Introduction
Most deep ice-coring projects of various nations have, because of cost and logistical reasons, used a variety of fuel oil-based drilling fluids, e.g. Reference Ueda and GarfieldUeda and Garfield, 1969. In virtually all cases a densifier such as PER (perchloro-ethylene) or TCE (trichloroethylene) has had to be added to bring the fluid density up to that of ice in order to prevent closure of the borehole. We are aware of two variations from this practice: one is the use of alcohol, about which we will briefly comment but plan to report in a companion paper, and two, a field test of an oil well-drilling fluid consisting of a refined hydrocarbon known as LVT-200, densified with PBBE (polybrominated biphenyl ether) to form a dilute solution known by the trade names “Permoil” or “Bromoil”. LVT appears, by gas chromato-graphy–mass spectrometry, to be a mixture of saturated branch chain and saturated cyclic compounds. It should be noted that bromoil was developed for high-temperature well-boring, and not low temperature ice-coring. Use of PBBE as the densifier is less acceptable than that of PER or TCE, all of which are hazardous to health and the environment. Furthermore, the viscosity of hydrocarbon solutions is too high to permit timely operation at deep
(≥1000 m) drill sites. The chemical structures of butyl acetate, anisole and several other compounds mentioned in this paper are shown in Figure 1.
The use of PER and TCE pose significant health safety and environmental risks. Both PER and TCE have known and suspected carcinogenic properties (PICO TR 89–2 and references therein), and add to the already undesirable load of atmospheric organic chlorides. PBBE is a viscous semi-solid material (Reference Brackenridge and McKinzieBrackenridge and McKenzie, 1988), is on the United States Environmental Protection Agency’s (EPA) list of “extremely hazardous substances” and falls under the EPA’s “Community right to know” (Reference Sax and LewsSax and Lewis, 1989). Moreover, the brominated components are resistant to biodegradation as are DDT, PCB (polychlorinated biphenyl) and PBB (polybrominated biphenyls), all of which have been shown to concentrate in the food chain, to have deleterious long-term effects on the health of individuals who have been exposed to relatively low levels (e.g. Reference Sundström and HutzingerSundström and Hutzinger, 1976; Reference Watanabe, Kashimoto and TatsukawaWatanabe and others, 1987; Reference Mulligan, Caruso and FrickeMulligan and others, 1980), and are potent inducers of xenobiotic metabolism (Reference CarlsonCarlson, 1980a,b).
The Polar Ice Coring Office (PICO) conducted a chemical literature survey in an effort to identify a drilling fluid suitable for deep ice-coring that would have the appropriate viscosity and density characteristics, a fluid that would minimize potential health and safety risks for workers, cause minimal environmental impact and maintain the integrity of the ice core for scientific analysis. The results were published in two technical reports (Reference GosinkGosink, 1989; Reference Gosink, Turneo, Koci and BurtonGosink and others, 1989). This paper briefly reviews these reports, and presents a case for the use of butyl acetate as a deep ice-coring drilling fluid. Our investigation of alcohol is reported in the paper by Reference Gosink, Koci and KelleyGosink and others (in press).
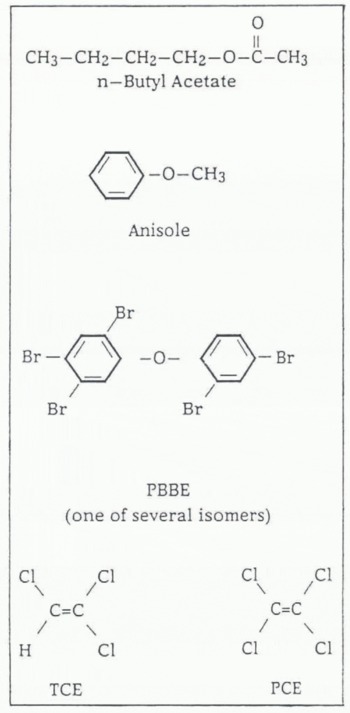
Fig. 1. Chemical structures for butyl acetate, anisole PBBE, TCE and PCE.
