INTRODUCTION
Skin and subcutaneous tissue infections are a heterogeneous group of superficial bacterial infections, most frequently caused by opportunistic skin pathogens: Staphylococcus aureus and Streptococcus pyogenes [Reference Sladden and Johnston1]. These infections are common and are usually adequately treated within the community; however, in a number of cases serious skin infections develop which require hospitalization for often invasive treatments. An increase in the incidence of serious skin infections in children has been recognized worldwide [Reference Koning2–Reference Hersh4]. In New Zealand (NZ) this trend has been particularly marked with the rate of paediatric cellulitis double that of Australia and the USA [Reference Hunt5]. The reasons for this high and increasing disease burden are not understood.
In 2007 a comprehensive report on the health and wellbeing of NZ children and young people was published by Craig et al. [Reference Craig, Jackson and Han6]. It found the incidence of serious skin infections in children had doubled during the last two decades, with the highest rates occurring in preschool-aged children, Māori and Pacific children, boys and children living in areas of greatest deprivation. There have been a number of regional reports with similar observations [Reference Hunt5, Reference Lawes7–Reference Leversha10]; however, the only study in this field to have been published in the peer-reviewed medical literature is a 1-year retrospective audit of paediatric skin infection admissions to a South Auckland hospital [Reference Finger11]. None of these studies have investigated whether changing epidemiology over time could be contributing to the increasing incidence of infections.
Furthermore, recent work has shown the case definition for serious skin infection currently in use is deficient in several areas, with a sensitivity of just 61% when tested against a set of clinically defined serious skin infections [Reference O'Sullivan and Baker12]. As this narrower case definition was used in the work by Craig et al. [Reference Craig, Jackson and Han6] currently reported national incidence rates are likely to underestimate the true burden of infection, and epidemiological trends are potentially different to those described.
This study aimed to use a newly developed and validated case definition of serious skin infection to describe the incidence of these conditions in NZ children during the period 1990–2007. It also aimed to investigate if there have been changes in the distribution of disease over time that could help explain the increasing infection rates.
METHODS
This study was based on hospital discharge data obtained from the NZ Ministry of Health. It selected children aged 0–14 years, admitted overnight to a NZ public hospital between 1 January 1990 and 31 December 2007, with a principal or additional discharge diagnosis from a defined list of serious skin infection International Classification of Disease (ICD) codes (see Appendix).
This case definition was developed in recent work which found its validity and clinical relevance was markedly improved by including categories of infection previously overlooked [Reference O'Sullivan and Baker12]. With the addition of skin infections of atypical anatomical sites, those secondary to either primary skin disease or trauma, and those recorded as additional diagnoses (see Appendix), the sensitivity of the case definition increased from 61·0% to 98·9%, with little loss in specificity.
Each discharge record included a unique patient identifier (encrypted National Health Index number) enabling transfers and readmissions within 30 days with the same principal diagnosis code to be removed. To ensure a better match with the census population overseas visitors were removed. Day cases were excluded due to inconsistencies in data recording over time.
Cases were assigned rurality and deprivation levels based on their home domicile census area units (CAUs). Rurality assignment used a Statistics NZ classification which defines seven grades of rurality on the basis of population size and employment status. Assigning levels of socioeconomic deprivation used the New Zealand Deprivation Index (NZDep) which is based on nine variables extracted from census data [Reference Salmond, Crampton and Atkinson13]. NZDep 1 indicates least deprivation and 10 indicates highest deprivation. In 2·21% of cases domicile codes could not be linked to CAUs due to retired codes and addresses outside classification. To reduce the impact of these ‘missing CAUs’, retired domicile codes were linked to new codes using files from the Ministry of Health and Statistics NZ (R. Bishop, Statistics New Zealand, personal communication; CAU changes 1991–2006, Wellington, 2009; C. Lewis, New Zealand Health Information Service, personal communication; Domicile code mapping, Wellington, 2009).
