1.1 Introduction
Once thought to be an immune-privileged site, we now know that there is a complex and essential bidirectional interplay between the central nervous system (CNS) and the immune system (Reference Savitz and Harrison1). Technological advances in imaging, genomic medicine and immunology have resulted in major revisions to some of the most fundamental and long-held assumptions in neuroscience, and we now understand that the immune system is critically involved not only in brain pathology, but also in the normal processes of brain development and homeostasis.
‘Immunopsychiatry’, namely the study of the interactions between neuroscience, mental health and the immune system, has rapidly become a major priority for psychiatric research. Accumulating evidence indicates roles for the immune system in the pathophysiology of many neurodegenerative and psychiatric disorders including Alzheimer’s disease (AD), schizophrenia, major depressive disorder (MDD), multiple sclerosis (MS) and others. In particular, pro-inflammatory cytokines associated with inflammation, which is common to many of these disorders, can influence the brain to bring about a host of physiological as well as behavioural alterations such as changes in mood and cognition. Raised pro-inflammatory cytokines are consistently reported in MDD (Reference Dowlati, Herrmann and Swardfager2) and schizophrenia (Reference Miller, Buckley, Seabolt, Mellor and Kirkpatrick3). Moreover, systemic inflammation brought about by acute infection, diabetes, obesity and atherosclerosis can accelerate cognitive decline in the elderly and present significant risk for the development or acceleration of AD and delirium (Reference Cunningham and Hennessy4). With this new knowledge come new targets to be exploited as biomarkers for diagnosis or informing treatment. A number of immune-modulating therapeutics are currently being trialled for the treatment of MDD, schizophrenia, AD and others.
In this chapter we aim to provide an overview of the key concepts in immunology and lay the foundations for understanding the role of the immune system in normal CNS homeostasis, and its contribution to neurodegeneration and psychiatric illness.
1.2 Overview of the Immune System
The most fundamental function of the immune system is to prevent and eradicate threats to bodily integrity. Frequently these threats are exogenous, invading microbes for example; but can also be endogenous in the form of damaged or dying tissue and malignancy.
The immune system can be broadly classified into two arms: the innate immune system and the adaptive immune system. Innate or natural immunity is always present in the individual. It consists of barriers that physically prevent entry of invading microbes, and a range of cells and non-cellular products that recognize and respond to threat. The innate immune system is critical for conveying messages to the brain warning of infection, resulting in the fever response. Additionally, components of the innate immune system enhance the adaptive immune response to pathogens. The adaptive immune system consists of T and B cells and their products. Where the innate immune system recognizes structures that are shared by many classes of microbes, and mounts the same response on repeated exposure, the adaptive immune system recognizes a wide variety of molecules with exquisite specificity, generates memory, and mounts a stronger response with each subsequent exposure. The innate and adaptive arms of the immune system work co-operatively to respond to threats and preserve bodily integrity. Failure can result in overwhelming infection, autoimmunity, or tissue damage, such as occurs in neurodegeneration. The immune system is increasingly recognized for its additional non-immune functions: wound healing, organ development and maintenance of homeostasis. The innate and adaptive immune systems and their contributions to immunopsychiatry will be discussed in this chapter.
1.3 Innate Immune System: Barriers
The first line of defence against invading microbes are the skin and mucosal epithelium, including the digestive tract, lungs and urogenital tract, which together provide a continuous physical barrier preventing the entry of microbes into the bloodstream. Within the healthy CNS, the blood-brain-barrier (BBB) and the blood-cerebrospinal fluid barrier (BCSFB) play analogous roles, blocking entry of microbes and selectively restricting the entry of immune cells. However, it is important to recognize that they are highly dynamic interfaces that communicate with the adjacent environments and serve the needs of the CNS (Reference Banks5). The BBB is a complex system comprising highly specialized endothelial cells that are joined by tight junctions, an underlying basement membrane embedded with pericytes, perivascular antigen presenting cells (APCs) and astrocytic endfeet (Reference Engelhardt and Sorokin6). The BCSFB on the other hand consists of the choroid plexus, which provides a physical interface between the blood and the cerebrospinal fluid (CSF). The choroid plexus epithelial cells generate approximately two-thirds of the CSF and help to control its composition by regulating the passage of ions, metabolites and molecules between the blood and the CSF (Reference Liddelow7). Tight junctions between the cells of the BBB and BCSFB inhibit the unrestricted diffusion of water soluble molecules, and both interfaces express active and passive cellular transporters that allow passage of select molecules, such as cytokines, hormones, amino acids and peptides between the blood and the CNS (Reference Banks5) (Figure 1.1).

Figure 1.1 Schematic representation of the Blood Brain Barrier and the blood cerebrospinal fluid barrier (BCSFB). A. The BBB consists of highly specialized endothelial cells joined by tight junctions. They deposit an endothelial basement membrane (yellow) in which pericytes are embedded. The parenchymal basement membrane (orange) is deposited by astrocytes and together with astrocytic endfeet forms the glia limitans perivascularis. The endothelial layer prevents the unrestricted movement of large solutes, antibodies and immune cells while allowing the passage of smaller solutes, cytokines and other proteins through dedicated transport systems.B. The BCSFB is made up by the choroid plexus epithelial cells which similarly prevent the unrestricted passage of molecules between the blood and the CSF. Central memory T cells are the predominant immune population in the CSF, and it is thought that they may cross the BCSFB, although the molecular mechanisms behind this are still unclear.
The BBB should not be thought of as an uninterrupted physical barrier. Certain regions of the brain, such as the circumventricular organs (CVOs) and the nucleus tractus solitarius have a specialized BBB with fenestrated capillary walls that enhance sensing of circulating molecules. Others, like the large vessels of the pial and subarachnoid space, and the sensory ganglia of the spinal and cranial nerves, contain ‘functional leaks’, allowing passage of molecules and substances into the Virchow–Robin spaces and participation in glymphatic flow. While the amounts of material entering the CNS through these pathways is small, it is probably the route through which natural and therapeutic antibodies gain access to the brain, such as anti-amyloid-β antibodies currently in trials for the treatment of AD (Reference Sevigny, Chiao and Bussière8).
