It is important to recognise that mental health and physical health both affect each other. Patients with serious mental health problems are known to have higher levels of mortality, and it has become a key priority to improve the physical health of patients with mental illness (NHS England 2014). Obesity and depression are just one area of concern, and the following statistics indicate the scale of the problem:
-
• 13% of the world's population are obese (WHO 2017a)
-
• More that 300 million people worldwide have depression (WHO 2017b)
-
• 23% of obese individuals have comorbid depression (Carey Reference Carey, Small and Lin Yoong2014)
-
• the prevalence of obesity among adults with severe mental health problems is as high as 55% (NICE 2014)
-
• as independent risk factors, depression confers a 37% relative risk of obesity and obesity confers an 18% relative risk of depression; however, the excess risk of obesity in depression is small (Mannan Reference Mannan, Mamun and Doi2015)
-
• the risk of development of type 2 diabetes attributable to obesity is between 30 and 70% (Royal College of Physicians 2013).
Obesity is a known risk factor for many conditions, including cardiovascular, renal and mental health problems. Guidelines now suggest that there should be more services dedicated to obesity management in multidisciplinary settings (Royal College of Physicians 2013). Obesity and depression have both been linked to high use of healthcare resources and, in combination, they can result in increased duration of illness and longer in-patient stays (Opel Reference Opel, Redlich and Grotegerd2015; Robinson Reference Robinson, Grabner and Rao Palli2016). The key learning points from this article are summarised in Box 1.
Box 1 Key learning points
In comorbid depression and obesity, the two disorders should be considered together to make the most effective treatment decisions
Medication can affect weight and mood
It is important to consider weight-related side-effects when making prescribing decisions
The obesity–depression cycle
Obesity and depression are thus common conditions, with significant implications individually. When considered together, obesity with depression has been shown to negatively affect quality of life in a synergistic manner (Mannan Reference Mannan, Mamun and Doi2015; Nigatu Reference Nigatu, Reijneveld and de Jonge2016). A bi-directional relationship between the two is proposed, although not all studies support this, suggesting that the relationship is a complex one (Askari Reference Askari, Hassanbeigi and Khosravi2013; Singh Reference Singh, Jackson and Dobson2014; Jantaratnotai Reference Jantaratnotai, Mosikanon and Lee2017).
