Cellulose ethers including methyl cellulose (MC, CAS-9004-65-5), a type of dietary fibre, have been evaluated as safe for human consumption, though the acceptable daily intake has not been specified( 1 ). The main reason for the evaluation of this dietary fibre as safe is that the digestive tract is unable to absorb it. A viscous MC solution, for example 0·5 % (w/v) Methyl Cellulose 400cP Solution, Sterilized™, may therefore be used as a vehicle for oral administration instead of an edible oil( Reference Irwin 2 ). However, it has been reported that the administration of dietary fibre may have a variety of effects, including the induction of a change in mucosal formation in the proximal digestive tract( Reference Langhout, Schutte and Jong 3 – Reference Van Nevel, Dierick and Decuypere 5 ), the suppression of fat absorption( Reference Vissia and Beynen 6 , Reference Dikeman, Murphy and Fahey 7 ), an influence on the intestinal flora( Reference Hopwood, Pethick and Hampson 8 – Reference Lalles, Boudry and Favier 10 ), and the induction of an increase in intestinal mucin( Reference Montagne, Piel and Lalles 11 – Reference Tanabe, Ito and Sugiyama 14 ) and the number of goblet cells in the small intestine( Reference Piel, Montagne and Seve 15 , Reference Tanabe, Sugiyama and Matsuda 16 ). These reports have focused on the effects on the formation and function of the digestive tract; there have been few studies regarding changes induced in the stomach. When we used the viscous MC solution as a vehicle for food chemical administration via a stomach tube, we observed that the stomach became stiff and difficult to open evenly. In preliminary experiments, we noted that the gastric mucosa was thickened by the continuous administration of the viscous MC solution. We therefore investigated the effect of viscous MC solution on the stomach mucosa in the present study.
Materials and methods
A 0·5 % (w/v) Methyl Cellulose 400cP Solution, Sterilized™ was purchased from Wako Pure Chemicals Industries Limited and was used as the viscous MC solution in the present study. The viscosity of this solution, which is available online at http://www.siyaku.com/, was 7·3 mPa × s. Histofine Mousestain Kit and DAB Substrate Kit were purchased from Nichirei Biosciences, Inc. Porcine serum was purchased from Gibco. HIK1083 reagent was purchased from Kanto Chemical Company, Inc. Proteinase K, nuclease P1, alkaline phosphatase from Escherichia coli and ribonucleases T1 and A were purchased from Sigma Chemical Company.
Animals and diet
Animal studies were performed in compliance with the regulations of the University of Shizuoka. Male ICR mice (4 weeks old) were purchased from Japan SLC, Inc. The animals were housed at 23 ± 2°C and in 50–70 % humidity in a room with a 12 h light–12 h dark cycle, and were maintained on an Oriental MF pellet diet (Oriental Yeast Company Limited) and tap water ad libitum. Oriental MF contained the following ingredients (per 100 g): 8·0 g moisture; 23·1 g crude protein; 4·9 g crude fat; 5·7 g crude ash; 2·8 g crude fibre; 55·5 g N-free extract (a detailed composition is available online at http://www.oyc-bio.jp/pages/animal_products/feed/ingredient). Mice were acclimatised for 1 week before the commencement of the study.
Experimental procedure
A total of forty-eight mice were divided into four groups: a control group, which was administered 0·2 ml of sterilised water/d for 4 weeks, and experimental groups I, II and III, which were orally administered 0·2 ml of viscous MC solution per d for 2, 3 and 4 weeks, respectively. Both water and MC solution were administered via a stomach tube. The administration period for all groups was terminated at 9 weeks of age. An MC solution dosage of 0·2 ml/animal was chosen for the following reasons. First, it had already been observed in a preliminary experiment that a significant difference in body weight between the control and experimental groups was not induced by the 0·2 ml administration. Therefore, it was thought that the experimental results were unaffected even if the dosage was fixed to 0·2 ml/animal. Second, 0·2 ml was a sufficiently small dosage compared with a commonly administered oral dosage( Reference Diehl, Hull and Morton 17 ).