The data were analysed using Microsoft Excel®. Denominators in rate calculations were derived from usually resident population counts from the 1991, 1996, 2001, and 2006 censuses. Counts from each census were used to approximate the population in the preceding and subsequent two years. Trends over time and between populations were explored by the calculation of rate ratios (RRs) with 95% confidence intervals (95% CIs) calculated using the log-transformation method [Reference Clayton and Hills14]. Changes in the distribution of disease over time were measured by the difference in RRs for each variable between 1990–1999 and 2000–2007, with statistical significance indicated by a two-tailed P value <0·01.
The first part of the analysis describes the incidence of serious skin infections for the entire period 1990–2007. To compare trends over time, the more detailed descriptive analyses have split the data into two periods, corresponding to the changeover from ICD-9 to ICD-10 in mid-1999.
RESULTS
Selection of cases, incidence and impact
A total of 82 408 hospitalizations met the case definition. From this we excluded 213 private hospital admissions, 955 overseas visitors, 3109 transfers, 12 353 day cases, and 1210 readmissions.
Of the remaining 64 568 cases, 12 were reported to have been discharged dead from hospital (case fatality 0·04%) from 1990 to 1999 and 17 (0·05%) from 2000 to 2007. Hospitalization data recorded a total of 213 141 hospital days over the study period. The median stay was 2 days and mean was 3·3 days in both 1990–1999 and 2000–2007.
Based on a 2003 estimate of hospitalization costs per case of NZ$2180 [Reference Hunt5], the direct cost of these infections for District Health Boards (DHBs) in 2007 alone was almost NZ$15 million (based on an inflation-adjusted cost per case of NZ$2434·21).
Table 1 shows the incidence of serious skin infections over 1990–1999 and 2000–2007. As recommended by the previous work developing the case definition [Reference O'Sullivan and Baker12], and to provide an indication of the level of certainty of these estimates, these data are disaggregated by category and level of diagnosis (see Appendix for details). This analysis shows that the distribution of disease between principal and additional diagnosis categories changed little over this period (64·8% principal diagnosis in 1990–1999 and 62·8% in 2000–2007).
Table 1. The incidence of serious skin infections in children aged 0–14 years in New Zealand, 1990–1999 and 2000–2007, disaggregated by category and level of diagnosis
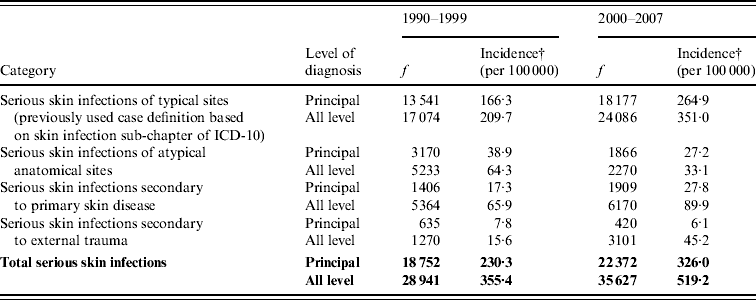
f, Frequency is number of cases in 1990–1999 and 2000–2007.
† Average annual incidence per 100 000 based on usually resident population (from NZ Census).
Incidence by year and season, 1990–2007
Over the 18-year period analysed the average annual incidence rate of serious skin infections in NZ children almost doubled, from 298·0/100 000 in 1990 to 547·3/100 000 in 2007 (see Fig. 1). In the first two years of the study there was a largely stable incidence around 300/100 000, then from 1992–2002 infection rates steadily rose to over 500/100 000. Since 2002 the incidence has been relatively steady again. These trends were a direct reflection of changes in the incidence of serious skin infections of typical sites, with the rates of infections of atypical sites and those secondary to primary skin disease and trauma fairly stable over time.