1.4 Innate Immune System: Cells
In the periphery, a range of innate immune cells interact to prevent or limit the spread of infection. These cells include phagocytic neutrophils and monocytes, dendritic cells (DCs), mast cells and eosinophils, as well as specific subsets of lymphocytes such as innate lymphoid cells (ILCs), natural killer (NK) cells and NK-T cells. Within the CNS the range of innate immune cells is more limited, the most prevalent being the tissue-resident macrophage population known as microglia, which make up 10–15% of all brain cells (Reference Ransohoff and Cardona9). These cells populate the CNS early in embryonic development, performing typical phagocytic functions such as clearance of apoptotic cell debris and sensing local tissue damage, as well as tissue-specific functions including synapse elimination, regulation of neurogenesis and remodelling of neural circuits (Reference Li and Barres10). While microglia are the resident immune population of the brain parenchyma, they are not the only innate cells to populate the CNS. Macrophages, NK cells, mast cells and DCs also reside within the choroid plexus, perivascular spaces of the CNS and its meningeal coverings, sampling local debris and communicating with surrounding cells (Reference Ransohoff and Engelhardt11). Within the body, histamine released from mast cells plays an important role in dilating postcapillary venules and increasing blood vessel permeability leading to the oedema, warmth and redness associated with classical inflammation. Within the CNS, mast cells also play a critical role in regulating BBB permeability and have been implicated in increases in BBB permeability associated with stress (Reference Esposito, Gheorghe and Kandere12).
While the innate immune system comprises a diverse array of cells with specific functions in host defence, this discussion will focus on selected cell types and their contributions to immunopsychiatry.
1.4.1 Macrophages/Microglia
Macrophages and monocytes are closely related professional phagocytes that engulf and digest microbes and dead and dying host cells. During an inflammatory reaction, circulating blood monocytes can extravasate into tissues where they differentiate into specialized macrophages. The majority of tissues in the body contain phenotypically distinct tissue-resident macrophage populations which act as immune sentinels and perform additional tissue-specific functions. Langerhans cells (skin), Kupffer cells (liver), alveolar macrophages (lungs) and microglia (brain) are examples of tissue-resident macrophage populations. Fate mapping studies indicate that most tissue-resident macrophage populations derive from yolk sac or foetal liver origins, with blood monocytes existing in tissues only transiently (Reference Davies, Jenkins, Allen and Taylor13). The microglia of the brain are established in their niche prenatally, and derive from cells in the yolk sac (Reference Ginhoux, Greter and Leboeuf14). Experimental evidence suggests that microglia undergo self-renewal via local proliferation; however under certain circumstances infiltrating blood monocytes can differentiate into a microglial-like phenotype within the brain parenchyma (Reference Ajami, Bennett, Krieger, Tetzlaff and Rossi15,Reference Mildner, Schmidt and Nitsche16).
Macrophages are a highly dynamic cell population which undergo significant structural and functional changes depending on their local environment. They are critically important not only in the innate immune recognition of foreign and self-antigens, but also in the presentation of antigen to T cells and in guiding the outcome of the adaptive immune response through the secretion of specific cytokines.
Several studies have associated peripheral monocyte/macrophage numbers or activation status with psychiatric disorders. Monocytes/macrophages in both the blood and CSF may be increased in patients with schizophrenia or during acute psychotic episodes (Reference Nikkilä, Müller and Ahokas17,Reference Jackson and Miller18). While the number of monocytes does not appear to be altered in bipolar disorder, gene expression studies suggest that monocytes may be shifted towards a pro-inflammatory phenotype (Reference Padmos, Hillegers and Knijff19).
Traditionally, microglia have been thought of as existing in two states: ‘resting’ or ‘activated’, a misleadingly simplistic classification. Imaging studies have shown that ‘resting’ microglia are in fact highly active, continuously sampling the local environment with extremely motile processes (Reference Nimmerjahn, Kirchhoff and Helmchen20). Microglia express an array of receptors including: purinergic, neurotransmitter, cytokine, complement and Fc receptors, as well as pattern recognition receptors (PRRs), that allow them to detect subtle changes in their microenvironment (Reference Nimmerjahn, Kirchhoff and Helmchen20). In this ‘resting’ state microglia can secrete many neurotrophic factors including brain-derived neurotrophic factor (BDNF), transforming growth factor (TGF)-β, insulin-like growth factor 1 (IGF1), as well as pro- and anti-inflammatory cytokines (Reference Nimmerjahn, Kirchhoff and Helmchen20). In response to homeostatic disruption such as injury, infection or increased neuronal activity, microglia become activated and switch from undirected monitoring to targeted movement of microglial processes towards the site of insult (Reference Nimmerjahn, Kirchhoff and Helmchen20,Reference Davalos, Grutzendler and Yang21). Additional changes to morphology, motility, cell number, phagocytic capacity and cytokine secretion can also occur. Importantly, the shift from ‘resting’ (M1) to ‘activated’ (M2) phenotypes is accompanied by increased expression of the translocator protein (TSPO) which can be detected with positron emission tomography (PET) tracers such PK11195, FEPPA and DTA714 (Reference Setiawan, Wilson and Mizrahi22). Microglial dysregulation is associated with a number of psychiatric and neurodegenerative disorders including amyotrophic lateral sclerosis (ALS), AD, Parkinson’s disease, MDD and schizophrenia (Reference Li and Barres10,Reference Tay, Béchade and D’Andrea23).
A range of cytokines are secreted by microglia in both the healthy and the diseased brain. Microglial-derived IL-1β appears to be critical for normal hippocampus-dependent cognition, learning and memory formation (Reference Williamson, Sholar, Mistry, Smith and Bilbo24), whereas abnormally high levels of IL-1β and tumour necrosis factor (TNF)-α profoundly impair memory and are associated with the development of AD and other neurodegenerative disorders (Reference Marin and Kipnis25,Reference Holmes, Cunningham and Zotova26).