Increasing severity of depression has been shown to be associated with decreased physical activity and increased calorie intake, with a consequent increase in the risk of obesity (Simon Reference Simon, Ludman and Linde2008). Obesity can be used to predict a poorer response to antidepressants and a worse depression outcome over 1 year; one particular factor implicated is the development of metabolic syndrome (García-Toro Reference García-Toro, Vicens-Pons and Gili2016; Jantaratnotai Reference Jantaratnotai, Mosikanon and Lee2017). A summary of the links proposed is demonstrated in Fig. 1.
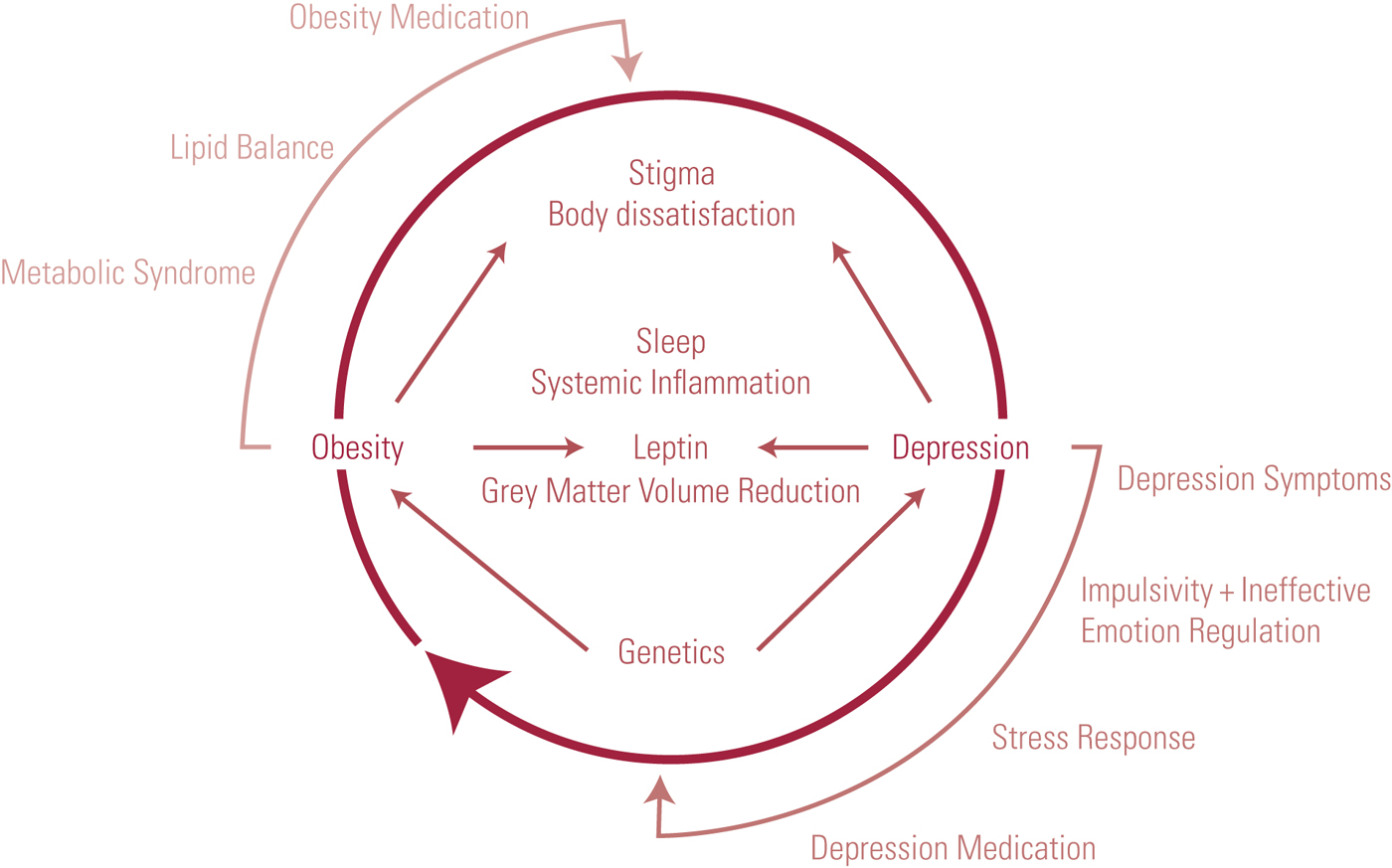
FIG 1 The cycle of depression and obesity.
The effect of depression
Depression as an illness is characterised partially by changes in diet. Anecdotes suggest that depression can be related to overeating or self-neglect and undereating. This is supported by the higher prevalence estimates of depression in both obese and underweight groups (Carey Reference Carey, Small and Lin Yoong2014). Greater severity of depression has been associated with poorer diet quality overall, particularly in relation to higher intake of components related to weight gain, such as sugar and saturated fats (Appelhands Reference Appelhands, Whited and Schneider2012).
Stress response
Acute and chronic stressors are often associated with depression. Chronic stress has been shown to influence dietary intake and the desire for more high-energy foods, leading to increased abdominal fat mass. The mechanism for this is thought to be mediated by increased cortisol and repeated activation of the hypothalamic–pituitary–adrenal (HPA) axis (Torres Reference Torres and Nowson2007). Excess glucocorticoids have been linked to insulin resistance, leading to leptin resistance, and leptin is part of the satiety signal pathways (Michopoulos Reference Michopoulos2016). This glucocorticoid effect therefore links leptin to the stress response.
One study of a weight-loss programme for obese adolescents with depression investigated the role of leptin. After 1 year on this programme, participants demonstrated weight loss, decreased leptin levels and an improvement in depressive symptoms. It was therefore concluded that a change in leptin levels was a significant predicting factor for changes in depressive symptoms (de Carvalho-Ferreira Reference de Carvalho-Ferreira, Landi Masquio and da Silveira Campos2014).