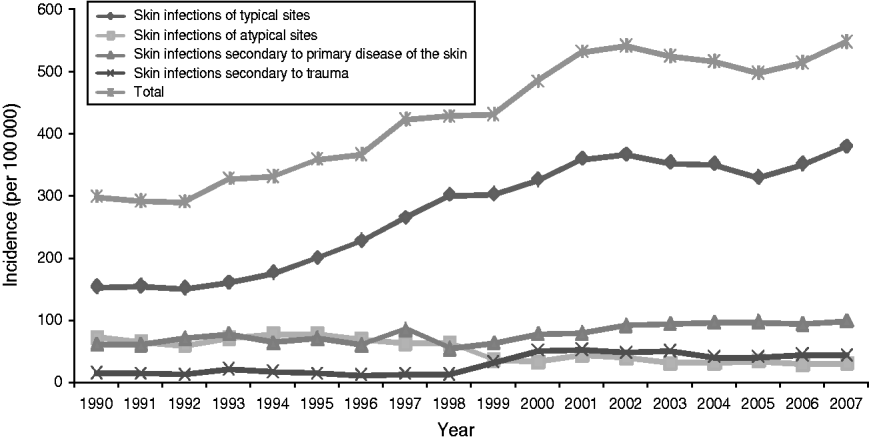
Fig. 1. Incidence of serious skin infection in children aged 0–14 years in New Zealand by ICD-10 code category 1990–2007.
Table 2 shows the incidence of serious skin infections by season of admission. Infections were significantly more frequent during summer and autumn compared to winter (summer: RR 1·08 in 1990–1999 and 1·15 in 2000–2007; autumn: RR 1·11 in 1990–1999 and 1·14 in 2000–2007). There was no change in this seasonal trend over time (P>0·01 for all seasons).
Table 2. Childhood serious skin infection frequency, incidence and rate ratios by season, gender, age, ethnicity, rurality and deprivation, 1990–1999 and 2000–2007
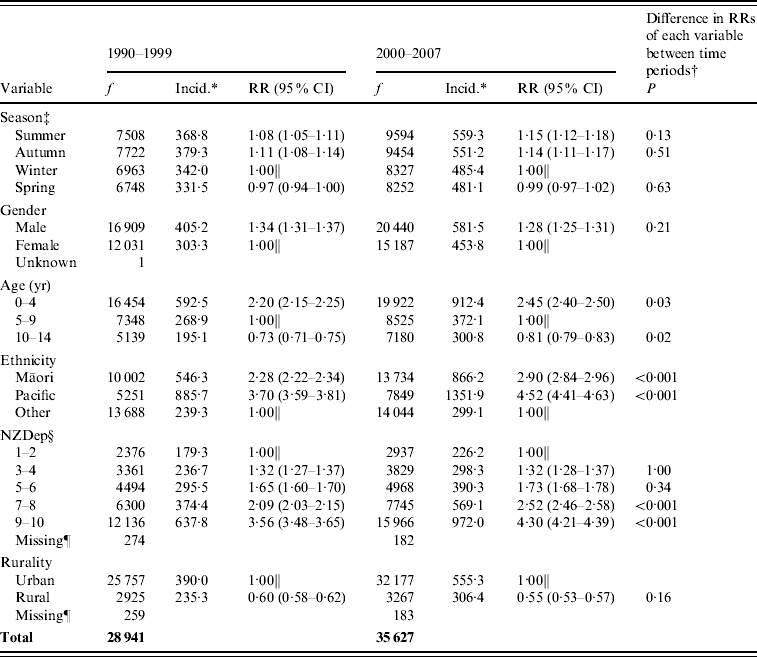
RR, Rate ratio; CI, confidence interval.
f, Frequency is number of cases in 1990–1999 and 2000–2007.
* Average annual incidence per 100 000 based on usually resident population (from NZ Census).
† The P value is comparing the RRs of each variable across the two time periods. P<0·01 indicates a statistically significant difference in the RR of the variable between 1990–1999 and 2000–2007.