Microglia are critically dependent on colony stimulating factor 1 receptor (CSF1R) signalling for survival (Reference Erblich, Zhu, Etgen, Dobrenis and Pollard27,Reference Elmore, Najafi and Koike28). Congenital absence of CSF1R in mice results in abnormal postnatal brain development, with enlarged ventricles, increased neuronal density in the cortex, elevated numbers of astrocytes and reduced oligodendrocytes, with these animals rarely surviving to adulthood (Reference Erblich, Zhu, Etgen, Dobrenis and Pollard27). Whereas adult mice briefly depleted of microglia using CSF1R inhibitors show no readily apparent behavioural abnormalities or cognitive deficits, suggesting that microglia may not be essential for these functions in the fully developed brain (Reference Elmore, Najafi and Koike28). Pharmacological inhibition of CSF1R in mice with AD-like pathology halts microglial proliferation, prevents synaptic degeneration and improves cognitive performance (Reference Olmos-Alonso, Schetters and Sri29). Interestingly, microglial activation as a result of cranial irradiation for the treatment of brain cancer is associated with progressive and severe cognitive dysfunction. Experimental evidence suggests that elimination of microglia prior to cranial irradiation of mice ameliorates these cognitive defects (Reference Acharya, Green and Allen30). Therapies designed to deplete microglia using CSF1 R inhibitors or similar in the human brain could have efficacy for disorders associated with microglial activation and cognitive decline.
1.4.2 NK Cells
NK cells detect the absence of major histocompatibility complex (MHC) Class I proteins on infected, damaged or malignant cells and respond by inducing apoptosis of the target cell, independent of the adaptive immune system. Reduced NK cell numbers have been repeatedly found in patients with MDD, with some studies suggesting that reduced NK cell number or activity is associated with treatment resistance (Reference Grosse, Hoogenboezem and Ambrée31–Reference Grosse, Carvalho and Birkenhager33). Abnormal NK cell activity has also been associated with schizophrenia; however, the direction of association is less consistent, possibly owing to clinical heterogeneity in the patient cohorts and small sample sizes (Reference Yovel, Sirota and Mazeh34). Of general relevance to psychiatry is the concept that psychological stress, a common feature across psychiatric disorders, is often accompanied by reduced NK cell activity (Reference Schedlowski, Jacobs and Stratmann35–Reference Duggal, Upton, Phillips, Hampson and Lord37).
1.5 Innate Immune System: Molecules
The innate immune system employs a network of soluble and cell surface associated molecules to detect and combat invading microbial pathogens, remove potentially damaging dead and dying cells, and promote tissue repair. Like much of the immune system, these molecules are understood to also play roles in the maintenance of homeostasis, during development and in various aspects of normal and pathological mental function.
Innate immune cells express PRRs that recognize distinct microbial molecules known as pathogen-associate molecular patterns (PAMPs), or molecules released from damaged or dying host cells known as damage-associated molecular patterns (DAMPs). PAMPs are generally microbial structures that are essential for the survival or infectivity of the microbe, making this feature of innate immune recognition highly effective.
1.5.1 Toll-Like Receptors
TLRs are expressed on the cell surface or endosomal membranes of innate immune cells, including macrophages, NK cells, and DCs, as well as some non-immune cells such as endothelial cells. Importantly, TLRs are expressed by microglia, astrocytes, oligodendrocytes, neurons, neural progenitor cells and the BBB endothelium (Reference Okun, Griffioen and Mattson38). They are recognized for their importance in innate immune defence, and more recently they have been acknowledged for their roles in CNS plasticity and regulation of cognitive function in the absence of pathogen-derived ligands.
Individual TLRs recognize PAMPs that are shared across various classes of pathogen, and host-derived DAMPs. For example, TLR4 recognizes bacterial lipopolysaccharide (LPS) that is common to the outer membrane of all Gram-negative bacteria, as well as endogenous proteins such as heat shock protein-70 (HSP-70). In the CNS, TLR2 and TLR4 are expressed predominantly by glial cells including microglia, and both have been implicated in neuropathic pain (Reference Tanga, Nutile-McMenemy and DeLeo39,Reference Hutchinson, Zhang and Brown40). Interestingly, opioids such as morphine are capable of activating microglia by binding to the TLR4 accessory protein MD2 (Reference Hutchinson, Zhang and Shridhar41), and by stimulating the release of HSP-70 from neurons which in turn potentiates the TLR4 mediated inflammatory response (Reference Qu, Tao and Teng42). Furthermore, activation of TLR4 signalling by opioids is thought to contribute to the development of opioid tolerance and dependence, supporting a role for immune signalling in drug reward and opening up new possibilities for the treatment of addictions (Reference Hutchinson, Northcutt and Hiranita43). TLR2, TLR3 and TLR4 are expressed by human neural progenitor cells and influence cell proliferation and differentiation (Reference Okun, Griffioen and Mattson38). Increased gene expression of TLRs has been found in the brains of depressed suicide victims (Reference Pandey, Rizavi, Ren, Bhaumik and Dwivedi44), and peripheral blood of patients with MDD (Reference Hung, Kang, Huang and Huang45).
1.5.2 NOD-Like Receptors and the Inflammasome
The innate immune system utilizes a number of other PRRs in addition to TLRs. The nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) are a family of cytosolic receptors that sense both PAMPs and DAMPs in the cytoplasm of host cells. Engagement of NLRs by their ligand results in activation of the nuclear factor κ-light-chain-enhancer of activated B cells (NFκB) transcription factor and upregulation of pro-inflammatory cytokine gene expression. The NLRs form an important component of most inflammasomes. These are multimeric protein complexes that consist of an inflammasome sensor (often an NLR), the adaptor protein ASC, and caspase 1. Activation of the inflammasome generally results in cleavage of the inactive cytokine precursors, pro-IL-1β and pro-IL-18 and the subsequent release of their active forms, IL-1β and IL-18, respectively (Reference Latz, Xiao and Stutz46).
Of particular importance for immunopsychiatry is the NLR family pyrin domain containing 3 (NLRP3) inflammasome, a cytosolic inflammasome complex that responds to a diverse range of PAMPs and DAMPs by enhancing production of the pro-inflammatory cytokines IL-1β and IL-18 and has been implicated in the pathophysiology of MDD (Reference Alcocer-Gómez, de Miguel and Casas-Barquero47,Reference Zhang, Liu and Liu48), bipolar disorder (Reference Kim, Andreazza, Elmi, Chen and Young49) and AD (Reference Heneka, Kummer and Stutz50). Psychological stress can activate the NLRP3 inflammasome via adenosine triphosphate (ATP) stimulation of the P2X7 receptor in the mouse hippocampus, ultimately leading to an increase in IL-1β secretion and increased neuroinflammation (Reference Iwata, Ota and Li51). Interestingly, deletion of the NLRP3 gene from mice renders them resistant to the depressive behaviour normally induced by chronic unpredictable stress, possibly implicating the NLRP3 inflammasome in the well-established links between stress and increased risk of MDD (Reference Iwata, Ota and Li51). Together, these studies identify potential therapeutic targets for the treatment of depressive and neuroinflammatory disorders.
