Introduction
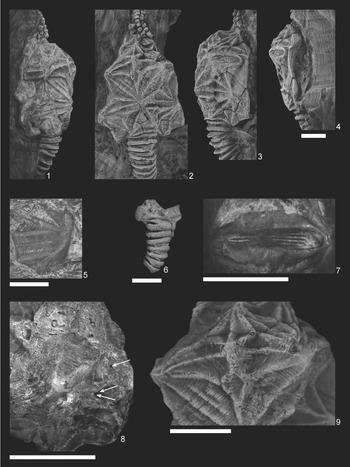
Among fossil echinoderms, glyptocystitoid rhombiferans are noted to have relatively conservative thecal plating. Excluding a few Cambrian stem taxa, glyptocystitoid thecae are constructed of five plate circlets in the following proximal-to-distal arrangement: seven oral plates, five to six (rarely four or ten) radials, five laterals, five infralaterals, and four basals (Sumrall and Sprinkle, Reference Sumrall, Sprinkle, Carnevali and Bonasoro1998; Zamora et al, Reference Zamora, Sumrall, Zhu and Lefebvre2017). These plate circlets and their constituent thecal plates are recognizable across glyptocystitoid taxa. The position and homology of these plates can be pinned to the developmental axes of the organism both at the basal circlet that bears the zygous plate B4 and by the position of the radials with respect to the 2–1–2 ambulacral symmetry (Sumrall and Waters, Reference Sumrall and Waters2012). Plate circlets are plesiomorphically closed, meaning plate circlets are contiguous around the theca without interruption. Even in derived taxa with open plating, as in Callocystites, where the infralateral and lateral circlets interfinger and the radial circlet is interrupted by a suture between L5 and O5 (Fig. 1.1), plating remains uncontroversial. Finally, different configurations of plating along the periproctal border, ranging from one to eight plates, can have a strong influence on interplate relationships (Kesling, Reference Kesling and Moore1967), yet the identities of the main thecal plates except among the radials and orals are clear among taxa.

Figure 1. Plate diagram of Callocystites canadensis. (1) Juvenile specimen CMC-PUC 36206B showing open-circlet plate configuration. (2) Adult specimen CMC-PUC 36206A, note the division of L4 into two plates. Scale bars = 5 mm. B = basal plate; IL = infralateral plate; L = lateral plate; R = radial plate; O = oral plate. Letters A–E at the top of each panel represent the ambulacral identifications under the Carpenter system (Carpenter, Reference Carpenter1884).
Within glyptocystitoids, there is not a clade more modified from the plesiomorphic bauplan than pleurocystitids, which bear a flattened theca with greatly expanded ventrally oriented periproctal membrane (Parsley, Reference Parsley and Sprinkle1982). Most taxa exhibit an expanded periproctal border to include as many as eight thecal plates while shifting the remaining plates onto the dorsal surface (Sprinkle, Reference Sprinkle1974; Sumrall and Sprinkle, Reference Sumrall and Sprinkle1995). This results in a theca that is longer in the plane parallel to the substrate and shorter in the perpendicular aspect. The five plate circlets common to glyptocystitoids are present in pleurocystitids despite this change in proportion, and while some uncertainty remains concerning the homology of a few plates in the radial and oral series, the remainder of plate homologies remain undisputed (Parsley, Reference Parsley1970; Broadhead, Reference Broadhead1974; Paul, Reference Paul1984).
The aim of this paper is to describe a new species of pleurocystitid from the Benbolt Formation. Due to the plate anomaly in the holotype, a brief discussion of teratology is included to address the validity of the specimen and contextualize its occurrence in echinoderm teratogenesis. This new species is an important addition to our understanding of pleurocystitid echinoderms as it contains features of both genera.
Materials and methods
Locality information
Holotype CMCIP95726 and isolated plate CMCIP95728 are from the Upper Ordovician (Sandbian) Benbolt Formation near Thorn Hill, Grainger County, Tennessee (36.3869°N, 83.4479°W) (Fig. 2). The Benbolt is interpreted as a sequence of supratidal, intertidal, and shallow subtidal zones (Walker, Reference Walker1985). The outcrop is an exposed dip slope along US 25E that exposes a series of thin interbedded shales with highly fossiliferous limestones. The fauna consists of a variety of blastozoan echinoderms, especially paracrinoids, glyptocystitoid rhombiferans, and crinoids, as well as brachiopods, trilobites, and receptaculitids. The unit is interpreted as a shallow open marine environment.