Having been fasted for 12 h after the final MC administration, animals were killed by anaesthesia with diethyl ether. A 12 h fasting period was used to ensure that the stomach was not overly full, and thus to avoid any potential damage to the gastric mucosa during dissection, which could be caused by pressure between the tweezers and the gastric contents. The stomachs of seven animals from each group were used for the measurement of stomach weight, gastric mucosal weight and the pH of the stomach contents. After the stomach contents were removed, a larger cut was made and the stomach was rinsed gently with an isotonic NaCl solution. The mucosa was then scraped using a scalpel. The mucosa was stored at − 80°C until the measurement of 8-oxo-2′-deoxyguanosine (8-oxodG), a biomarker of tissue oxidative stress. The stomachs of the remaining five animals per group were used for histological analysis.
Histological examination
Corpus ventriculi obtained from the stomach were immediately fixed in 10 % formalin and cut horizontally into three pieces (cardiac, middle and pyloric), and the pieces were dehydrated with ethanol, cleaned in xylene, embedded in paraffin and cut into 4 μm-thick slices. From each of the three pieces, three slices were mounted on a glass microscope slide and subjected to haematoxylin and eosin staining. Mucosal thickness was measured every 200 μm on the slides, using an Olympus BX60 microscope and a DP20-5 digital camera (Olympus Corporation), and 150 points were measured per slide.
In addition, three slides, prepared as described previously, were randomly selected from each of the control and three experimental groups, and subjected to immunohistochemical staining. Paraffin-embedded sections were dewaxed in xylene and rehydrated with ethanol and PBS, and endogenous peroxidase activity was blocked using 1·3 % (w/v) H2O2–methanol solution. Slides were then processed using a Histofine Mousestain Kit and DAB Substrate Kit (Nichirei Biosciences, Inc.). HIK1083 monoclonal antibody medium (a 1:50 mixture of HIK1083 reagent and PBS containing 2 % (v/v) of porcine serum on a volume basis) was used as a primary antibody. Peroxidase activity was visualised using diaminobenzidine–H2O2 solution. Counter-staining was performed using haematoxylin. A control experiment was completed, omitting the primary antibody, and no non-specific staining was seen. The monoclonal antibody HIK1083 specifically reacts with the gland mucous cell-derived mucin localised in the gland mucous cells of the corpus, pyloric gland cells and Brunner's gland cells of the duodenum( Reference Nakamura, Ota and Katsuyama 18 , Reference Hayashida, Ishihara and Ichikawa 19 ). The measurement of the size of the HIK1083-stained area was performed using Scion Image software (available online from the National Institutes of Health at http://scion-image.software.informer.com/). An HIK1083-stained area of 250 μm in length was measured in the mucosal regions, in each of which the mucosal thickness was approximately equal to the mean value of the control and experimental groups. As a result, twenty-two, fifty-seven, thirty-two and fifty-two measurements from the control group and groups I, II and III were obtained, respectively. The HIK1083-stained area was expressed as a percentage of the mucosal region.
Measurement of 8-oxo-2′-deoxyguanosine
Measurement of 8-oxodG was carried out using the method of Kaneko et al. ( Reference Kaneko, Tahara and Takabayashi 20 ). Stomach mucosa was homogenised and centrifuged to remove mitochondria. Following hydrolysis by proteinase K, the tissue was hydrolysed by ribonucleases T1 and A to completely remove RNA. DNA was then extracted using chloroform–isoamyl alcohol and digested by nuclease P1 and alkaline phosphatase. These processes were performed under an Ar atmosphere. The mixture was filtered through an Ultrafree-MC filter (Millipore Company) and the filtrate was applied to an HPLC system with a Symmetry C18 column (4·6 × 150 mm; Kanto Chemical Company, Inc.) and an ESA Coulochem II 5200 electrochemical detector (ESA) with a guard cell 5020 (400 mV) and an analytical cell 5011 (350 mV). The amount of 8-oxodG is expressed as the molar ratio to 105 2′-deoxyguanosine, which was calculated from absorption at 260 nm in the same measurement using a UV detector.