The final group of PRRs of importance to immunopsychiatry are the secreted forms, particularly C-reactive protein (CRP). CRP is an acute-phase protein synthesized in the liver with the primary function of activating the complement cascade. CRP is commonly measured in the clinic as an indicator of inflammation, and has consistently been shown to be raised in a proportion of patients with MDD (Reference Smith, Au, Ollis and Schmitz52–Reference Wium-Andersen, Ørsted, Nielsen and Nordestgaard54). CRP shows promise as a peripherally accessible biomarker to predict the development of depression (Reference Khandaker, Pearson, Zammit, Lewis and Jones55) and for predicting therapeutic response to serotonergic and noradrenergic antidepressants (Reference Uher, Tansey and Dew56). CRP has additionally been implicated in schizophrenia (Reference Inoshita, Numata and Tajima57,Reference Fernandes, Steiner and Bernstein58); bipolar disorder, where it appears to be particularly increased during periods of mania (Reference Fernandes, Steiner and Molendijk59); and autism (Reference Khakzad, Javanbakht and Shayegan60).
1.5.3 The Complement System
The complement system is a network of more than 30 circulating and membrane-associated proteins responsible for induction of the complement cascade and inflammatory response. While complement components are synthesized predominantly by the liver, they can also be produced selectively by various other cell types including monocytes, fibroblasts, epithelial cells and, notably, all brain cell populations (Reference Orsini, De Blasio, Zangari, Zanier and De Simoni61). Initially recognized for its role in innate immune defence against pathogens, the complement system is now also known for its importance in the maintenance of brain homeostasis, synaptic plasticity and regeneration. Over-activation of the complement system has been implicated in the pathology of stroke, traumatic brain injury, AD, Parkinson’s disease and ALS (Reference Orsini, De Blasio, Zangari, Zanier and De Simoni61). More recently, excessive complement activity in the brain has been functionally linked to genetic risk for schizophrenia (Reference Sekar, Bialas and de Rivera62). Sekar et al. found that gene expression of complement component 4 (C4A) is elevated in the brains of individuals with schizophrenia and that higher levels of C4A expression are associated with a greater risk of schizophrenia. In addition to its critical role in innate immunity, many complement components including C4 are involved in synaptic pruning. Frequently documented pathological finding in the brains of individuals with schizophrenia include cortical grey matter loss and reduced numbers of synaptic structures (Reference Haijma, Van Haren and Cahn63,Reference Osimo, Beck, Reis Marques and Howes64). Given that microglia express the majority of complement receptors in the brain and are critically important in synaptic pruning, Sekar and colleagues offer an intriguing mechanistic explanation: that excessive synaptic pruning by microglia, driven by C4 deposition at the synapses, contributes to the pathology of schizophrenia (Reference Sekar, Bialas and de Rivera62). While C4 and other complement components have potential as diagnostic biomarkers or therapeutic treatment targets, much work still needs to be done before complement therapies reach the clinic.
1.6 Linking Innate and Adaptive Immunity: Cytokines and Chemokines
Cytokines are small proteins secreted by immune and non-immune cells that are responsible for intercellular communication, mediating many of the cellular responses of both innate and adaptive immunity. In innate immunity, most cytokines are produced by mast cells, DCs and macrophages, whereas the main cytokine producers of the adaptive immune system are T helper cells. Chemokines are a class of cytokines that act as chemoattractants for immune cells. Together, the cytokine network includes the interleukins, interferons, tumour necrosis factors, TGFs, colony stimulating factors and chemokines (CCL and CXCL nomenclature). Under steady-state conditions these molecules circulate in very low (picomolar) concentrations, but during infection or trauma blood concentrations can increase up to 1,000-fold. Cytokines can be pro- or anti-inflammatory, and can act in an autocrine, paracrine or endocrine manner. Examples of the latter include the systemic fever response triggered by IL-1β and TNF-α acting on the hypothalamus, a component of sickness behaviours (Reference Dantzer, O’Connor, Freund, Johnson and Kelley65) (covered in detail in Chapter 8), and IL-6 acting on the liver to stimulate production of acute-phase proteins such as CRP. Cytokines typically display a high degree of redundancy and pleiotropy and can enhance or inhibit the action of other cytokines in complex interactions, impeding simple functional classification. Nevertheless, one clinically useful division is between pro-inflammatory cytokines such as IL-1β, TNF-α, IL-6, IFN-γ and IL-12 – which share the common function of promoting inflammation; and anti-inflammatory cytokines – such as IL-4 and IL-10 that reduce inflammation and promote healing and tissue regeneration. Classification of cytokines can also be based on their roles in T helper cell polarization and function. IFN-γ and IL-12 are typically considered Th1 cytokines; IL-4, IL-5 and IL-13 are regarded as Th2 cytokines; and IL-17 and IL-22 as Th17 cytokines.
Some cytokines may cross the BBB through dedicated transporter systems or when barrier function is disrupted (Reference Banks66). Within the CNS, microglial activation results in the production of a number of pro-inflammatory cytokines and chemokines such as IL-1β, TNF-α, IL-6 and CCL2. Like many features of the immune system, cytokines are now known to play additional non-immunological roles, such as in regulation of complex cognitive processes like sleep (Reference Krueger, Rector and Roy67) and hippocampus-dependent learning and memory formation (Reference Rachal Pugh, Fleshner, Watkins, Maier and Rudy68). There is also extensive evidence that some CNS cytokines, IL-1β in particular, are directly involved in the pathogenesis of neurodegenerative disorders (Reference Allan, Tyrrell and Rothwell69).
CCL2, also known as monocyte chemoattractant protein 1 (MCP1) controls monocyte and macrophage migration. CCR2, the receptor that binds CCL2, is constitutively expressed by immune cells and can also be expressed by microglia, astrocytes, and dopaminergic and cholinergic neurons (Reference Conductier, Blondeau, Guyon, Nahon and Rovère70). Increased CCL2 gene expression by monocytes or astrocytes has been linked to a number of neuroinflammatory disorders including MS, stroke, AD, epilepsy, traumatic brain injury and schizophrenia (Reference Conductier, Blondeau, Guyon, Nahon and Rovère70). Elevated peripheral CCL2 has been consistently found in MDD and schizophrenia (Reference Eyre, Air and Pradhan71–Reference Stuart and Baune73).
