INTRODUCTION
Infection with the hepatitis A virus (HAV) remains a significant public health problem in certain geographical regions, especially developing countries. HAV has a high prevalence in South East Asia and is considered endemic to this region [Reference Fiore, Wasley and Bell1]. A population-based survey conducted in 1992–1995 reported that the seroprevalence of HAV antibodies was 80·9% in China [Reference Dai2]. In 1990, there were 637 717 cases of hepatitis A in China, and the incidence rate was 55·7/100 000 [Reference Li and Liang3]. Shanghai, which is on the eastern coast of China, experienced a serious HAV outbreak in 1988 due to the consumption of infected raw clams, and more than 300 000 residents developed hepatitis A [Reference Halliday4–Reference Kang6]. This is the largest reported outbreak of hepatitis A.
Hundreds of new sporadic cases of HAV infection are reported every year in Shanghai. Due to its coastal location, consumption of possibly infected shellfish and other seafood is common. A recent study detected HAV in aquatic products collected from local markets in Shanghai, and that the rate of HAV positivity in seafood was as high as 13·83% [Reference Zhu7]. Control of HAV in patients and seafood is necessary to prevent future outbreaks [Reference Pinto, Costafreda and Bosch8].
Previous studies [Reference Wasley, Samandari and Bell9, Reference Chodick10] have demonstrated that HAV vaccination has clear effects on the incidence rate of HAV infection. HAV vaccines have been available in Shanghai since the early 1990s. School children are given priority and other residents pay out-of-pocket for the vaccination. The incidence rate in Shanghai and other eastern regions was significantly lower than the national average in recent years [Reference Li and Liang3, Reference Zheng11]. Since 2009, free hepatitis A vaccinations were offered to all children in Shanghai born after 1 July 2006.
The purpose of the present study was to determine the anti-HAV seroprevalence of children and young adults in Shanghai in order to provide a basis for the development and implementation of immunization policies for the prevention of hepatitis A epidemics in a high-risk population.
METHODS
Study design and population
This was a clustering cross-sectional study of people aged 0–30 years who lived in six of the 18 districts of Shanghai, representing urban and suburban areas. The population of the six surveyed districts (∼8 million residents) represented 41·5% of the total population of Shanghai. The estimated sample size was 4378 at the 68·7% prevalence of HAV infection according to historical surveillance data. Considering the possibility of non-response (20%), the sample size was enlarged to 5253. Three communities were randomly selected in each district and 300 subjects were selected from each community, with a total of 10 subjects of each age from 0 to 30 years. Subjects were recruited continuously from community residents who visited the community centreduring the period January–December 2009.
Specimen and data collection
A 2-ml blood sample was collected from each study subject, centrifuged for separation of sera, and stored at −20 °C before testing. Each study participant also completed a questionnaire that assessed demographic information, history of previous hepatitis A vaccination, and history of hepatitis diagnosis and treatment. Participants were asked to bring their immunization records during interview. Hepatitis A vaccination status was determined by vaccination records and self-report. Questions about vaccination status on the questionnaire was grouped as yes/no/unknown. For children aged 1–14 years, the questionnaire was completed by their parents or guardians.
Laboratory assays
All blood samples were tested for anti-HAV IgM and total anti-HAV. Anti-HAV IgM was measured qualitatively with an ELISA detection kit (Kehua Company, China). Total anti-HAV (including anti-HAV IgM and anti-HAV IgG) was measured quantitatively with an ELISA kit (ETI-AB-HAVK Plus, DiaSorin S.p.A., Italy). Anti-HAV positivity was defined at a cut-off value of 20 mIU/ml.
Ethical considerations
The Institutional Review Board of Shanghai Municipal Centre for Disease Control and Prevention approved the study protocol. All adult study participants and the parents or guardians of minors provided written informed consent.