The HPA axis has been further linked to functional gastrointestinal symptoms that can also have an impact on diet and weight (Karling Reference Karling, Wikgren and Adolfsson2016). Animal models suggest that the increase in glucocorticoids can increase ghrelin and neuropeptide Y (NPY), both of which stimulate food intake. This can lead to fat growth as well as an increase in melanin-concentrating hormone (MCH). Increase in MCH is demonstrated to increase appetite (Michopoulos Reference Michopoulos2016).
Acute stress creates biological changes that are greater in the context of obesity. For example, a study of responses to acute stress (Benson Reference Benson, Arck and Tan2009) showed a higher cortisol response following public speaking in obese women. Heart rate and diastolic blood pressure took longer to recover following the stressor in obese compared with non-obese participants. Other factors implicated in the acute stress response were interleukin 6 (IL-6) and circulating leucocytes, granulocytes and CD3+ T-lymphocytes, all of which were present at higher levels in the obese compared with the non-obese women at baseline. IL-6 was particularly interesting, as this level also increased significantly more in the obese participants after the acute stressor. A redistribution of lymphocytes was uncovered in response to acute stress, but this did not differ between obese and non-obese participants. Similarly, the emotional response to the acute stressor was not significantly different in obese and non-obese participants – in fact, anxiety levels were generally lower during the test.
Body dissatisfaction and stigma
Another factor often discussed as a possible contributor to the obesity–depression cycle is body dissatisfaction, which is associated with both depression and obesity (Benson Reference Benson, Arck and Tan2009). Social stigma associated with obesity, revealing itself in insulting comments, negative media representations and discriminatory behaviour. Self-stigma can also exacerbate feelings of body dissatisfaction, which is a significant cause of emotional distress, low self-esteem and mood changes (Taylor Reference Taylor, Forhan and Vigod2013).
Sleep, obesity and depression
Figure 2 demonstrates how sleep fits into the cycle of depression and obesity. Insufficient sleep can increase the risk of obesity and metabolic syndrome, and short sleep duration is associated with obesity in both children and adults. Alongside increases in calorie intake, physical activity levels decrease when sleep is restricted, further exacerbating weight gain (Schmid Reference Schmid, Hallschmid and Shultes2015).

FIG 2 Depression, obesity and sleep.
Some studies have identified higher levels of ghrelin in sleep loss, and these have been associated with increased food intake (Schmid Reference Schmid, Hallschmid and Shultes2015). Studies of ghrelin in depression have reported mixed results, with some suggesting that the hormone has a possible antidepressant effect, but others finding no significant effect on depressive symptoms (Kluge Reference Kluge, Schüssler and Dresler2011). The effect of ghrelin in sleep and depression therefore needs more investigation and clarification. Low levels of sleep are also associated with lower levels of leptin, resulting in increased calorie intake in some studies, although evidence for this is again conflicting. Research therefore suggests a complex set of mechanisms linking sleep with ghrelin and leptin changes (Schmid Reference Schmid, Hallschmid and Shultes2015).
Although poor sleep has been related to obesity, the opposite is also true: individuals with higher weights have been shown to have an increased level of sleep problems (Palm Reference Palm, Janson and Lindberg2015). Weight gain was independently demonstrated in this work to be linked to difficulty maintaining sleep, excessive daytime sleepiness and insomnia.
So how does sleep link to depression? One large study of an adolescent population linked insomnia and disturbed sleep with an increase in the severity and prevalence of depressive symptoms, and discusses associations with anxiety, suicidal ideation and substance misuse. Levels of depressive symptoms increased between the normal and disturbed sleepers groups and further increased in the insomnia group. These patients are also likely to be at a higher risk of ongoing sleep problems into their adult lives (Amaral Reference Amaral, Garrido and Pereira2016). Other studies, including one of participants with mild obstructive sleep apnoea who had a mean BMI of 24.7 kg/m2, demonstrated that poor sleep hygiene was linked to depressed mood and higher levels of daytime sleepiness through poor sleep quality (Lee Reference Lee, Paek and Han2015).