Statistical analyses
Results are expressed as means and standard deviations, and statistical comparisons among groups were carried out using Dunnett's test. Analyses were performed using the Pharmaco Analyst software computerised statistical analysis program (Humanlife). P <0·05 was considered to be statistically significant.
Results
Effect of viscous methyl cellulose solution on body weight, food intake, stomach weight, the ratio of mucosal weight:stomach weight, the pH of the stomach contents and the length and weight of the digestive tract
There were no significant differences between the groups with respect to any of the parameters shown in Table 1. The administration of 0·2 ml of viscous MC solution/d via a stomach tube for 4 weeks caused no alteration in body weight, food intake or digestive tract length and weight. However, it was found that the ratio of mucosal weight:stomach weight in groups II and III tended to increase compared with that of the control group, and that there was an apparent difference between the values of groups I and II.
Table 1 Body weight, food intake, stomach weight, stomach mucosal weight, ratio of mucosal weight: stomach weight, pH of the stomach contents and the length and weight of the digestive tract (Mean values and standard deviations for twelve or seven mice per group)

Control, 0·2 ml of water/d for 4 weeks; groups I, II and III, 0·2 ml of methyl cellulose/d for 2, 3 and 4 weeks, respectively.
* Body weight at the end of the experimental period. The experimental period was ended for all groups at 9 weeks of age.
† Values represent means obtained from seven mice that were used for the measurement of 8-oxo-2′-deoxyguanosine. The remaining five mice in each group were used for histological examinations; therefore, the stomach mucosal weight was not measured in these mice.