‡ Autumn (March, April, May); winter (June, July, August); spring (September, October, November); summer (December, January, February).
§ The New Zealand Deprivation Index (NZDep) is a measure of socioeconomic deprivation based on nine variables extracted from census data [Reference Salmond, Crampton and Atkinson13]. NZDep 1 indicates least deprivation and 10 indicates highest deprivation.
∥ Arbitrary reference category.
¶ Missing refers to cases with domicile codes that could not be linked to CAUs.
Incidence by gender, age, ethnicity, deprivation level, and rurality, 1990–2007
Table 2 details serious skin infections in NZ children by a range of patient characteristics, across two time periods 1990–1999 and 2000–2007.
Boys had a significantly greater risk of infection than girls, with an incidence rate of 405·2/100 000 compared to 303·3/100 000 (RR 1·34) in 1990–1999 and 581·5/100 000 compared to 453·8/100 000 (RR 1·28) in 2000–2007. There was no significant difference in the RRs between the two time periods (P=0·21).
The incidence of skin infections decreased with increasing age. Children aged 0–4 years had more than double the risk of infection than those aged 5–9 years (RR 2·20 in 1990–1999 and 2·45 in 2000–2007) and the 10–14 years age group had the lowest rates of infection overall. Between the two time periods there were increases in the proportion of cases in both the youngest and oldest age groups relative to the reference 5–9 years age group; this difference in RRs approached but did not reach statistical significance (P=0·03 and P=0·02).
The rate of serious skin infections was significantly higher in Māori and Pacific children than those in other ethnic groups. In 1990–1999 the incidence rate was 2·28 times higher in Māori children, and 3·70 times higher in Pacific children, compared to those of other ethnicities. By 2000–2007 that difference had increased to 2·90 times higher in Māori children and 4·52 times higher in Pacific children. The difference in RRs over time was statistically significant (P<0·001).
The incidence of serious skin infections was lowest in areas of least deprivation and increased markedly with rising deprivation levels. During the period 1990–1999, the rate of infection in children from NZDep 9–10 areas was 3·56 times greater than for children from NZDep 1–2 areas (179·3/100 000 and 637·8/100 000, respectively). By 2000–2007 this difference had increased significantly to 4·30 times higher (P<0·001).
Serious skin infection rates were more than 1·5 times higher in children from urban areas compared to children from rural areas (see Fig. 2). Of the three urban classifications, the incidence of infection steadily decreased as areas became increasingly rural. The gradient of this trend appeared steeper in 2000–2007 compared to 1990–1999, but there was no statistically significant difference found (P=0·16). The incidence of infection in the four rural areas was similar, with no change over time.
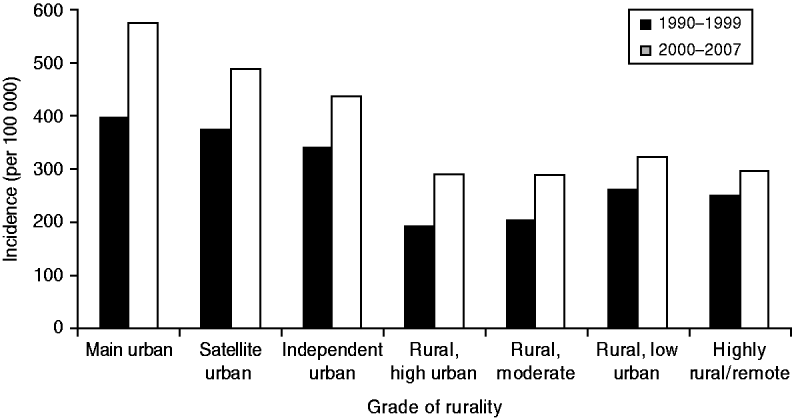
Fig. 2. Incidence of serious skin infections in children aged 0–14 years in New Zealand by rurality, 1990–2007.