Of nearly 250 000 compounds electronically surveyed, 11 potential drilling fluids tentatively were identified as suitable. Of these 11, only two, butyl acetate and anisole, fully meet the constraints imposed by technical, scientific, health and safety concerns previously mentioned. This paper discusses the chemical and physical characteristics of butyl acetate and anisole with respect to their use as a drilling fluid, and presents some important technical considerations with respect to health, safety and the environment surrounding the use of these compounds. Butyl acetate alone at lower temperatures (–20° to –78°C) meets and exceeds the desired physical characteristics of density and viscosity. The cost of butyl acetate is reasonable ($0.95kg−1). Both butyl acetate and anisole (>$2.00kg−1) are totally synthetic, i.e. derived from petroleum products, thus presenting no carbon-14 complications. Recommendation of butyl acetate is also based on the fact that even-numbered carbon chains (e.g. butyl-4) are less toxic (narcotic) than the odd-numbered (e.g. propyl-3 or amyl-5), and it is more readily available. These production costs, however, become minor compared to the delivery charges of $3–4-kg−1 for any drill fluid delivered to remote polar sites. Ethyl alcohol would have considerable cost advantage in that only half the weight would have to be shipped, with the remainder being dissolved snow, achievable without the addition of heat.

Fig. 2. Density (Mgm−3) of potential ice-drilling fluids versus temperature (°C). Fuel oil (×); pure ice (
Discussion
Density
The hydrostatic pressure, which is a function of density and depth, is of major importance in the selection of ice-core drilling fluids. The density of butyl acetate rapidly increases with decreasing temperature (Fig, 2) and at temperatures below –15°C is sufficient, being greater than the firn-ice-layer density of 900kgm−3 (Reference PatersonPatterson, 1981).

Fig. 3. Calculated difference in pressure (∆ kg m−2) curve for butyl acetate in a glacial borehole, based on temperature, density and compressibility.
Firn-ice density may also be as low as 900 kg m−3. At –30°C the density of butyl acetate (Fig. 2) is more dense than pure ice (Reference AshtonAshton, 1986). A 10% mixture of anisole in butyl acetate at 1 bar is as dense as pure ice at –15°C. Typically, it is only necessary to keep the top of the fluid level 40–100 m below the ice surface to balance the ice-mass pressure in the lower reaches of the borehole. Since the minimum internal temperature in the upper kilometer of the Greenland ice sheet has been observed to be –31°C, and is expected to be significantly colder in Antarctica, an added densifier will not be required. If a densifier is required for more temperate glacier-drilling operations, anisole is much more desirable than PCE, TCE or PBBE with respect to water pollution, human toxicity and air pollution. In deep holes, the slight compressibility of butyl acetate (about 50% greater than that of water) will compensate for the loss of density (at 1 bar) with warmer temperatures.
Compressibility and internal pressure
Figure 3 is a plot of the pressure difference between butyl acetate and the ice-overburden pressure (∆kgm−2) as a function of depth. Temperatures for the calculations are based on the model of Reference WaddingtonWaddington (1989) and the 1990 GISP-2 depth–density profile. Butyl acetate density is taken from Figure 2. The drill fluid is asumed to be 70 m below the surface. Data supplied by Eastman Kodak, the manufacturer of butyl acetate, suggest a bulk modulus of 12 381 bar, which results in a density increase of nearly 3% at 3 km depth. The density increase more than compensates for the lower density of butyl acetate at the warmer temperatures (Fig. 2) expected in the lower levels of glaciers indicated in Figure 3. Curve 3a ignores compressibility while curve 3b considers it. It can be seen from the figure that pressure neutrality will occur at 1500–1900 m depth. In the deeper regions of uncertainty, the pressure sensor on board the drill and caliper measurements of the holewill have to be monitored. Diesel fuel and ethanol have similar to greater compressibility problems,respectively.
Viscosity
The viscosity of the fluid is important to the travel time of the drill string, particularly at greater depths, and thus to the overall cost of the project. Either a low-viscosity fluid must be employed, or alternatively, larger-diameter (more expenditure of time and energy) holes with greater clearance must be bored to accommodate the high-viscosity fluids. Figure 4a shows the effect of drill-fluid viscosity on velocity of the free fall of a 6.6 in diameter, 85 ft long, 12501b drill string through a 7.1 in diameter borehole. This equipment was employed in the 1990 Greenland Ice Sheet Project and provided a 5.2 in diameter core. Given a fluid viscosity of 10cp, the free-fall velocity is about 0.65 ms−1, at 2cp about 3.1 ms−1, thus cutting this part of the operation time by a factor of nearly 5. Butyl acetate’s viscosity is < 2 cp; hydrocarbons have a viscosity of c. 15–20cp. Aqueous ethanol viscosities at low temperatures rapidly go to 50 cp. Figure 4b shows the round-trip time for the drill string going to 1000 m depth. It is calculated to be about 10 min with butyl acetate, but nearly 1.5 h with a fluid having a viscosity of about 15cp. Depths of 3000 m are anticipated. It was assumed in the calculation that the return velocity is the same as that of the free fall. Attempted velocities greater than that would place undue strain on the cable, experiencing about a 1 ton load (includes weight of cable and sample), and would be tantamount to lifting the whole liquid column above the drill string.