A large meta-analysis concluded that there is a bidirectional relationship between sleep disturbances and depression in which both problems are mutually exacerbating, and that it is therefore important to consider interventions to help reduce these comorbidities (Bao Reference Bao, Han and Ma2017).
Overall, it is clear that sleep has a great impact on the cycle of depression and obesity.
Emotion regulation and neurological changes
Difficulty in regulating emotion and impulses might be another link between depression and obesity, operating through poor food choices (Privitera Reference Privitera, McGrath and Windus2015). A functional magnetic resonance imaging (fMRI) study demonstrated that individuals with increased weight did not as effectively regulate negative emotions compared with those in lower weight categories. The scans also revealed an abnormal neural activation pattern for those in the higher weight category, demonstrating differences in the regulation of emotion. The area of the brain primarily involved was the insula, which is known to have roles in emotion regulation, but changes were also noted in other areas, including prefrontal regions (Steward Reference Steward, Picó-Pérez and Mata2016). Both depression and obesity are independently associated with grey matter volume reductions in overlapping regions in the medial prefrontal cortex (Opel Reference Opel, Redlich and Grotegerd2015). This shows that there are both emotion regulation and physical grey matter components linking depression and obesity neurologically.
Systemic inflammation
Research into other possible biochemical mechanisms focuses on markers of systemic inflammation such as C-reactive protein (CRP). CRP has been shown to be at a higher baseline level in obese people. Analysis shows that there is an association between depression and elevated CRP that is modified by abnormal body weight (Benson Reference Benson, Arck and Tan2009; Liu Reference Liu, Al-Sayegh and Jabrah2014).
Inflammatory markers such as CRP, the interleukins IL-1, IL-2 and IL-6, tumour necrosis factor-alpha (TNF-α) and interferon-gamma (IF-γ) are among systemic inflammatory markers noted to be more active in people with depression (Lopresti Reference Lopresti, Hood and Drummond2013). Some of these factors, including IL-6 and TNF-α, are also involved in the development of insulin resistance and changes in leptin levels (Michopoulos Reference Michopoulos2016). Other cytokines noted to be raised in depression are soluble receptors of IL-2 (sIL-2R) and the interleukin-1 receptor antagonist (IL-1RA) (Pennix Reference Pennix, Milaneschi and Lamers2013).
Systemic inflammation has a direct effect on neurogenesis, reducing it via the action of IL-6 and TNF receptors. Pro-inflammatory cytokines have been shown to reduce the availability of precursors for serotonin and melatonin synthesis and to increase tryptophan catabolites, leading to neuron damage (Pennix Reference Pennix, Milaneschi and Lamers2013). White adipose tissue produces further inflammatory cytokines and this stimulates lipid release to provide energy to support the immune response. Abdominal obesity and these lipid disturbances provide a significant link between depression and the development of metabolic syndrome (Pennix Reference Pennix, Milaneschi and Lamers2013).
Genetic factors
Gender differences have been noted in some of the proposed links between depression and obesity. The association between leptin resistance and depression seems to be stronger for females (de Carvalho-Ferreira Reference de Carvalho-Ferreira, Landi Masquio and da Silveira Campos2014). Gender has been noted to modify the relationship between CRP, sleep and depression (Liu Reference Liu, Al-Sayegh and Jabrah2014; Palm Reference Palm, Janson and Lindberg2015; Amaral Reference Amaral, Garrido and Pereira2016). Hormones such as oestradiol have been implicated as a factor in the difference in responses sometimes demonstrated between male and female participants, in part because of their influences on various biological pathways. Often oestradiol was found to be opposing the actions of cortisol, although under chronic stress these effects appeared to be halted or possibly even reversed. In depression, there is a noted difference in oestradiol receptor expression, although currently these mechanisms are unclear (Michopoulos Reference Michopoulos2016).