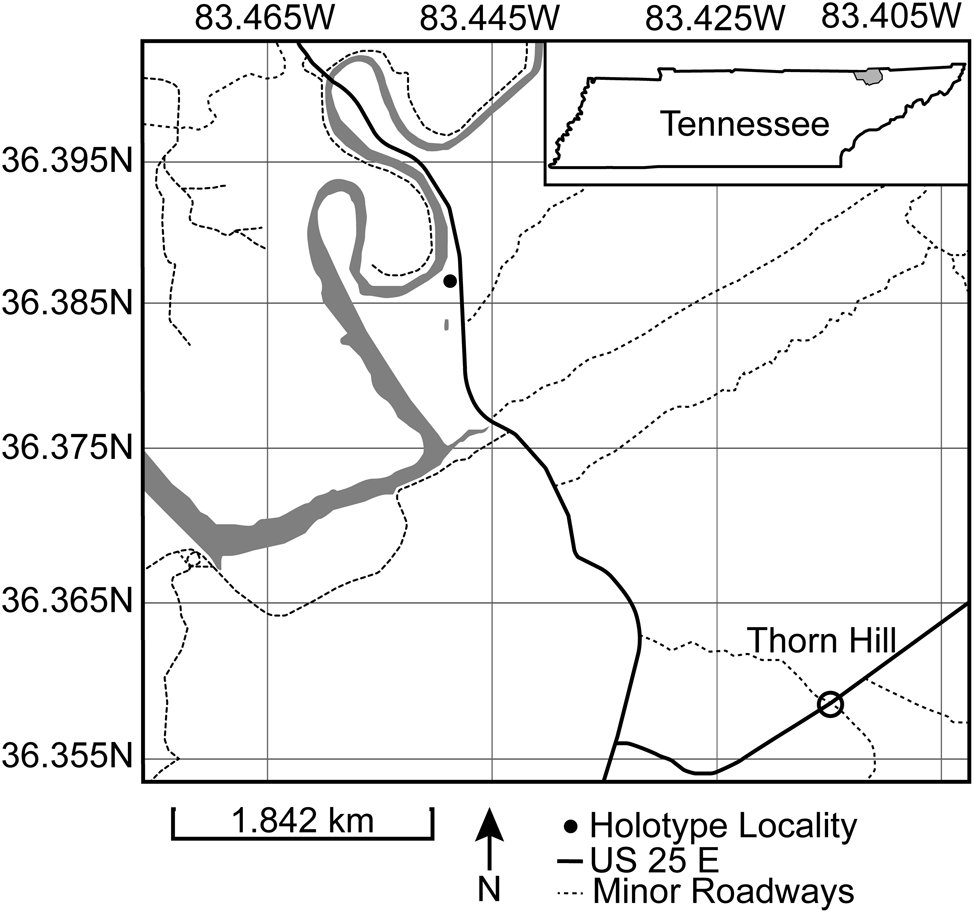
Figure 2. Map of locality near Thorn Hill, Tennessee. Holotype CMCIP95726 collection site indicated by filled circle.
CMCIP95727 is from the Upper Ordovician (Sandbian) Benbolt Formation north of Knoxville along Racoon Valley Road from the site described by Broadhead (Reference Broadhead1984) (36.1916°N, 83.9250°W). The unit consists of interbedded argillaceous packstone and wackestone, interpreted as a small-scale deepening to shoaling carbonate shelf regime in a broader transgressive sequence, followed by a regression (Broadhead, Reference Broadhead1984). The fauna is like that seen at the Thorne Hill locality, with the addition of the early stem blastoid Macurdablastus (Broadhead, Reference Broadhead1984; Bauer et al., Reference Bauer, Waters and Sumrall2019) and the unusual glyptocystitoid Sprinkleocystis (Broadhead and Sumrall, Reference Broadhead and Sumrall2003).
Terminology
Dorsal and ventral are used to describe the orientation of thecal sides. Dorsal is synonymous with the upper, abanal, or rhomb-bearing side. Dorsal and ventral descriptors are used because pectinirhombs are oriented toward the water column, and the periproct is oriented toward the substrate (Sumrall, Reference Sumrall2000). Posterior, rectal, and anal lobes are morphologically synonymous. “Posterior lobe” is used in this description.
Repository and institutional abbreviation
Types and figured specimens examined in this study are deposited in the Invertebrate Paleontology Collection at the Cincinnati Museum Center (CMCIP), Cincinnati, USA.
Systematic paleontology
Class Rhombifera Zittel, Reference Zittel1879 emend. Paul, Reference Paul1968
Order Dichoporita Jaekel, Reference Jaekel1899 emend. Paul, Reference Paul1968
Superfamily Glyptocystitida Bather, Reference Bather and Murray1899
Family Pleurocystitidae Neumayr, Reference Neumayr1889
Subfamily Pleurocystitinae Sumrall and Sprinkle, Reference Sumrall and Sprinkle1995
Genus Pleurocystites Billings, Reference Billings1854
Type species
Pleurocystites squamosus Billings, Reference Billings1854.