Statistical analysis
Data were entered by use of double-entry and verified in EpiData 3.0 (EpiData Association, Denmark). Statistical analyses were performed using Stata v. 10 (StataCorp, USA) and SAS 9.2 (SAS Institute Inc., USA.). Continuous variables were expressed as means and categorical variables as percentages. Pearson's χ2 test and a χ2 test for trend were used to identify associations of categorical and ordinal data, respectively. Student's t test was used to compare the means of numerical data. Two-tailed tests were performed with a significance level of 0·05.
RESULTS
Demographic characteristics of the study population
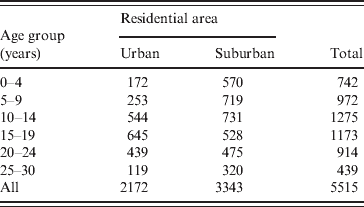
A total of 5515 subjects were enrolled in the study, which included 2172 subjects from urban areas and 3343 from suburban areas. Table 1 shows the distribution of study subjects by age and residential area. The mean age of the subjects was 14·3 ± 7·4 years (range 2 months to 30 years). A total of 2556 (46·3%) subjects were male and 2953 (53·7%) female.
Table 1. Age ranges and residential status of the study population
History of HAV infection
A total of 13 participants reported a previous diagnosis of hepatitis. Ten of these claimed to have been diagnosed and treated clinically as hepatitis A and the other three participants as hepatitis B or unidentified hepatitis. All 10 of these HAV-infected subjects were seropositive for anti-HAV, had an average anti-HAV concentration of 115·6 ± 7·7 mIU/ml (range 107·4–130·3 mIU/ml), and a mean age of 25·1 ± 4·2 years (range 18–30 years). One of the ten subjects was co-infected with HCV. The other three subjects were infected with unidentified hepatitis viruses, and all were anti-HAV negative.
HAV seroprevalence according to gender, age, and residential area
The overall seroprevalence of total anti-HAV was 52·9% [95% confidence interval (CI) 51·5–54·2]. Table 2 shows the anti-HAV seroprevalence by gender, age, residential area, and immunization history. The highest anti-HAV seroprevalence was observed in children aged 0–4 years (66·9%, 95% CI 63·3–70·2) and those aged 5–9 years (74·6%, 95% CI 71·7–77·3). There was a significant trend for lower anti-HAV seroprevalence in the older age groups (trend χ2 = 244·1, P < 0·0001). A review of the immunization history of the study population indicated that anti-HAV seroprevalence was 87·4% (95% CI 85·7–88·9) in subjects with HAV vaccination and only 37·0% (95% CI 35·5–38·6) in those without HAV vaccination or with unknown vaccination history [odds ratio (OR) 0·08, 95% CI 0·07–0·10]. In the vaccinated group, HAV vaccination was based on self-report for 139 participants and on vaccination records for 1595 participants. For those with no or unknown vaccination, it was judged by records for 2343 subjects and by self-report for 1440 subjects.
Table 2. Seroprevalence of anti-HAV according to gender, age, residential area, and immunization history

OR, Odds ratio; CI, confidence interval.
All subjects were born between 1980 and 2008, and the percentage of anti-HAV seroprevalence varied with age (Fig. 1). For males and females, the prevalence was 70–80% in subjects aged 2–8 years who were born during 2000–2007, declined to about 50% in teenagers born in 1990s and fell to almost 30% in subjects aged 20–23 years born around 1988. It then increased to nearly 60% in subjects born in early 1980s. There were no statistically significant differences in the seroprevalence of males and females (Pearson's χ2 = 0·4, P = 0·372).

Fig. 1. Anti-HAV seroprevalence of male and female subjects by age.
The distribution of seroprevalence of anti-HAV was remarkably different for subjects from urban and suburban areas (Pearson's χ2 = 43·2, P < 0·0001) (Fig. 2). For subjects aged 2–8 years born after 2000, the seroprevalence was >80% in suburban areas, but only 50% in urban areas. During the study period, urban areas had a significantly lower prevalence of anti-HAV than suburban areas (Pearson's χ2 = 55·8, P < 0·0001).