Numerous studies show increased levels of circulating pro-inflammatory cytokines, particularly IL-6 and TNF-α, and the acute-phase protein CRP, in MDD and schizophrenia (Reference Dowlati, Herrmann and Swardfager2,Reference Haapakoski, Mathieu, Ebmeier, Alenius and Kivimäki74,Reference Rodrigues-Amorim, Rivera-Baltanás and Spuch75). Importantly, peripheral levels of IL-6 are thought to precede depressive episodes (Reference Khandaker, Pearson, Zammit, Lewis and Jones55,Reference Miller and Cole76) and are often higher in individuals with a history of childhood adversity (Reference Baumeister, Akhtar, Ciufolini, Pariante and Mondelli77). There is considerable interest in exploiting IL-6 as a therapeutic target, with a number of humanized monoclonal antibodies targeting IL-6 or the IL-6 receptor currently in clinical trials. Evidence linking pro-inflammatory cytokines and depression will be covered in detail in Chapter 10. Another group of cytokines important to immunopsychiatry are the interferons, key players in the antiviral response that have been used therapeutically for the treatment of certain cancers and the chronic viral infection hepatitis C. Although it has been an effective treatment for hepatitis C, sustained therapeutic use of IFN-α precipitates major depressive episodes in approximately one-third of patients, although the risk can be minimized through pretreatment with the antidepressant paroxetine (Reference Musselman, Lawson and Gumnick78).
1.7 Adaptive Immune System
In contrast to the innate immune system, which functions through the recognition of shared microbial structures and does not possess any form of memory, the adaptive immune system is characterized by the generation of memory responses by lymphocytes that recognize microbes with exquisite specificity. The adaptive immune system can be broadly classified into two functional arms: cellular immunity which is mediated by T cells, and humoral immunity which is mediated by B cells and their secreted antibodies. Key features of the adaptive immune system are the antigen receptors: namely, membrane-bound antibodies in the case of B cells, and the T cell receptor (TCR) complex on T cells. These receptors recognize antigens with a high degree of specificity. The ability of the immune system to discriminate self from non-self, and thereby avoid mounting a pathological response to self antigens, is known as tolerance and is achieved through two processes, termed central and peripheral tolerance. Central tolerance largely occurs in the primary lymphoid organs (thymus and bone marrow) and is a process whereby lymphocytes that strongly recognize self-antigens presented by MHC molecules are removed before they enter the circulation. Additionally, some of the immature CD4+ T cells that do recognize self-antigen develop into T regulatory cells (Treg) that are important for peripheral tolerance (discussed in Section 1.8.1 below). If self-reactive T cells do leave the primary lymphoid organs, they can be further controlled by peripheral tolerance mechanisms through which mature self-reactive T cells are rendered functionally inactive (anergic) or their response is controlled by Tregs. Autoimmunity occurs when immune tolerance breaks down, causing the adaptive immune system to mount a pathogenic response against self-antigens. While the precise aetiology of the inflammatory demyelinating disorder MS is incompletely understood, breakdown of immune tolerance leading to auto-reactive T and B cells plays a major role.
1.7.1 Major Histocompatibility Complex (MHC)
All nucleated cells express MHC Class I, while professional APCs such as DCs and B cells express MHC Class II. CD4+ and CD8+ T cells can only recognize antigen bound to MHC Class I or Class II, respectively. The MHC locus contains more than 200 genes and includes those that encode the MHC Class I and Class II molecules themselves, and other genes collectively known as the Class III genes which include complement components, cytokines, and some genes with no known immune function. The MHC genes are highly polymorphic, with over 10,000 Class I alleles and 3,000 Class II alleles estimated in the human population. The polymorphic gene variants are inherited and determine which peptides can be presented by the MHC molecules. Given their crucial role in immunity it is unsurprising that single nucleotide polymorphisms (SNPs) in the MHC region have been linked to a diverse range of diseases. Over the last decade, several independent genome wide association studies (GWAS) have revealed persuasive evidence for involvement of MHC genes in schizophrenia susceptibility (Reference Corvin and Morris79,80).
1.7.2 Adaptive Immunity: T Cells
Microbial antigens that enter the body are transported to and concentrated within peripheral lymphoid organs where they are most likely to come into contact with lymphocytes bearing antigen receptors that can recognize and respond to them. Lymphocytes continuously circulate between the bloodstream and the peripheral lymphoid organs with perhaps only 2% present in the blood. T cells are functionally classified into three main subsets; CD4+ T helper cells (Th), CD4+ Tregs and CD8+ cytotoxic T cells (Tc).
Th cells do not kill infected cells or microbes directly, but as their name implies, help other cell types to eliminate microbes. They are the primary source of cytokines in the adaptive immune system. Following antigen recognition, naïve Th cells rapidly proliferate (a process known as clonal expansion) and differentiate into effector cells of various subtypes that perform distinct and targeted functions in host defence. The three main subsets of Th cells are Th1, Th2 and Th17 cells, each distinguished by their cytokine profiles, chemokine receptor expression and targeted functions. There is considerable plasticity in the cytokine profile of T cells, allowing one subset to convert to another given the right conditions (See Figure 1.2).
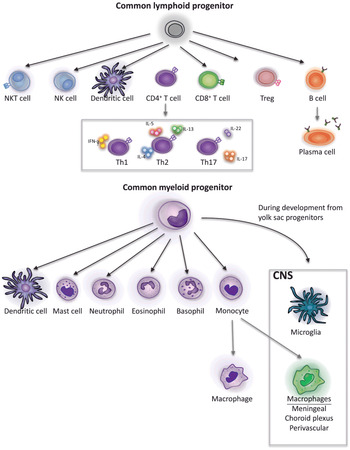
Figure 1.2 Developmental origins of immune cells. Lymphocytes and some dendritic cells derive from the common lymphoid progenitor cell line. CD4+ T cells can differentiate into T helper cells with specific functions, defined by their cytokine profiles. Activated B cells give rise to antibody secreting plasma cells. Granulocytes (mast cells, neutrophils, eosinophils and basophils), monocytes, some dendritic cells and microglia derive from the common myeloid progenitor cell line. However, microglia are established in the brain prenatally during development from yolk sac progenitors and are transcriptionally distinct from other myeloid cells. Monocytes give rise to macrophages, some in peripheral tissues and others in the CNS.