Fig. 4. (a) Drill-string free-fall velocity as a function of the drill-fluid viscosity. See text for parameters used in the calculation, (b) Drill-string round-trip travel time as a function of drill-fluid viscosity.
The general desire is that the viscosity of the drill fluid be less than 5 cp. As can be seen in Figure 5, the viscosity of butyl acetate (and the 10% anisole mixture) remains well below 3 cp, even at –50°C, whereas the viscosity of 0.91Footnote *bromoil is substantially above 15cp at anticipated ice temperatures. With the exception of the three points labeled “Lit.”, all of the data in Figure 5 were determined in this laboratory using a falling-ball type viscometer. The viscosity estimated by the manufacturer of LVT-200 at –40°C is also indicated in Figure 5 as “Lit.”, is comparable to other fuel-oil mixtures, and fits well with our experimental data. It can also be seen that the addition of a small quantity of PBBE to LVT-200 in the formulation of bromoil raises the viscosity even higher. Alcohol and water mixtures have substantially higher viscosities at low temperatures (Reference Gosink, Koci and KelleyGosink and others, in press).
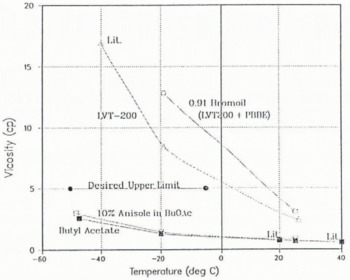
Fig. 5. Viscosity (cp) of potential ice-drilling fluids versus temperature (°C). Butyl acetate (

Fig. 6. Vapor pressure versus temperature of butyl acetate, indicating the temperature required to exceed the lower flame pressure / concentration at sea level and at 3300 m [10820ft] altitude. (Most conservative data employed.)
Volatility/flammability
The greater volatility of butyl acetate raises fire-safety questions, but it is unlikely that under the conditions experienced in ice-core work that this will be a problem. There is confusion in the technical literature on the flash point of butyl acetate. Reference Sax and LewsSax and Lewis (1989) reported 72°F [22°C]. On a bottle of high-quality butyl acetate the label indicated 92°F [33°C], and the Merck index (1976) quoted 38°C [100°F]. Determination of the flash point by a local independent testing laboratory produced a value of 85°F [29.4°C]. Our observations in the following paragraph support the higher numbers. In Figure 6, the handbook vapor pressure of s-butyl acetate is plotted against temperature. The curve for s-butyl acetate is reasonably estimated from the fact that the boiling point of the n-butyl isomer is 2% higher, on the absolute temperature scale, than that of the s-butyl isomer. The published safety data (1988–89 CRC handbook) states that the lower flame-limit concentration of n-butyl acetate in air is 1.4%. This corresponds to a vapor pressure of 10.6 mmHg, achievable only in an unventilated closed room or container at 20°C. At-20°C [–4°F], an average to cool day on the drill site, the maximum possible air concentration of butyl acetate in an unventilated area would be approximately 1300ppm (0.13%), well below the flame limit. Figure 6 further indicates that at the higher altitudes where drilling operations will occur (3300 m), the lower flame limit is still about +11°C.Footnote * The warmest part of the drill-site days, according to the 1989 Greenland drill-site temperature record, rises to about 0°C. Odor detection and irritation both occur below the permissible exposure limit (150ppm) and thus provide both adequate physiological and fire-warning properties. This is, of course, in addition to monitoring equipment available to avoid these problems.
The theory was tested by applying a lit paper match to a small beaker of butyl acetate at laboratory temperature (c. 24°C). The butyl acetate ignited only by briefly touching the flame to the surface, but it went out as the match was immediately withdrawn. The experiment was repeated twice more outside where the temperature was approximately 4°C. The butyl acetate would not ignite, and in fact, extinguished the match. There was a slight flare as the glowing match head touched the surface, but all of the flame was extinguished as the match was plunged below the cold liquid surface.
Side benefits of the greater volatility of butyl acetate are: no oily residue will remain on work clothes between shifts of drilling crews, residual odor will disappear rapidly depending on degree of ventilation, the core will be much less “greasy” to handle, and the lower viscosity and higher volatility should pose less of a hazard for making the driller’s deck slippery.