Remarks
The holotype of Pleurocystites? scylla n. sp. has conjunct pectinirhombs, excluding it from Praepleurocystis. Amecystis and Deltacystis are also excluded as pectinirhombs are absent in these genera. Unlike the single pectinirhomb of Turgidacystis, Regulaecystis, and Coopericystis, P? scylla has three pectinirhombs. The plating of the periproctal margin of P? scylla differentiates it from Pygecystis. A designation of Pleurocystites is then preliminarily supported on the basis of pectinirhomb number and dichopore type, as well as the plating of the periproctal margin.
However, the tumidity of the theca (~11.19 mm) and thecal outline in P? scylla do not align with other members of Pleurocystites. Excluding the slight inflation shown in P. beckeri (Foerste, Reference Foerste1921), Pleurocystites are generally flat in the dorsal–ventral plane, while Praepleurocystis are highly inflated. The deformation required to achieve the tumidity shown in the holotype of P? scylla without some base level of thecal inflation would completely distort the plate boundaries and stem attachment. This is not demonstrated in the holotype, and some degree of tumidity is inferred to be natural. The overall outline is also more in line with Praepleurocystis, but this character varies across Pleurocystitida (Parsley, Reference Parsley1970).
The original description of Praepleurocystis distinguished it from Pleurocystites by the nature of the pectinirhombs and plating around the gonopore and hydropore (Paul, Reference Paul and Millott1967). The plating of the gonopore/hydropore is not known in this specimen and, as such, cannot be used in diagnosis. The placement of the pectinirhombs within pleurocystitids is largely stable, excluding Plethoschisma, where the dominant rhomb is on the IL3/IL4 suture (Sumrall and Sprinkle, Reference Sumrall and Sprinkle1995), but the number of total pectinirhombs varies from zero to three. Pleurocystites differs from Turgidacystis, Regulaecystis, and Coopericystis in the presence of pectinirhombs on the B2/IL2 suture and the L1/L2 suture. But even within Pleurocystites, P. rugeri (Paul, Reference Paul1984) lacks a pectinirhomb on the B2/IL2 suture. Pectinirhombs are likely convergent structures and, as such, should not be considered as the sole diagnostic character (Sheffield et al., Reference Sheffield, Limbeck, Bauer, Hill and Nohejlová2022).
The infralaterals that border the periproctal margin have been interpreted as taxonomically significant (Parsley, Reference Parsley1970; Sumrall and Sprinkle, Reference Sumrall and Sprinkle1995). In the holotype of P? scylla, the periproctal margin consists of plates B1, B4, IL4, IL5, L4, and L1. This plating aligns with Pleurocystites rather than the B1, B4, IL1, IL3, IL4, IL5, L1, and L4 margin assigned to Praepleurocystis. However, specimens fitting the Praepleurocystis-type margin include Pleurocystites distans (Parsley, Reference Parsley1970), Pleurocystites anglicus (Bather, Reference Bather1913), and Pleurocystites toddi (Sprinkle et al., Reference Sprinkle, Henry, Zimmer, Kelley and Whiteley1985). In addition, Pleurocystites squamosus (Hussey, Reference Hussey1928) and Pleurocystites beckeri (Foerste, Reference Foerste1921) exhibit variable marginal plating. The simplest explanation for variance in marginal plating is that pleurocystitids, like other glyptocystitids, grow through the addition of peripheral plates (Kesling, Reference Kesling and Moore1967). Due to the flattening of the theca, these areas of peripheral growth would presumably correlate with the periproctal margin. Some changes in the marginal plating appear to be the result of the presence of the anal lobe (Sprinkle, Reference Sprinkle1974). As shown by P. anglicus. P. distans, and P. toddi, a greater number of marginal plates does not have a direct correlation with the presence of the anal lobe. Variance in marginal plating may then be a result of ontogeny as the periproct increases in size during ontogeny (Brower, Reference Brower1999).
While most characters align with Pleurocystites, P? scylla is placed provisionally in this genus due to concerns surrounding the consistency of marginal plating and the disparity in tumidity between the holotype and other species of Pleurocystites. Other characters, including the plating of the gonopore and hydropore, would be able to solidify this assignment. Until another specimen is found with an exposed ventral surface, P? scylla should remain provisionally in Pleurocystites.
Holotype
CMCIP95726 preserved in a small slab in dorsal aspect.