The FTO (fat mass and obesity-associated) gene has been linked to common forms of obesity. Research has identified single-nucleotide polymorphisms that are significantly associated with increased BMI in patients with depression but not in controls (Rivera Reference Rivera, Cohen-Woods and Kapur2012). The study team concluded that the effect of the FTO gene on increased BMI is modified by the presence of depression.
Medication and the implications of prescribing choices
Antidepressant medication is often suggested as a contributing factor to weight gain and this can be of great concern to patients. Antidepressant use in general is associated with increased energy intake: one study reported that individuals taking antidepressant medication consumed 215 kcal/day (S.E. = 73 kcal/day) more than those not taking it (Jensen-Otsu Reference Jensen-Otsu and Austin2015). There are many proposed mechanisms by which antidepressant medications affect weight. Examples include (Bazire Reference Bazire2016: 6.6):
-
• sedation and the resulting decrease in physical activity
-
• reduction in body metabolic rate
-
• increased appetite due to receptor blockade of histamine, serotonin and leptin
-
• increased thirst due to dry mouth caused by anticholinergic side-effects
-
• increase in prolactin, promoting adiposity
-
• sleep changes resulting in changes to hunger, fatigue and thermoregulations, leading to altered energy requirements.
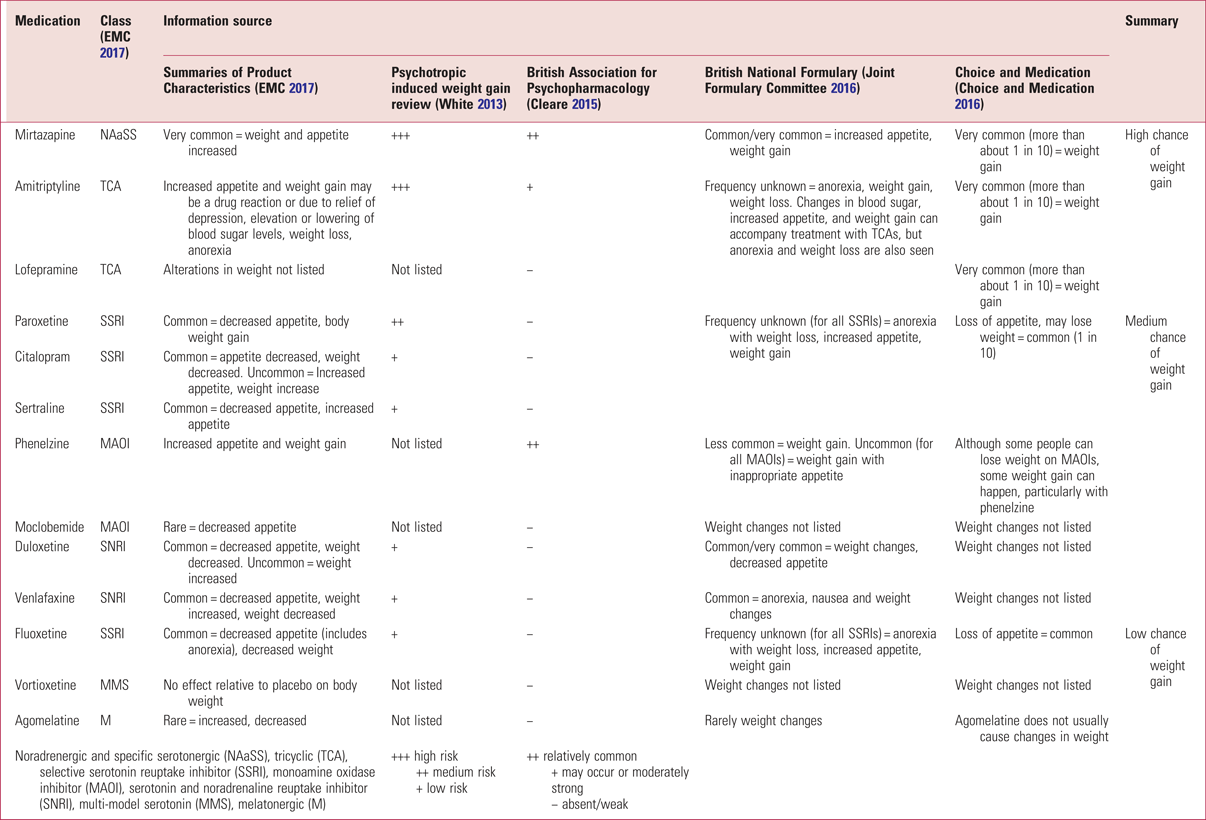
At the receptor level, there are a variety of proposed mechanisms governing antidepressant-related weight changes. The most important receptors are the dopamine D2, serotonin 5-HT2c and histamine H1 receptors, as demonstrated in Fig. 3 (White Reference White, Elmore and Luthin2013). Of the commonly prescribed antidepressants, mirtazapine, amitriptyline and lofepramine are associated with the highest chance of weight gain, and fluoxetine, vortioxetine and agomelatine with the lowest. For further details, together with information sources, on the weight-change effects of these and other antidepressants see Table 1.