Incidence by DHB
Figure 3 shows the incidence of serious skin infections across all 21 geographically assigned NZ DHBs. There was a rough north–south gradient with higher rates generally observed in North Island DHBs (Northland-Wairarapa) compared to South Island DHBs (Nelson Malborough-Southland). The incidence of infection increased over time in all DHBs except West Coast, where a small decrease was observed. Tairawhiti DHB had the highest incidence rate during both time periods studied, approaching double the national rate with an incidence of 644·9/100 000 in 1990–1999 rising to 961·4/100 000 in 2000–2007.
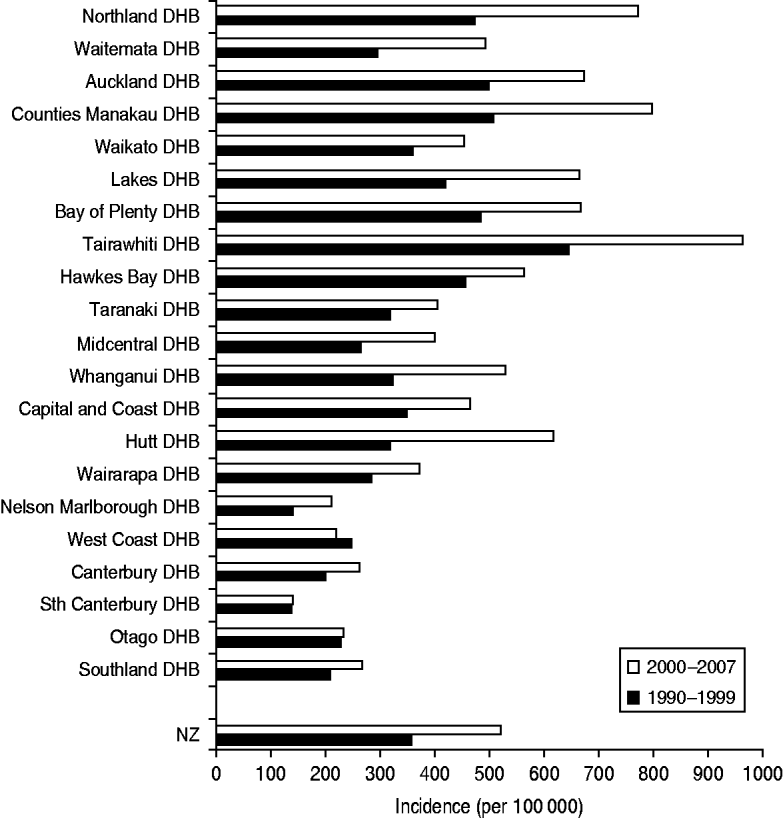
Fig. 3. Incidence of serious skin infections in children aged 0–14 years in New Zealand by District Health Board (DHB), 1990–2007.
DISCUSSION
Serious skin infections are an important and increasing challenge to the health of NZ children, and are making a growing contribution to ethnic and socioeconomic health inequalities.
Recent work has shown the case definition for serious skin infections used in previous publications has been deficient in a number of areas, lacking sensitivity, standardization and clinical relevance. By including several categories of skin infections previously overlooked, the sensitivity of this definition could be increased from 61·0% to 98·9% with minimal loss of specificity [Reference O'Sullivan and Baker12]. This is the first study to apply this standardized case definition to the NZ paediatric population. Findings showed that between 1990 and 2007 the incidence of serious skin infections almost doubled, from 298·0/100 000 to 547·3/100 000. Besides the considerable medical and social impact, this incidence equates to a sizable public expenditure on health services, even without accounting for other direct and indirect economic costs.
Furthermore, this is the only study we are aware of that has investigated whether there have been changes in the distribution of serious skin infections over time which could account for the increasing incidence of disease. Between 1990 and 2007 the highest infection rates were observed in boys, children aged <5 years, Māori and Pacific children, those living in deprived neighbourhoods, in urban areas and in northern districts of the country. While these high-risk groups have remained the same between 1990–1999 and 2000–2007, ethnic and deprivation-related disparities have significantly increased. This change in the distribution of serious skin infections over time is contributing to the increasing incidence of disease; however, it cannot explain the whole increase as rates have risen across all population groups.
