda.nih.gov/nda/webinars-and-tutorials. R codes for this analysis are available at https://osf.io/nvwam/ after acceptance.