Fig. 2. Anti-HAV seroprevalence of urban and suburban subjects by age.
Anti-HAV quantitation by age, gender, residential area, and immunization status
We also analysed the total anti-HAV concentrations of subjects according to gender and residential area (Table 3). The results indicate that the mean concentration of anti-HAV was significantly higher in females than males (55·9 ± 1·0 mIU/ml vs. 52·9 ± 1·0 mIU/ml, t test = −2·2, P = 0·027). In addition, significantly more males had received hepatitis A vaccination than females [33·3% (95% CI 31·5–35·2) vs. 30·0% (95% CI 28·15–31·5); Pearson's χ2 = 7·9, P = 0·005]. The mean anti-HAV concentration was higher in subjects from suburban than urban areas (58·7 ± 0·9 mIU/ml vs. 48·1 ± 1·1 mIU/ml, t test = 7·5, P < 0·0001). There were also more subjects who had received vaccinations in suburban areas than in urban areas [35·9% (95% CI 34·3–37·6) vs. 24·4% (95% CI 22·7–26·3); Pearson's χ2 = 99·1, P < 0·0001].
Table 3. Total anti-HAV concentration according to gender and residential area

Table 4 shows the total anti-HAV concentrations for the different age groups. A total of 65·8% (95% CI 62·8–68·8) of subjects aged 5–9 years had a concentration >80 mIU/ml, but only 33·8% (95% CI 25·4–31·3) of subjects aged 20–24 years had a concentration >80 mIU/ml. There was a statistically significant trend for lower total anti-HAV concentration in the older age groups (trend χ2 = 669·6, P < 0·0001).
Table 4. Total anti-HAV concentration of different age groups

DISCUSSION
Population-based studies of HAV infection in different geographical regions of the world have reported significant differences in the prevalence of HAV infection [Reference Jacobsen and Wiersma12]. In middle- and low-income regions, where hepatitis A vaccination is not routine, HAV seroprevalence usually increases with age. Children are more frequently exposed to HAV and 100% seroprevalence of anti-HAV has been reported in some adult populations [Reference Li and Liang3]. Many epidemiological studies of HAV have focused on children because of their higher risk of transmission, the greater clinical significance of HAV infection in this age group, and because immunization recommendations often focus on this age group [Reference Singleton13]. A previous study in central Tunisia reported an overall anti-HAV seroprevalence of 60%, with significantly greater seroprevalence in adults than infants [Reference Letaief14]. A study in Lebanon reported a HAV seroprevalence of 11% in those aged 0–4 years, 28% in those aged 5–9 years, and 78% in adults aged ⩾21 years [Reference Sacy15]. In contrast, our study indicated that the anti-HAV seroprevalence was maximal (74·6%) in children aged 5–9 years and declined with age, with a minimum (34·4%) for those aged 20–24 years. In 1988–1989, there was an outbreak of hepatitis A in Shanghai, resulting in an estimated 310 000 cases [Reference Dai2, Reference Xiao5, Reference Kang6, Reference Xu16]. Subjects in our study who were older than 25–30 years were elementary school students at the time of this outbreak, and this age group had an anti-HAV seroprevalence of 56·3%. After this outbreak, there was a marked decrease in the incidence of hepatitis A, with only periodic small hepatitis A outbreaks, and a volunteer-based hepatitis A vaccination programme was implemented [Reference Xu17]. The lower infection rate of HAV and the effects of vaccination may explain the unique pattern of age-specific anti-HAV seroprevalence in our population, which is markedly different from those previously reported in developing regions [Reference Ceyhan18–Reference Mukomolov20] and developed regions [Reference Chen21–Reference Broman23] of the world.