Reactivity with ice
Several experiments were performed in which a 20 g cube of ice was placed in a covered beaker with about 100 ml of solvent. The results are shown in Figure 7. The handbook published solubility (summarized in Reference GosinkGosink (1989)) of butyl acetate in water is about an order of magnitude lower than ethyl acetate, and is essentially the same as that for PER, about 0.7%. The solubility of water in butyl acetate is 1.6%. Note that the weight loss of ice in wet butyl acetate was not noticeable in 7 h contact time. Soviet drillers have used alcohol as the drilling fluid at their sites (Reference Morev, Manevskiy, Yakolev and BagorodnovMorev and others, 1982; Reference ZagorodnovZagorodnov, 1982). However, our experiments show that pure ethanol causes extremely rapid attack on the ice (Fig. 7). We have subsequently found that any alcohol–water mixture having even 5% too much alcohol rapidly attacks the ice, but stops when the equilibrium for that temperature is reached. Unfortunately, we have also found that alcohol penetrates the core of the ice at the 10–20ppm level, thus posing a threat to some analyses (Reference Gosink, Koci and KelleyGosink and others, in press). Limited testing with hydrophobic butyl acetate shows little or no ice penetration.

Fig. 7. (a) Per cent evaporation of drilling fluids ( at 22°C) versus time (5 ml of solvent in a 50 ml beaker); (b) per cent weight loss of ice in potential ice-drilling fluids at −19°C (approximately 20 g of ice in c. 100 ml of solvent).
Solvent effect on polymers
The effect of butyl acetate and several other solvents on a variety of polymers employed in the construction of the drill and cable is shown in Table 1. Both butyl acetate and anisole have no effect on the various polymeric materials employed in the PICO deep drill string. Recent enquiries to manufacturers about various epoxy resins and other plastics also indicate no deleterious effects. A letter from the manufacturer, Corland Co., in October 1989 stated that the tensile strength of the Kevlar sheathed electric cable used in PICO’s drilling operations is not affected by butyl acetate. The wires in the electric motor, even at temperatures of 125°C, were not affected by either butyl acetate or the 10% mixture of anisole. The drill motor, which contains brushes, has recently been operated successfully at full power immersed in butyl acetate at c. 25°C for over 700 h. Aqueous ethanol solutions are not expected to pose problems for drill-construction materials, but electrical motors would have to be scaled, and electrical connections potted.
Trace contaminants
A recent analysis of butyl acetate (≥99.5%) indicated contaminant concentrations of: <0.01% water, 0.07% butanol and < 0.01 % acetic acid. Assuming 0.01% (100ppm) free acetic acid in butyl acetate, and assuming se, slight effect; ss, soften and/or swell; +, more; -, less, i. e. a very slight effect of softening; ne, no effect (did not swell, soften or become tacky).
Table I. Solvent effect on polymers (24 ± 3h)

*Lacquer on wire, 125°C for 2 h.
†Plastic jacket on wire.
A blank area indicates no data.
the unlikely penetration of hydrophobic butyl acetate into the ice core at the 10 ppm level, the resultant free-acetate contamination would be at the ppb level and thus should pose no problem for acetate analyses. There may, however, be a chemical matrix problem which should be surmountable with procedure modification.
A trace of butyl acetate odor can be detected on the core after 48 h. Analysis of a piece of Greenland ice core immersed in butyl acetate at –31°C for 2 h showed the following: surface 0.5 mm, 1.9; 1 mm, 1.0; 5 mm, 0.4 ppm butyl acetate in the melted ice samples. An analysis based on a 1:1 water extraction was reported as: chloride, < 0.5 ppm; sulfate < 0.1 ppm, i.e. below the routine detection limits of the analyzer, and therefore of no threat to trace-element analyses of ice cores even if 1 % of butyl acetate was trapped in the core. No data are available on the residual trace inorganic pollutants in fluids such as fuel oil, LVT-200 or PBBE, etc.
Indoor ambient air quality
Butyl acetate odors are detectable at 10 ppm. At approximately 100 ppm ambient concentration, some nasal irritation may be noticed, well below the Occupational Health and Safety Administration’s (OSHA) limit of 150 ppm (710 Mg m−3). Kerosene fumes, it should be noted, are only permitted to be 100 Mg m−3 of air. Ethanol vapors are permitted in the work place at 1000 ppm. Anisole odors are detectable at 0.2 ppm, but no OSHA standards have been set. Therefore, before any significant health hazard would be present, odors would be noticeable and irritating. Given that anisole, if used, will be used in such low amounts, no special precautions will be necessary above those which should be instituted for butyl acetate.