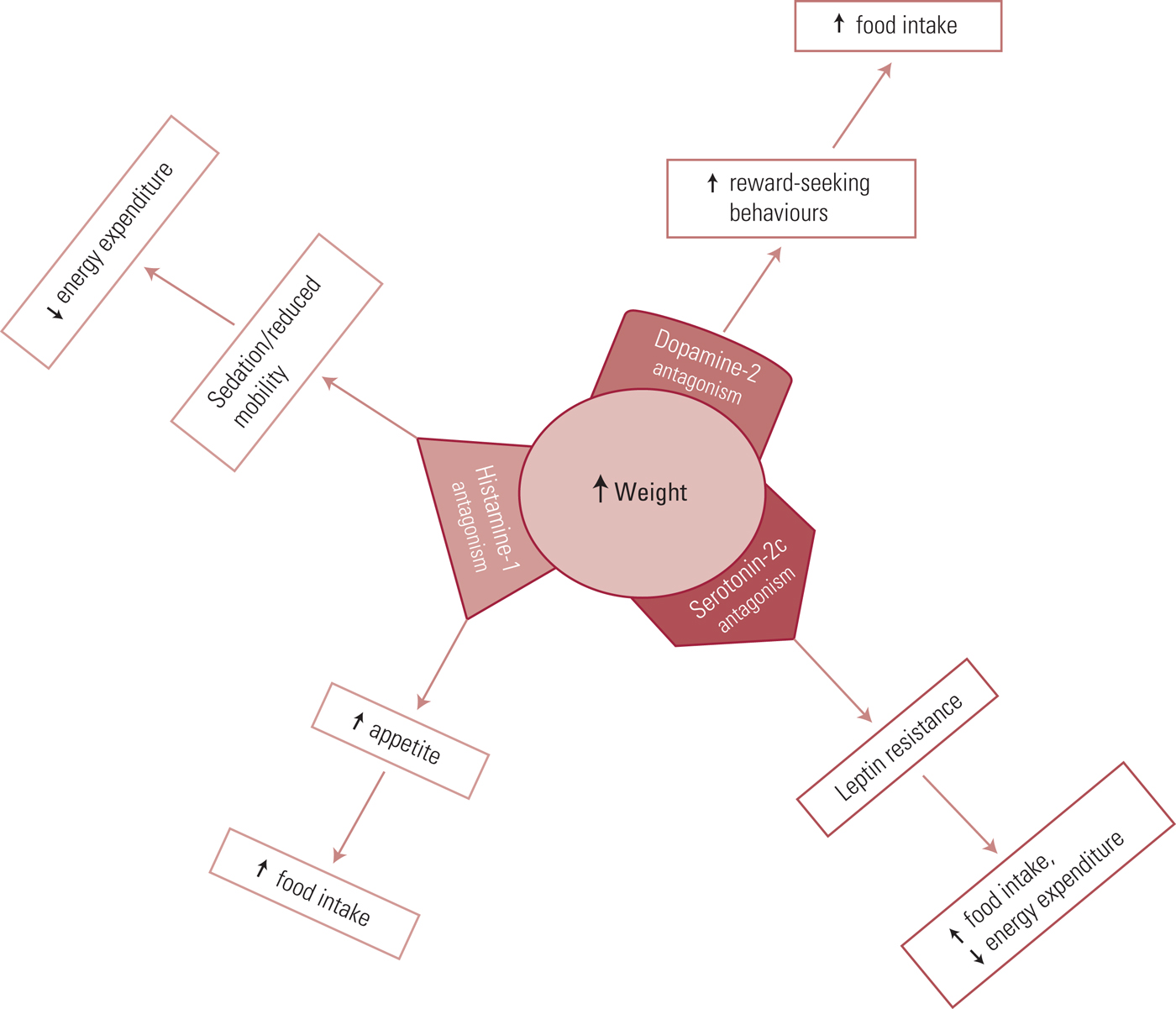
FIG 3 Receptors linking antidepressants and weight change.