Public health officials in Shanghai have developed strategies for hepatitis A prevention during the past two decades. In 1992, the first vaccine with attenuated HAV was licensed and made available to school children in Shanghai during 1994–1996. Since 1996, vaccines with inactivated HAV have been available for both children and adults in Shanghai. In 2000, HAV vaccination was recommended by the Shanghai Health Authority for all citizens of Shanghai on the basis of out-of-pocket payment. In 2008, the Expanded Programme on Immunization (EPI) for HAV was implemented nationwide in China, and in 2009 Shanghai offered free inactivated hepatitis A vaccine to all children born after 1 July 2006. Children should be vaccinated according to a 0-, and 6-month schedule after 18 months. Two doses of inactivated HAV vaccine are given at ages 18 and 24 months, respectively. According to the Notifiable Infectious Disease Surveillance data in Shanghai, the incidence rate of hepatitis A declined rapidly after the initiation of these vaccination programmes [Reference Xu17], from over 20/100 000 in 1992, to 6·98/100 000 in 2000, and then 1·31/100 000 in 2009.
In our study, subjects with histories of hepatitis A vaccination had a much higher (89·3%) seroprevalence of anti-HAV than those without vaccination (33·9%). This is strong evidence that hepatitis A vaccination significantly impacted the distribution of anti-HAV seroprevalence in Shanghai. During 1992–1995, there was a nationwide serological survey of hepatitis A in China. The survey reported that the age-specific anti-HAV seropositivity rate in subjects aged 0–15 years in Shanghai was 23·7% [Reference Dai2], significantly lower than the 60·5% in our study. This provides additional evidence of the beneficial impact of HAV vaccination strategies in Shanghai.
Adults were always supposed to have acquired immunity through natural infection at an early age in developing countries [Reference Jacobsen and Koopman24]. However, we found that the total anti-HAV seropositivity rate of subjects aged 20–24 years and 25–30 years was relatively low (34·4% and 56·3%, respectively) and that the vaccination rate for these two age groups was also low (4·8% and 7·1%, respectively) in Shanghai. After the Shanghai outbreak in 1988, behavioural changes appear to have contributed to the low rate of HAV infection in young adults. The relatively low anti-HAV seroprevalence in these age groups indicates that the risk of HAV infection in young adults should be considered in policy decisions regarding HAV vaccination. In subjects aged 10–14 and 15–19 years (the groups that grew up in the early stage of HAV vaccination), the seropositivity rate was 46·1% and 46·5%, and the vaccination rate was 30·2% and 13·3%, respectively. Either one dose of attenuated vaccine or two doses of inactivated vaccine of HAV could have been applied. It was suggested that booster doses might be considered for single-dose vaccinated participants in these age groups [Reference Zheng and Cui25].
A previous study in Bangladesh reported that the anti-HAV seroprevalence in children and adolescents was higher in rural areas than in developed urban areas [Reference Saha26]. We found similar results in our study of HAV in Shanghai. Although hepatitis A vaccination may play a critical role in the greater seroprevalence of HAV antibodies (vaccination rate 35·9% in suburban and 24·4% in urban areas), it seems unlikely to explain all of our reported regional differences, especially for subjects born in the 2000s. Other social and environmental factors presumably have important influences on the risk of natural infection.
The main result of our study is that the anti-HAV seroprevalence in Shanghai increased significantly following implementation of HAV immunization strategies for children and adolescents. Future longitudinal observations are required to examine the effect of the EPI of HAV vaccination to further evaluate the effectiveness of hepatitis A vaccination strategies. It is likely that multiple approaches to hepatitis A prevention, such as immunization, health promotion, and improvement of socioeconomic conditions, will all contribute to more effective control of hepatitis A in Shanghai.
The anti-HAV seroprevalence and total anti-HAV level varied with age and residential area for subjects aged 0–30 years in Shanghai. The highest (67–75%) seroprevalence was in children. Social and economic development, changes in behaviour, and implementation of hepatitis A vaccination programmes appear to explain our results. Introduction of hepatitis A vaccination into the EPI would further help to control hepatitis A in Shanghai.
ACKNOWLEDGEMENTS
This study was approved by the Institutional Review Board of Shanghai Municipal Centre for Disease Control and Prevention and supported by grants from Shanghai Health Bureau.
DECLARATION OF INTEREST
None.