The differentiation of Th effector cells into Th1 cells occurs in the presence of IL-12 and IFN-γ. Th1 cells secrete an abundance of IFN-γ and enhance the efficiency of macrophage killing of ingested microbes. In contrast, Th2 cells are induced during parasitic worm infections and allergy, and are characterized by the secretion of IL-4, IL-5 and IL-13. Th17 cells are important for the control of extracellular bacteria and fungi, but also play a detrimental role in many chronic inflammatory disorders. They are induced by IL-6, IL1-β, IL-23 and TGF-β and characterized by their secretion of IL-17 and IL-22.
Autoimmune Th cells of both the Th1 and Th17 subsets have been well described in MS, where they cross the BBB and accumulate in inflammatory lesions (Reference Kebir, Kreymborg and Ifergan81,Reference Reboldi, Coisne and Baumjohann82). Notably, the ratio of Th1:Th17 cells appears to determine where in the CNS the inflammation will occur. Myelin-specific Th cells infiltrate the meninges regardless of lineage; however, inflammation of the brain parenchyma only occurs when the balance is shifted towards Th17 cells, resulting in an increase in IL-17 in the brain (Reference Stromnes, Cerretti, Liggitt, Harris and Goverman83). While each of the Th subsets has been variously implicated in psychiatric disorders (Reference Grosse, Hoogenboezem and Ambrée31,Reference Avgustin, Wraber and Tavcar84,Reference Brambilla, Bellani and Isola85), their roles are still unclear and many studies regarding Th17 cells in schizophrenia report contradictory findings (Reference Debnath and Berk86–Reference Fernandez-Egea, Vértes and Flint88). The discrepancies in these studies could be attributed to a number of factors such as inadequate sample size, ill-defined disease status (e.g., chronic versus recent onset psychosis), age, sex, body mass index (BMI) and treatment history. Moreover, antipsychotics may well have an effect on the inflammatory profile of immune cells (Reference Røge, Møller, Andersen, Correll and Nielsen89–Reference Chen, Tsai and Wang91). Risperidone and clozapine, two antipsychotics commonly used for the treatment of schizophrenia, have shown therapeutic potential in ameliorating experimental autoimmune encephalitis (EAE), an experimental mouse model of MS (Reference O’Sullivan, Green and Stone92). The immunomodulatory activity of these antipsychotics occurs primarily in the CNS, significantly reducing microglial activation in EAE mice. Although the precise molecular mode of action of most antipsychotics is incompletely understood, they are generally thought to exert their therapeutic effects through the blockade of dopamine and serotonin receptors, both of which have immunomodulatory properties themselves. It is thus conceivable that antipsychotics may have therapeutic potential in non-psychotic inflammatory disorders.
In contrast to Th cells, Tc cells recognize antigen bound to MHC Class I, present on all nucleated cells including neurons (Reference Shatz93). Following activation, naïve Tc cells differentiate into cytotoxic T lymphocytes (CTLs) that are adept at killing infected cells, thereby eliminating the reservoir of infection. Tc cells have been less well-studied in immunopsychiatry, although there is some evidence that increased proportions of Tc cells may predict non-responsiveness to antidepressant treatment (Reference Grosse, Carvalho and Birkenhager33).
Following resolution of infection, a fraction of Th and Tc cells persist as long-lived memory cells. These cells circulate throughout the lymphoid organs, mucosa, peripheral tissues and the blood stream, and upon exposure to their antigen they rapidly and vigorously respond. Memory cells are defined by the lack of CD45RA and by the expression of CD45RO. While the numbers of lymphocytes and leukocytes in the CSF of normal healthy humans are low, there is a predominance of central memory CD45RO+ Th cells (Reference de Graaf, Smitt and Luitwieler94). Few studies have investigated the phenotype of immune cells in the CSF of patients with psychiatric disorders. However, there is some evidence that these populations differ from those found in healthy CSF (Reference Nikkilä, Müller and Ahokas17,Reference Nikkilä, Müller and Ahokas95). The question of whether or not these cells are detrimental in the CNS is an interesting one. Early studies showed that in response to CNS injury, effector T cells and Treg cells become activated, with autoimmune brain-reactive T cells playing a protective role and preventing further neurodegeneration, and Tregs playing a detrimental role by dampening the effector response (Reference Moalem, Leibowitz-Amit and Yoles96–Reference Kipnis, Cardon and Avidan98). However, subsequent studies have shown that both depletion and administration of Tregs is associated with impaired neuronal survival (Reference Walsh, Zheng, Smirnov, Lorenz, Tung and Kipnis99). While this seems paradoxical, alterations in Treg numbers correlate with macrophage phenotype at the injury site. The broader relevance of this is yet to be defined. In addition to their preventative or pathologic roles in neurodegeneration, T cells also appear to be important in cognition and learning behaviour, as mice lacking T cells exhibit abnormal cognitive function. In the absence of T cells, meningeal myeloid cells acquire a pro-inflammatory phenotype which is associated with impaired learning behaviour (Reference Kipnis, Gadani and Derecki100). Taken together these studies suggest an important role for CNS T cells in maintaining homeostasis, that may have pathological implications when disrupted.
1.7.3 Adaptive Immunity: B Cells
B cells are able to recognize macromolecules of many types including proteins, lipids, carbohydrates and nucleic acids, without the requirement for MHC presentation. Activation of naïve B cells upon antigen exposure results in their clonal expansion and differentiation into antibody secreting plasma cells. The antibodies that are secreted have the same antigen specificity as the surface antibody receptor of the naïve B cell that initiated the response. Th cells are critical for the activation of B cells and the maturation of the humoral immune response. B cells can also have specificity for self-antigen, secreting antibodies that recognize self-antigens (autoantibodies) and mounting an unwanted immune response. In 2007, the first cases of antibody-mediated encephalitis and associated psychosis were reported by Josep Dalmau and colleagues (Reference Dalmau, Tüzün and Wu101) and since this seminal publication, autoantibodies to multiple neuronal antigens or synaptic proteins have been described (Reference Lennox, Palmer-Cooper and Pollak102). Psychiatric or behavioural manifestations are often seen in patients with these autoantibodies. However, they have also frequently been detected in healthy control patients without psychiatric symptomology. A recent study estimates approximately 9% of patients presenting with first episode psychosis have serum anti-neuronal antibodies, which may be treatable with immunotherapy (Reference Lennox, Palmer-Cooper and Pollak102). This will be discussed in further detail in Chapter 5. Some preliminary evidence suggests that maternal brain-reactive antibodies may contribute to the development of autism in the child (Reference Brimberg, Mader and Jeganathan103). Autoantibodies are also associated with Parkinson’s disease, where the presence of anti-α-synuclein antibodies is reduced compared with healthy controls (Reference Brudek, Winge and Folke104). While the function of these autoantibodies in the healthy CNS is currently unknown, it is possible that they help to clear pathological α-synuclein, a key characteristic of Parkinson’s disease. Thus, like CNS T cells, CNS autoantibodies may serve both protective and pathologic functions which could be exploited for therapeutic or diagnostic benefit.