Histological alteration induced by viscous methyl cellulose solution
The mucosal histology of the stomach sections of the different experimental groups stained with haematoxylin and eosin or the HIK1083 anti-mucin antibody is shown in Figs. 1 and 2, respectively. The thickness of the gastric mucosa and the percentage of the mucosal area stained with HIK1083 are shown in Table 2. The mucosa became thicker as the administration period of the viscous MC solution was extended, and there was a significant difference in thickness between the control group and groups II and III (P< 0·01). Although mucosal thickness increased with time during the MC treatment, neutrophil invasion was not observed (groups I, II and III). Moreover, the HIK1083-stained area was very small in the control group, but the size of the HIK1083-stained area increased with increasing duration of MC administration; a significant increase was shown in the percentage of the HIK1083-stained area between the control group and groups I, II and III (P< 0·01).
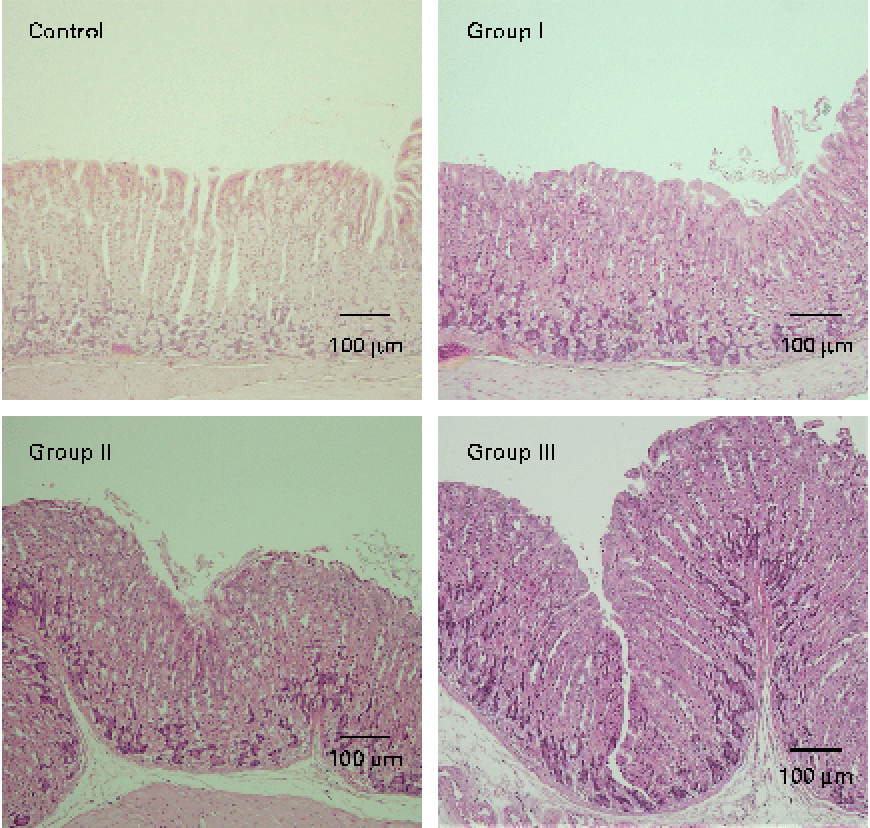
Fig. 1 Photomicrographs of mouse stomach samples (haematoxylin and eosin staining). Control, 0·2 ml of water/d for 4 weeks; groups I, II and III, 0·2 ml of methyl cellulose solution/d for 2, 3 and 4 weeks, respectively.
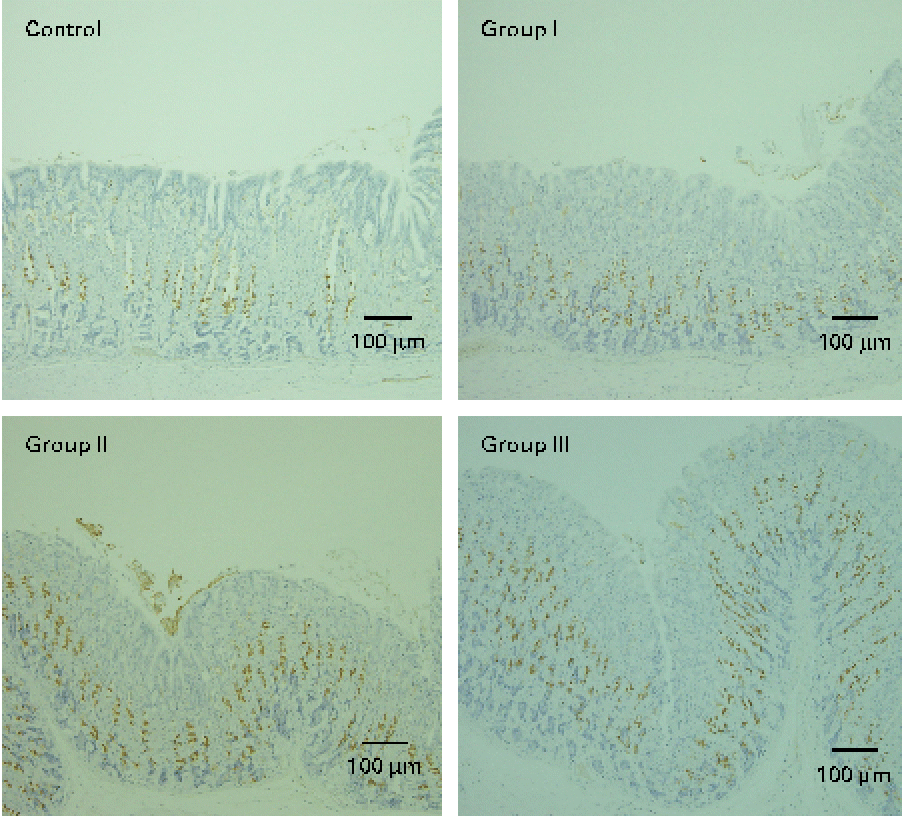
Fig. 2 Photomicrographs of mouse stomach samples (immunostained with the HIK1083 antibody). Control, 0·2 ml of water/d for 4 weeks; groups I, II and III, 0·2 ml of methyl cellulose solution/d for 2, 3 and 4 weeks, respectively. Brown colour indicates the HIK1083-stained area.