Potential problems of excessive butyl acetate concentrations in the air can be eliminated by proper design and use of ventilation systems in the drill enclosure, core-relaxation pit and science trench. Fans to keep the interior core-handling structures cold should be part of any management plan, and will thus insure that butyl acetate vapors will be kept to an acceptable minimum. Field-test results from the 1990 GISP-2 drilling season show that the vapors in the drilling dome were quite acceptable, even when the drill string was up and being manipulated. In the immediate vicinity of the carousel, vapor concentration went as high as 40 ppm. In the rest of the dome it was <10ppm. Butyl acetate vapors in the science trench generally were barely detectable at less than 10 ppm. The exhaust from the sump under the drill vent was also very good, containing 100–200 ppm of solvent vapors. The bulk of the butyl acetate appears to have evaporated from ice cores in about 12 h time, in keeping with our preliminary laboratory screening tests reported below.
Health hazards
Butyl acetate is slightly hazardous to health. It is a mammalian reproductive toxin. The risk, however, comes from prolonged inhaling vapors in excessive concentrations. Butyl acetate is a minor component of natural and artificial pear aroma. Anisole has no specific health hazards and is not a mammalian reproductive toxin. Neither butyl acetate nor anisole is listed as a carcinogen as are PBBE and TCE. Several inexpensive battery or a.c. operated alarms are available on the market at a cost of $300–1200 each. These alarms are sensitive enough to warn at the parts per million level for health purposes, or at higher ranges for fire-danger warning, and operate at –5°C without the need of a warmed housing. In addition, inexpensive chemical reaction tubes specific for butyl acetate are available. The tubes are sensitive to less than 10 ppm, but also give readings up to several tenths of a per cent level, and are routinely used in industry. The inexpensive reaction tubes, when corrected for the altitude, provided essentially the same readings as an expensive electronic device.
Environmental characteristics
From an environmental aspect, butyl acetate (and anisole) is a single, easily biodegradable compound, as opposed to the mixture of components in fuel oil, LVT-200 or PBBE. Both fuel oils and LVT-200 pose higher environmental risks since, in the event of a spill (or the long-term eventual release of residual material left in the glacier), they have a long residence time, particularly in a cold environment (Fig. 7). In a worst-case scenario, if all the butyl acetate leaked out the bottom and flowed into a fjord, most of it can be expected to evaporate in 1–3 d. An additional small amount would disperse in the water column, where it would be rapidly biodegraded. Butyl acetate will, contrary to all the other fluids proposed or in use, clean itself up by evaporation in a matter of days, even in a cold climate. Anisole would behave similarly. LVT-200 and other hydrocarbon solvents, on the other hand, are not readily biodegradable and would not evaporate appreciably. Thus, virtually all of the hydrocarbons would have to be physically removed from the water surface, particularly in view of the toxic densifier materials, whether it be PBBE or TCE, etc.
Anisole is not as biodegradable as butyl acetate, but should not have a high environmental residence time. If the use of a densifier is required in temperate glaciers (average internal temperature ≥-20°C), anisole should be acceptable in comparison to other commonly used densifiers from an environmental standpoint.
Alcohol, being a natural product, would pose the least environmental problems, but there are other factors that should be considered (Reference Gosink, Koci and KelleyGosink and others, in press).
Summary And Conclusions
Butyl acetate meets and exceeds all the desired physical properties of an ice-core drilling fluid (density, viscosity), docs not corrode the ice, and is available at reasonable cost. Butyl acetate is significantly better than hydrocarbons containing halogenated materials, with respect to the health safety for the workers and to the environment. The fire hazard of butyl acetate is greater than that of fuel oil, but at temperatures below 10°C the risk appears to be minimal. Contamination of the ice, for scientific studies by butyl acetate or by the trace contaminants in it, also seems to be minimal. First-year results (personal, environmental and scientific) after the use of butyl acetate at the GISP-2 site are all favorable.
Acknowledgements
The Polar Ice Coring Office (PICO) is operated at the University of Alaska Fairbanks under U.S. National Science Foundation contract DPP88–020948. We gratefully acknowledge the editorial and manuscript assistance of H. Stockholm, G. Tilton and V. Timm of the Institute of Marine Science, School of Fisheries and Ocean Sciences (SFOS). This is contribution number 90–2 of the Greenland Ice Sheet Project Two (GISP-2).