1.8 Glymphatics
Until recently the CNS was thought to be devoid of lymphatic vasculature, despite scattered reports over the years indicating its presence. With the 2012 demonstration of the ‘glymphatic system’ which functions as a fluid and solute clearance pathway (Reference Iliff, Wang and Liao105), and the subsequent 2015 discovery of meningeal lymphatic vasculature lining the dural sinuses and meningeal arteries to drain lymphatic fluid, immune cells and other solutes (Reference Louveau, Smirnov and Keyes106,Reference Aspelund, Antila and Proulx107), our mechanistic understanding of CNS drainage has undergone major revision. These two systems are now believed to work in concert to maintain homeostasis and contribute to CNS function and health (Reference Da Mesquita, Fu and Kipnis108).
In the periphery, toxic metabolites, cell debris, infiltrating microbes, DCs carrying microbial antigens, lymphocytes and interstitial fluid (IF) are drained from tissues via the lymphatic system. Located strategically along the lymphatic vessels are the lymph nodes through which the draining fluid passes before its return to the blood circulation. As lymphatic fluid passes through the lymph nodes its contents are sampled by B cells which can respond by producing antibodies, and APCs which internalize, process and present antigen to T cells, initiating the cell-mediated immune response. However, the CNS parenchyma lacks a classical lymphatic drainage system. Nedergard and colleagues demonstrated that CSF enters the brain within the periarterial spaces and then exchanges with IF, facilitated by aquaporin-4 (AQP4) water channels positioned within perivascular astrocyte endfoot processes. This fluid collects within perivenous spaces and drains to the subarachnoid CSF, which subsequently drains either to the venous circulation, extracranial lymphatic vessels or lymph nodes. Named the glial-associated lymphatic system, or ‘glymphatic system’, due to the dependence on AQP4 on astrocytes and the lymphatic function it serves. The glymphatic system allows nutrients such as glucose to diffuse through the brain parenchyma and functions to clear extracellular metabolites, waste products such as lactate, and protein aggregates such as amyloid-β from the brain parenchyma and into the CSF. Interestingly, glymphatic clearance of amyloid-β declines with age, and amyloid-β in turn suppresses glymphatic influx in a detrimental feedback loop. The drainage of amyloid-β is further impeded by APOE4, the most important genetic risk factor for late-onset AD, demonstrating the importance of the glymphatic system in the pathology of AD and highlighting its potential as a therapeutic target (Reference Iliff, Wang and Liao105).
The meninges that surround the brain and spinal cord are composed of three layers: the pia, arachnoid and dura maters. Despite scattered reports of the presence of lymphatic vessels within the meninges, it was not until 2015 that their presence gained wider attention thanks to work from Kipnis and Alitalo (Reference Louveau, Smirnov and Keyes106,Reference Aspelund, Antila and Proulx107). These two studies, published simultaneously, used advanced imaging techniques and fluorescent tracer experiments to demonstrate draining lymphatic vessels adjacent to arteries, major venous sinuses and cranial nerves (Figure 1.3).

Figure 1.3 CNS lymphatic and glymphatic systems. A. Schematic of the glymphatic system. CSF enters the brain parenchyma along paraarterial routes, exchanges with interstitial fluid and is cleared along paravenous routes. Convective flow is facilitated by AQP4 pores which are abundantly expressed on astrocytic endfeet. Clearance of solutes, proteins and waste products such as amyloid-β occur along this pathway. Draining fluid may be dispersed into the subarachnoid CSF, venous circulation or lymphatics.B. Schematic representation of lymphatic drainage of the brain. Meningeal lymphatic vessels (green) adjacent to the venous sinuses and arteries drain molecules and meningeal immune cells in the CSF into the deep cervical lymph nodes. Adapted with permission from Louveau et al. Neuron. 2016; 91:957–73 (Reference Louveau, Da Mesquita and Kipnis109).
Though still in the early stages, intensive research is underway to exploit these systems therapeutically for the treatment of disorders with CNS involvement.
1.9 Central and Peripheral Communication
Bidirectional crosstalk between the CNS and the peripheral immune system is now well established. An important example of immune-brain communication is the inflammatory reflex which consists of two arcs. The afferent or sensory arc involves visceral sensory neurons which detect and respond to inflammatory stimuli, such as changes in pro-inflammatory cytokines, by relaying information to the brain stem via the vagus nerve. Inflammation detected in the periphery can have profound effects on the brain, resulting in impaired learning and memory, cognitive abnormalities and altered mood (Reference Harrison, Brydon and Walker110,Reference Harrison, Doeller, Voon, Burgess and Critchley111) and this pathway is important in the generation of sickness behaviours (see Section 1.10 below). The efferent arc in which immunomodulatory signals are delivered from the brain stem to immune cells via the vagus nerve, is also known as the anti-inflammatory cholinergic pathway and signals through acetylcholine receptors expressed by immune cells, leading to NFκB activation and modulation of immune response (Reference Tracey112). Additionally, all primary and secondary lymphoid organs, including the thymus, bone marrow and spleen, receive direct sympathetic innervation (Reference Bellinger, Millar and Perez113). These sympathetic nerve fibres release the catecholamine norepinephrine (NE) in response to stress and other stimuli, which binds to β-adrenergic receptors on immune cells and modulates diverse immunological processes including lymphocyte activation, cytokine production and cell trafficking (Reference Sloan, Capitanio and Tarara114).