Table 2 Gastric mucosal thickness, percentage of HIK1083 antibody-positive cells and amount of 8-oxo-2′-deoxyguanosine (8-oxodG) (Mean values and standard deviations)
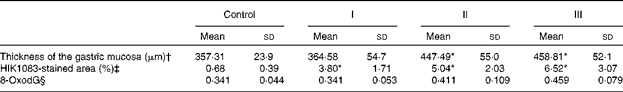
Control, 0·2 ml of water/d for 4 weeks; groups I, II and III, 0·2 ml of methyl cellulose solution/d for 2, 3 and 4 weeks, respectively.
* Mean values were significantly different compared with the control group (P< 0·01).
† Values represent the mean of five mice. The average value of mucosal thickness taken at 150 points in the corpus ventriculi was calculated for each mouse.
‡ Mean values represent the average of twenty-two to fifty-seven measurements of three mice selected randomly from five mice in each group.
§ Values represent the mean of seven mice and are expressed as the molar ratio to 105 2′-deoxyguanosine.
Amount of 8-oxo-2′-deoxyguanosine in the stomach mucosa
The amount of 8-oxodG in the nuclear DNA of stomach mucosa is shown in Table 2. Although a tendency for the amount of 8-oxodG to decrease was shown as the duration of administration extended, no significant differences were observed compared with the control group.
Discussion
Methyl cellulose, the methyl diethyl ether of cellulose, is included in the Japanese Pharmacopeia Sixteenth Edition and used as a distension-promoting laxative, and as an additive that increases the viscosity of a food. The safety of MC intake by human subjects was shown in a study that analysed MC intake at a dose of 250 mg/kg body weight for 23 d( Reference Eastwood, Brydin and Anderson 21 ). A solution of MC in water is therefore used as a vehicle for oral administration of various compounds in toxicity tests( Reference Irwin 2 ). In the present study, administration of a viscous MC solution showed no effect on body weight, food intake, stomach weight, the pH of the stomach contents or the length and weight of the distal digestive tract in mice (Table 1). These results suggest that 0·2 ml of MC viscous solution/d is not detrimental to mice at the whole-body level. Moreover, 0·2 ml for a mouse is equivalent to approximately 320 ml for a human (60 kg body weight) or 1·6 g MC. This is much less than the value shown in ‘Dietary Reference Intakes for Japanese – 2010’ (http://www0.nih.go.jp/eiken/info/dpf/dris2010en.pdf), in which dietary fibre intakes of more than 19·0 g/d (for males) and 17·0 g/d (for females) are recommended. It has therefore been estimated that ingestion of 320 ml of viscous MC solution/d for humans is safe.
Gastric mucosal thickness was investigated by haematoxylin and eosin staining of tissue slices. Previous studies have calculated an average value of gastric mucosal thickness by measuring the thickness at 60–180 arbitrarily selected points( Reference Irwin 2 , Reference Satchithanandam, Vargofcal-Apker and Calvert 22 , Reference Satchithanandam, Klurfeld and Calvert 23 ). In the present study, mucosal thickness was only measured in the corpus ventriculi at 150 points because mucosal thickness varies depending on which part of the stomach is studied; the mucosa is thin in the pyloric region, and there is no mucosa in the proventriculus. The data show that the increase in gastric mucosal thickness takes place essentially between weeks 2 and 3 of MC intake. It is thought that thickening of the mucosa is caused by an increase in the number of cells that constitute the gastric tissue, because mucosal histology was not observed to change. Piel et al. ( Reference Piel, Montagne and Seve 15 ) and Tanabe et al. ( Reference Tanabe, Sugiyama and Matsuda 16 ) have reported that administration of dietary fibre increases the number of goblet cells in the small intestine. They suggested that the increase in gastrointestinal mucous resulted from an increase in the number of excretory cells. Satchithanandam et al. ( Reference Satchithanandam, Vargofcal-Apker and Calvert 22 , Reference Satchithanandam, Klurfeld and Calvert 23 ) reported that dietary fibre feeding increased not only the amount of small-intestinal mucin but also the amount of gastric mucin. It was therefore presumed that a similar phenomenon (increasing the number of excretory cells) also occurred in the stomach.