TABLE 1 Antidepressant effects on weight change
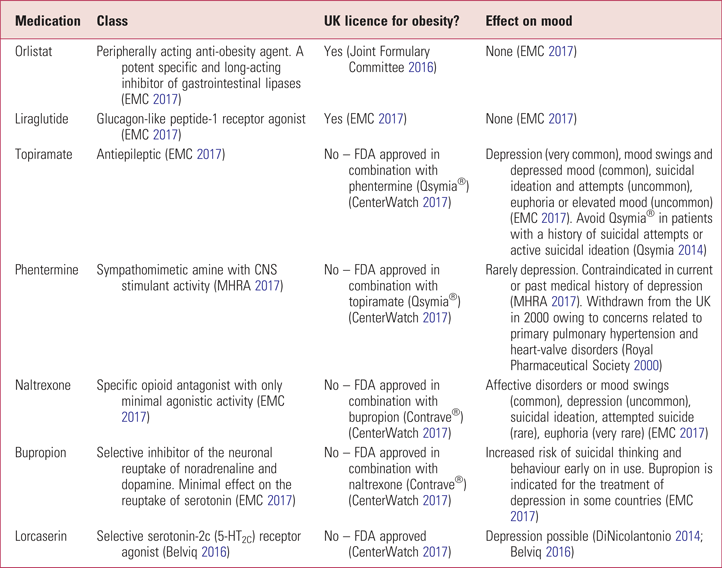
It is also important to consider whether weight-loss medication for obesity affects mood. At present, only orlistat, probably the most well-known weight-loss medication, and liraglutide are licensed to treat obesity in the UK. Neither is associated with depression, however orlistat has anxiety listed as a side effect (Joint Formulary Committee 2017; EMC 2017). However, depression has been listed as a side-effect of lorcaserin, phentermine–topiramate and bupropion–naltrexone (Bray Reference Bray and Perreault2016), which are used as part of weight-loss therapy in the USA, under licence of the Food and Drug Administration (FDA), and other countries. Further details on these and other anti-obesity medications are given in Table 2.
TABLE 2 Anti-obesity medications
Patients' perspectives on treatment
To best support patients, it is important to understand what factors have an impact on effective treatment. In a qualitative study of weight loss, patients identified their knowledge of nutrition, social support and creation of a structured approach through organisation and routine as helping them in weight reduction. They reported that negative social factors such as people's poor understanding of the challenges they faced in trying to lose weight could have the opposite effect (Rogerson Reference Rogerson, Soltani and Copeland2016). Other barriers to weight loss included dichotomous thinking and over-monitoring of progress. Dichotomous thinking is known to be linked to traits of perfectionism and anxiety (Rogerson Reference Rogerson, Soltani and Copeland2016). Interview participants have also expressed concerns about the safety and effectiveness of weight-loss medication (Xing Reference Xing, Sharp and Touchette2016).