It is clear that some classical neurotransmitters, like acetylcholine in the inflammatory reflex, are not only expressed by immune cells but also functionally important for immune system signalling. Dopamine signalling, in particular, has been linked to modulation of the immune synapse that forms between an APC, typically a DC and a naïve T cell (Reference Pacheco, Riquelme and Kalergis115). This interaction determines the fate of the T cell and is conditioned by dopamine released presynaptically from DCs activating dopamine receptors (DR) postsynaptically expressed by T cells. For example, activation of D3DR inhibits the suppressive capability of Tregs (Reference Kipnis, Cardon and Avidan98) which may be important for dysregulated cognition. Dysregulated dopaminergic neurotransmission has been implicated in the pathogenesis of Parkinson’s disease, schizophrenia, dementia with Lewy bodies and attention deficit hyperactivity disorder. In addition to these central defects in dopamine function, Parkinson’s disease has been replicably associated with increased (Reference Debnath and Berk86,Reference Ilani, Ben-Shachar and Strous116,Reference Brito-Melo, Nicolato and de Oliveira117) and schizophrenia with decreased (Reference Nagai, Ueno and Saeki118) peripheral expression of the gene coding the D3DR gene. Collectively these studies indicate that the peripheral immune system is capable of interacting with the nervous system through the secretion and recognition of neurotransmitters. With further research, the peripheral expression of neurotransmitter-related genes may prove useful as biomarkers for dopaminergic disorders such as schizophrenia and Parkinson’s disease.
1.10 Sickness Behaviour
In addition to immune activation, the physiological response to infection is accompanied by behavioural changes such as lethargy, malaise, irritability, impaired concentration, behavioural depression, decreased social activity, anhedonia and somnolence. Collectively these symptoms are known as ‘sickness behaviours’, notable for their remarkable similarity to common symptoms of depression. The main pro-inflammatory cytokines responsible for sickness behaviour are IL-1β and TNF-α, both of which are increased in the brain following peripheral infection or experimental administration of LPS in the absence of infection (Reference Dantzer, O’Connor, Freund, Johnson and Kelley65,Reference Thomson, McColl, Cavanagh and Graham119). Conversely, anti-inflammatory cytokines such as IL-10 limit sickness behaviour and attenuate the behavioural effects induced by LPS stimulation (Reference Dantzer, O’Connor, Freund, Johnson and Kelley65). Pro-inflammatory cytokines act on the brain to reorient motivation towards prioritizing wound healing and fighting infection. The behavioural phenotypes of social avoidance and anhedonia help to protect from further insult and pathogen exposure (Reference Miller and Raison120). When inflammation is severe or prolonged, as in chronic inflammatory conditions, major depressive episodes frequently arise. Roughly one-third of patients receiving recombinant IL-2 or IFN-α develop major depression, even previously euthymic individuals (Reference Musselman, Lawson and Gumnick78), and similar patterns of brain dysfunction are seen in both IFN-α induced depression and idiopathic MDD (Reference Harrison121). See Chapter 8 for an in-depth review of sickness behaviour.
1.10.1 Kynurenine Pathway
Cytokine immunotherapy in cancer patients is accompanied by a marked reduction in plasma tryptophan concentration that is positively correlated with depression scores (Reference Miller and Raison120,Reference Capuron, Gumnick and Musselman123). Actively transported into the brain, tryptophan is an essential precursor for the synthesis of serotonin, a neurotransmitter well known for its involvement in mood regulation. Decreased plasma tryptophan in patients undergoing immunotherapy could be due to the action of the tryptophan metabolizing enzymes tryptophan dioxygenase (TDO) or indoleamine 2,3 dioxygenase (IDO), both of which divert tryptophan metabolism away from serotonin synthesis and towards kynurenine synthesis. Under normal circumstances TDO is the dominant enzyme, however the enzymatic activity of IDO is enhanced during inflammation and is potently induced by several pro-inflammatory cytokines including IFN-γ and TNF-α (Figure 1.4). Kynurenine is readily transported across the BBB into the CNS where its metabolites can be either neuroprotective or neurotoxic (Reference Dantzer, O’Connor, Freund, Johnson and Kelley65). Kynurenine metabolites may prove useful as biomarkers; for example, a high kynurenine/tryptophan ratio predicted remission in a recent trial of the anti-inflammatory COX-2 inhibitor celecoxib (Reference Krause, Myint and Schuett124). Interestingly, previous clinical trials suggest that the antidepressant effects of celecoxib are linked to its capacity to reduce serum IL-6 (Reference Abbasi, Hosseini, Modabbernia, Ashrafi and Akhondzadeh125). While the causative role of IDO activation in MDD still needs to be thoroughly investigated in humans, there is convincing evidence from rodent studies that therapeutic targeting of IDO may help to alleviate inflammation-associated depression (Reference O’Connor, Lawson and André126).

Figure 1.4 The enzymatic activity of IDO is enhanced during inflammation and diverts tryptophan metabolism away from serotonin synthesis and towards the kynurenine pathway. Kynurenine is readily transported across the BBB where it can be metabolized to 3-hydroxykynurenine (3-HK), quinolinic acid (QA) or kynurenic acid (KA). 3-HK can increase oxidative stress and may be neurotoxic. QA is preferentially produced by microglia and is an NMDA-receptor agonist which may be neurotoxic, whereas KA is produced by astrocytes and is an NMDA-receptor antagonist which may be neuroprotective. In the periphery KA is thought to modulate immune cell phenotypes. Impaired glutamatergic and serotonergic neurotransmission are both associated with the development of depressive behaviour.
1.11 Conclusion
This chapter serves as a broad introduction to some of the immunological concepts relevant to the study of neurodegenerative and psychiatric disorders. A greater understanding of the causative role of the immune system in these disorders could generate novel immunotherapeutic targets for diagnosis and treatment. Convincing evidence from preclinical studies indicate that inflammation is a key contributor to MDD; several clinical trials using anti-inflammatory immunotherapies to treat MDD are in various stages of completion and are showing promising results. Recent advances in our understanding of how the immune system interacts with the brain to cause pathology offer a range of new therapeutic targets for researchers and clinicians to utilize. Although the field of immunopsychiatry is still relatively young, close collaborations between researchers, clinicians and industry will deliver high quality research and therapeutic targets for tackling what are currently some of the most common and debilitating human illnesses.