The incidence of infections showed marked and consistent seasonality. High disease rates in summer and autumn have been observed in other settings and are believed to result from warmer air temperatures leading to more frequent insect bites, deficiencies in hygienic precautions, and increased skin exposure resulting in greater skin-to-skin contact and minor trauma [Reference Koning2, Reference Loffeld3, Reference Hunt5, Reference Kristensen15–Reference Rogers20].
High rates of infection in preschool-aged children and boys have important implications for directing community prevention efforts. In both groups this increased risk could be attributable to more frequent injuries, poorer general hygiene, or longer delays in seeking medical attention. It is not known whether these trends reflect similar patterns in the community, or in the case of younger children, of lower hospital admission thresholds. Previous NZ reports have observed similar trends [Reference Hunt5–Reference Lawes7, Reference Finger11], but interestingly studies in the international literature report no gender predominance [Reference Koning2, Reference Elliot16, Reference Rogers20–Reference Dajani, Ferrieri and Wannamaker23]. As these studies mainly comprise primary-care and population surveys, it is possible that simple skin infections are experienced equally by both genders but boys are more likely to suffer progression to a serious skin infection.
Māori and Pacific children had higher rates of serious skin infections than children of other ethnicities. This finding is consistent with the wider observation that Māori and Pacific peoples generally experience high rates of infectious diseases [Reference Baker24]. The reasons for this pattern are complex and multifactorial; they include household crowding and a range of socioeconomic factors [Reference Baker25, Reference Grant26]. It is not known whether the high incidence of serious skin infections directly reflects higher community rates of disease, but it is known that Māori and Pacific families experience greater barriers to accessing primary healthcare including cost, cultural differences and longer travel distances [Reference Malcolm27–Reference Brabyn and Barnett29]. In addition, hospitalization data have been found to often undercount Māori [Reference Harris, Robson and Harris30, 31] which would result in an underestimation of ethnic inequalities. The significant increase in ethnic disparities between the two time periods is of particular concern. While some of this increase could be due to inconsistencies in how census (denominator) data has been collected over time, this factor is unlikely to completely account for the difference.
Socioeconomic deprivation was seen to be an important risk factor for serious skin infection with a steady increase in infection incidence with increasing neighbourhood deprivation. While this association is well established and thought to be mediated by hygiene, nutrition, household crowding, and the ability to afford timely medical treatment [Reference Hunt5, Reference Craig, Jackson and Han6, Reference Kakar17, Reference Bailie21], the evidence of increasing inequality has not been previously recognized. As there has been little change to the allocation and recording of deprivation status, these findings suggest truly worsening deprivation disparities over time.
The association between serious skin infection incidence and level of rurality has not been reported previously in NZ. The high incidence of infection in children from urban areas could be due to socioeconomic deprivation, household crowding and a higher frequency of skin contact with other children in more densely populated cities. It is possible that lower infection rates in rural areas could reflect more frequent treatment in the community due to reduced access to hospitals. However, as the management of serious skin infections in children frequently involves hospital-based treatments such as intravenous antibiotics and surgical debridement, it is unlikely that many true serious skin infections would be managed in an outpatient setting.
Within NZ, Tairawhiti DHB had the highest incidence of childhood serious skin infections. This finding could be a result of the large Māori population and high deprivation of the region, but requires greater investigation. The observed north–south gradient may in part reflect the distribution of population groups who experience higher disease rates, but could also relate to climatic differences, with northern districts of NZ experiencing relatively warmer weather compared to those in the south.