When considering treatment options for depression there are also possible barriers to effectiveness. In a study of patients’ perspectives on how treatment can impede their recovery from depression, key themes included a lack of clarity about their condition and treatment, a lack of perceived treatment personalisation and difficulty in accessing healthcare when needed. Clear goals and action plans, as well as a good relationship with clinicians, were thought to be of benefit, as was involving the patients in choosing the type of treatment, for example talking therapies and/or medication. Another important theme was the presence of social support and the involvement of significant others (van Grieken Reference van Grieken, Beune and Kirkenier2014).
What should clinicians be doing?
Given the many sources of evidence suggesting multifactorial links between depression and obesity it follows that consideration should be given to a combined treatment approach. Exercise interventions alongside treatment for depression may be of benefit to both conditions and, given the prevalence of depression in obese people, obesity might be seen as a trigger to screening for a mood disorder and vice versa (Carey Reference Carey, Small and Lin Yoong2014; Jantaratnotai Reference Jantaratnotai, Mosikanon and Lee2017). Dietary advice is also important, as depressive symptoms have been shown to be positively associated with consumption of sweets and high intake of fast food. A low-fat, calorie-restricted diet has been shown to improve mood even without weight loss (Lopresti Reference Lopresti, Hood and Drummond2013).
The National Institute for Health and Care Excellence (NICE) has published several guidelines relevant to this matter.
-
• In relation to obesity prevention in younger patients, NICE suggests that children's mental health services be involved to help reduce the risk of development of depression (NICE 2015).
-
• In relation to depression treatment in younger patients, NICE recommends that the benefits of exercise and a balanced diet should be discussed alongside other treatments (NICE 2005).
-
• Patients with obesity are at risk of developing physical health problems. For treatment of depression in adults with chronic physical health problems NICE recommends: a high-intensity psychological intervention in moderate depression; a combination of antidepressant treatment and individual cognitive–behavioural therapy in severe depression; and collaborative care if there is functional impairment (NICE 2011).
It is clear that there is room for further development of combined approaches, and we need to take into account and further research patients’ perspectives on these interventions and discuss any issues openly. Our suggestions for key good practice points are summarised in Box 2.
Box 2 Good practice points in treating obesity and/or depression
-
• Consider screening for depression in obese patients
-
• Dietary and exercise advice should be given to both groups of patients, and particularly to those affected by obesity and depression together
-
• When discussing obesity in children, consider the current and future mental health implications
Conclusions
Although links clearly exist between depression and obesity, it is not yet clear what the best treatment methods are. Should the conditions be tackled one at a time or in a programme focused on both together? If we plan to consider them together, work needs to be conducted on screening for the combination of depression and obesity and on developing a treatment programme with this specific focus that could be implemented across healthcare settings (Carey Reference Carey, Small and Lin Yoong2014).
MCQs
Select the single best option for each question stem
-
1 Which of the following medications would be categorised as conferring a low risk of weight gain?
-
a Citalopram
-
b Mirtazapine
-
c Fluoxetine
-
d Venlafaxine
-
e Amitriptyline.
-
-
2 What is the estimated prevalence of comorbid depression in people with obesity?
-
a 5%
-
b 8%
-
c 16%
-
d 20%
-
e 23%.
-
-
3 What change in daily calorie intake has been associated with initiation of antidepressant medication?
-
a Decreased by 105 kcal
-
b Decreased by 75 kcal
-
c No change
-
d Increased by 155 kcal
-
e Increased by 215 kcal.
-
-
4 Which best describes the link between depression and obesity?
-
a There are many mechanisms, but primarily biological mechanisms are the link
-
b Depression causes obesity
-
c Obesity causes depression
-
d The relationship is a complex one with many different factors contributing to the cycle
-
e They are unrelated.
-
-
5 What describes the effect of weight-loss medication on mood?
-
a Some medications have side-effects which include change in mood
-
b Orlistat and all other weight-loss medications do not affect mood
-
c All weight-loss medications have a negative effect on mood
-
d All weight-loss medications have a positive effect on mood
-
e Weight-loss medications are unrelated to mood.
-
MCQ answers
1 c 2 e 3 e 4 d 5 a










eLetters
No eLetters have been published for this article.