To clarify this, we carried out HIK1083 immunohistochemical staining. The epitope that reacts with HIK1083 is a mucinous carbohydrate chain whose non-reduced end is connected to N-acetylglucosamine in the α form( Reference Ishihara, Kurihara and Goso 24 ); this epitope is common to different species of vertebrates( Reference Kurihara, Ishihara and Ota 25 ). In the present study, most of the secreted mucous was removed in the process of preparing the specimen for analysis; thus, the part of the tissue that was stained with the HIK1083 antibody indicated gland mucous cells. The size of the stained area significantly increased with the duration of MC solution administration (Table 2), as can be seen in Fig. 2. This result suggests that an increase in gastric mucosal thickness is associated with an increase in the number of gland mucous cells. Montagne et al. ( Reference Montagne, Piel and Lalles 11 ) and Satchithanandam et al. ( Reference Satchithanandam, Vargofcal-Apker and Calvert 22 , Reference Satchithanandam, Klurfeld and Calvert 23 ) have suggested that an increase in intestinal mucin secretion is an adaptation to chronic mechanical irritation by the digesta containing dietary fibre. Furthermore, some reports have suggested that a rheological interaction between soluble dietary fibre macromolecules and mucin is important. Rossi et al. ( Reference Rossi, Bonferoni and Ferrari 26 ) reported that the interaction between a gelated polymer and gastrointestinal mucin was produced by the interpenetration of these molecules. Wapnir et al. ( Reference Wapnir, Wingertzahn and Teichberg 27 ) and Go et al. ( Reference Go, Harper and Sia 28 ) reported that contact of a 0·25–1 % (w/v) solution of MC or carboxymethylcellulose with the mucosal surface could lengthen the glycocalyx of gastrointestinal mucus. Because the concentration of the viscous MC solution used in the present study was 0·5 % (w/v), there might be an interaction between this solution and the gastric mucosal surface. Schmidt-Witting et al. ( Reference Schmidt-Witting, Enss and Coenen 29 ) reported that an inflammatory reaction is associated with an increase in mucinous cells. Matsuzawa et al. ( Reference Matsuzawa, Ota and Hayama 30 ) and Kaneko et al. ( Reference Kaneko, Ota and Hayama 31 ) reported that the secretion of gastric gland mucin was increased by Helicobacter pylori infection, which induced significant inflammation in the gastric mucosa. However, neutrophil invasion, which is both a sign of inflammation and the main source of reactive oxygen species, was not observed in gastric tissue in the present study (Fig. 1). Moreover, there was no significant increase in 8-oxodG in the groups administered with viscous MC solution compared with the control group. Mechanisms other than inflammation that induce an increase in the number of gland mucous cells should be further investigated.
Acknowledgements
We express our sincere appreciation to Dr Noboru Harada, Emeritus Professor of Gastrointestinal Surgery of University of Shizuoka, for his critical review of the histological examination. The present study was supported by a grant from the University of Shizuoka. F. T. designed the experiments, carried out the main experimental work and wrote the manuscript. H. S. carried out the 8-oxodG measurement experiment. Both authors declare that they have no personal or financial conflict of interest.