The descriptive data presented in this paper have limited potential for identifying factors that have caused the rise in serious skin infections and increases in ethnic and socio-economic inequalities. Other work in New Zealand over a similar time period has shown a marked increase in rates of infectious diseases generally, along with rising ethnic inequalities [Reference Baker24]. In addition, rheumatic fever incidence has shown worsening ethnic inequalities from 1996 to 2005 with this disease increasingly concentrated in Māori and Pacific children [Reference Jaine, Baker and Venugopal32]. The causes for these changes are not known.
There are several programmes monitoring health determinants and outcomes over time for NZ children and households [Reference Craig, Jackson and Han6, 33, 34]. This monitoring showed a mixed pattern over the last two decades. Mean income levels have been rising for New Zealanders but inequalities have increased, particularly during the 1990s [34]. While average levels of household crowding have generally decreased, there have been comparatively small declines in the proportion of children living in crowded conditions and persisting large ethnic inequalities [33].
Hospitalization data have strengths and weaknesses as a basis for surveillance of serious skin infections. The main limitation of these data is that, by definition, they only represent the ‘tip of the iceberg’ and cannot measure the incidence of mild to moderate skin infections in the community. This limitation is common to other areas of infectious disease epidemiology, such as acute gastroenteritis [Reference Lake35]. The advantages of this data source is that it is accessible and is likely to be relatively sensitive for serious skin infections as few paediatric cases would be treated outside of the public hospital setting due to the close monitoring and invasive treatments required. On this basis, and as by definition serious skin infections are those skin infections which require hospitalization, we used the term ‘incidence’ to describe hospitalization rates.
It is possible that the sensitivity of such surveillance has changed over time, for example, changes to the recording of day patients as admissions. We have attempted to minimize such effects by the use of a fairly high threshold for inclusion. A further consideration is modifications to the disease coding system; despite using standardized mapping tables, translating diagnoses between ICD-9 and ICD-10 was problematic in some areas with the incidence of several diagnoses markedly varying over time (see Appendix). However, as there was a steady increase in the total infection incidence over the years when the ICD revision occurred, the variation is more likely to reflect inter-code and inter-category drift, and further justifies our use of a more inclusive case definition than that used previously.
This study demonstrates an urgent need for action to prevent serious skin infections in NZ children. It highlights population groups with disparate rates of disease to which these efforts should be particularly focused. Future work could include extending this simple univariate analysis to a multivariate model to identify the independent effects of each risk factor. A retrospective case-note review or a case-control study would better elucidate the aetiological processes contributing to the development of serious skin infections. In order to allow clear and accurate comparison, any future work on hospitalization data could utilize the standardized case definition of serious skin infection used in this study. In addition, the epidemiology of skin infections in primary care is largely unknown; future study in this area could improve our understanding of whether inequalities in serious skin infection rates directly reflect community trends. In combination with such ongoing work, the findings of this present study indicate some priority areas for directing interventions to reduce the morbidity of serious skin infections in NZ children and to narrow health inequalities.
DECLARATION OF INTEREST
This work was supported by initial funding from Tairawhiti District Health as part of a larger piece of work made possible by a grant from the Ministry of Health Reducing Inequalities Budget.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the statistical advice and time given by Gordon Purdie.
APPENDIX. The incidence of serious skin infections in children aged 0–14 years in New Zealand, 1990–1999 and 2000–2007, disaggregated by ICD code, coding category and level of diagnosis
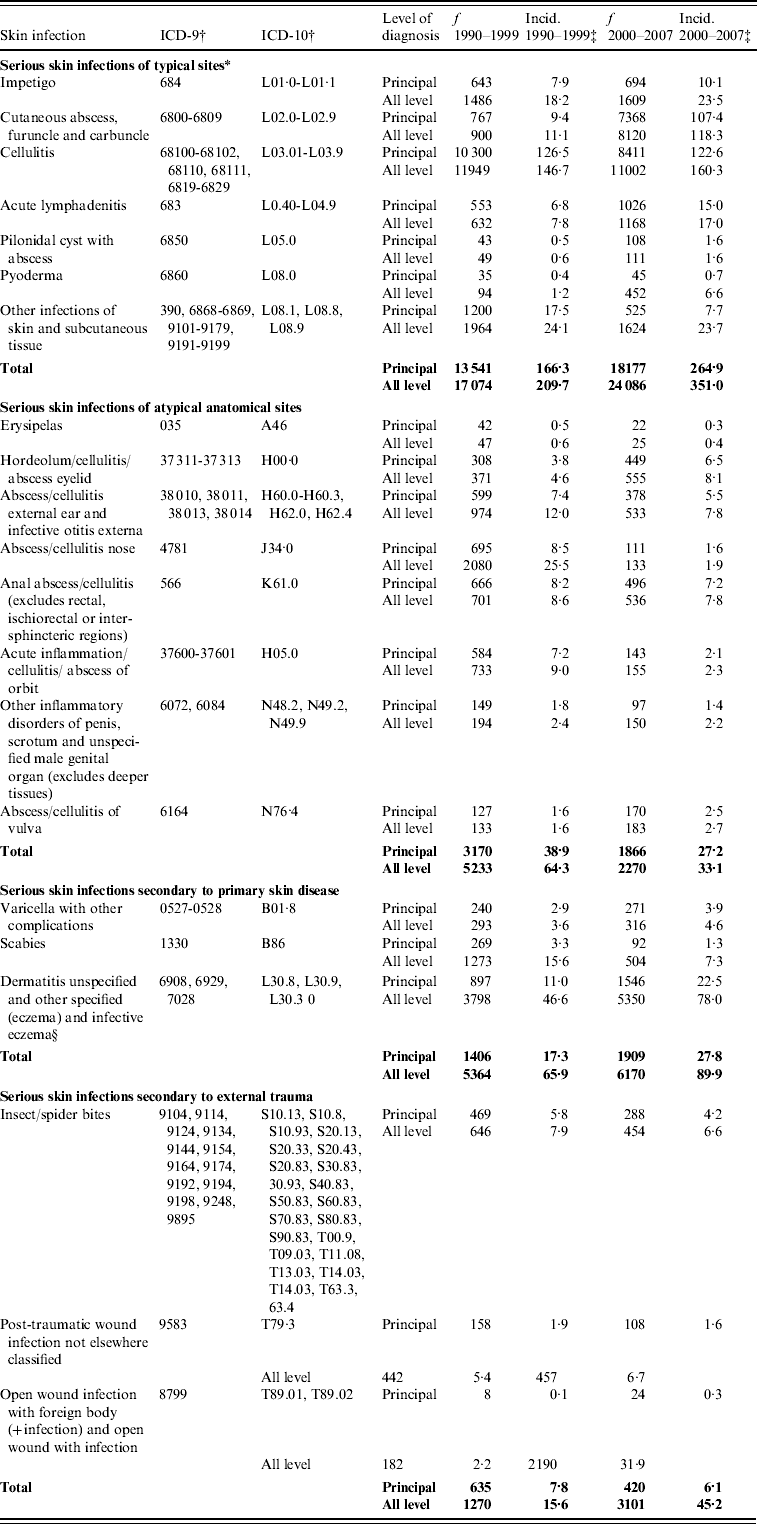
f, Frequency is number of cases in 1990–1999 and 2000–2007.
* Case definition based on skin infection sub-chapter of ICD-10.
† Cases after 1999 were identified using ICD-10 diagnostic codes, while cases prior to this year were identified by ICD-9 codes which were forward and backward mapped from ICD-10.
‡ Average annual incidence per 100 000 based on usually resident population (from NZ Census).
§ The medical definition of infective eczema (a primarily inflammatory condition) is not in keeping with the clinical description of a serious skin infection; however, due to similarities in terminology, this code is incorrectly used for eczema with a superficial bacterial infection.