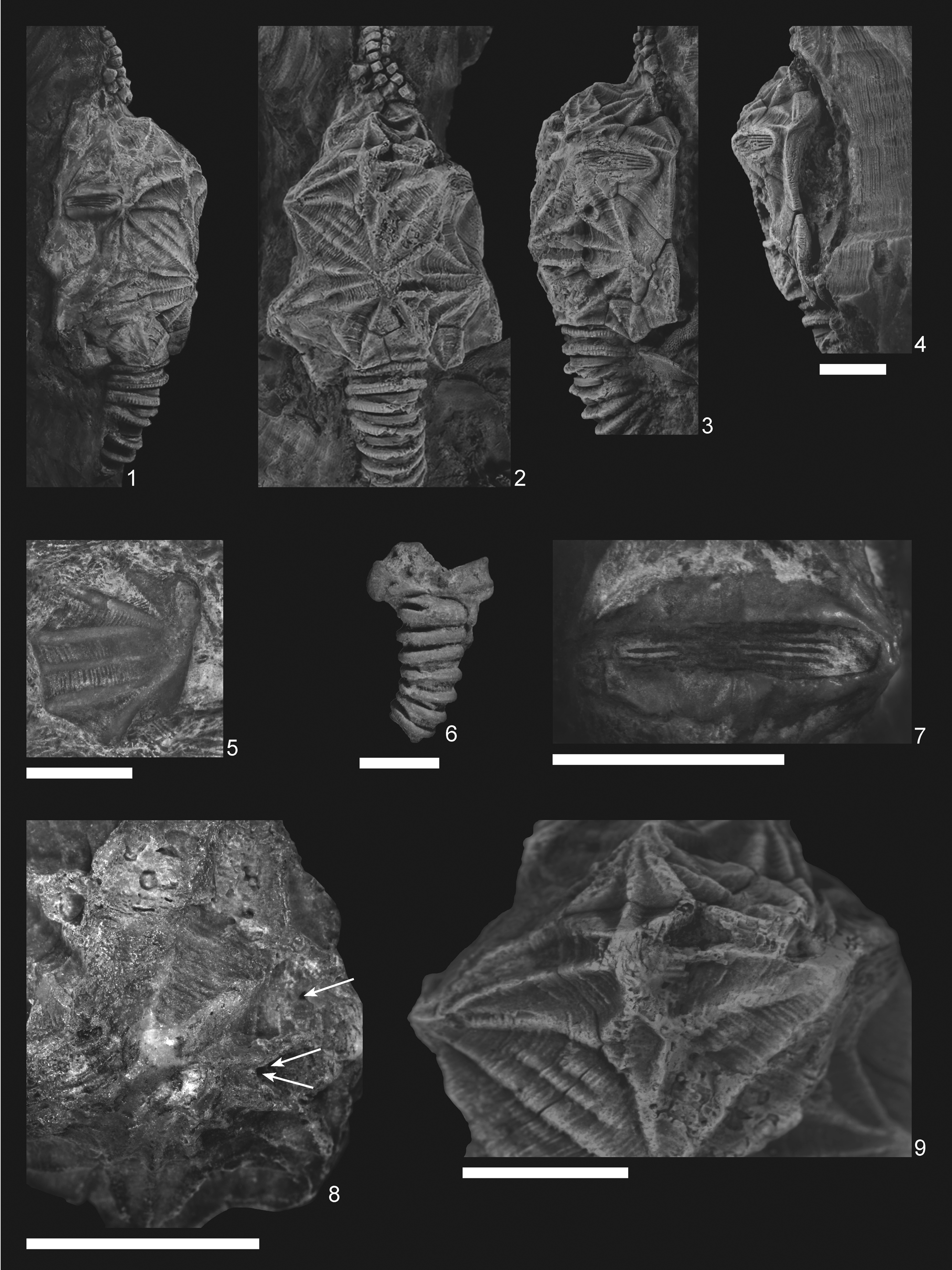
Figure 3. Pleurocystites? scylla n. sp. (1–4, 7–9) CMCIP95726 from the Benbolt Formation, upper Ordovician, near Thorn Hill, Grainger County, Tennessee: (1–3) dorsal and lateral views of holotype showing tumidity of theca and thecal outline; (4) oblique view of theca showing periproct; (7) L3/L4 conjunct pectinirhomb showing confluent dichopores; (8) small holes on the L2 plate (denoted by arrows) of a skeptically parasitic nature; (9) supernumerous L3′ plate showing clear suturing. (5) CMCIP95728 isolated plate from the Benbolt Formation. (6) Paratype CMCIP95727 showing partial basal circlet and proximal stem. Scale bars = 5 mm.
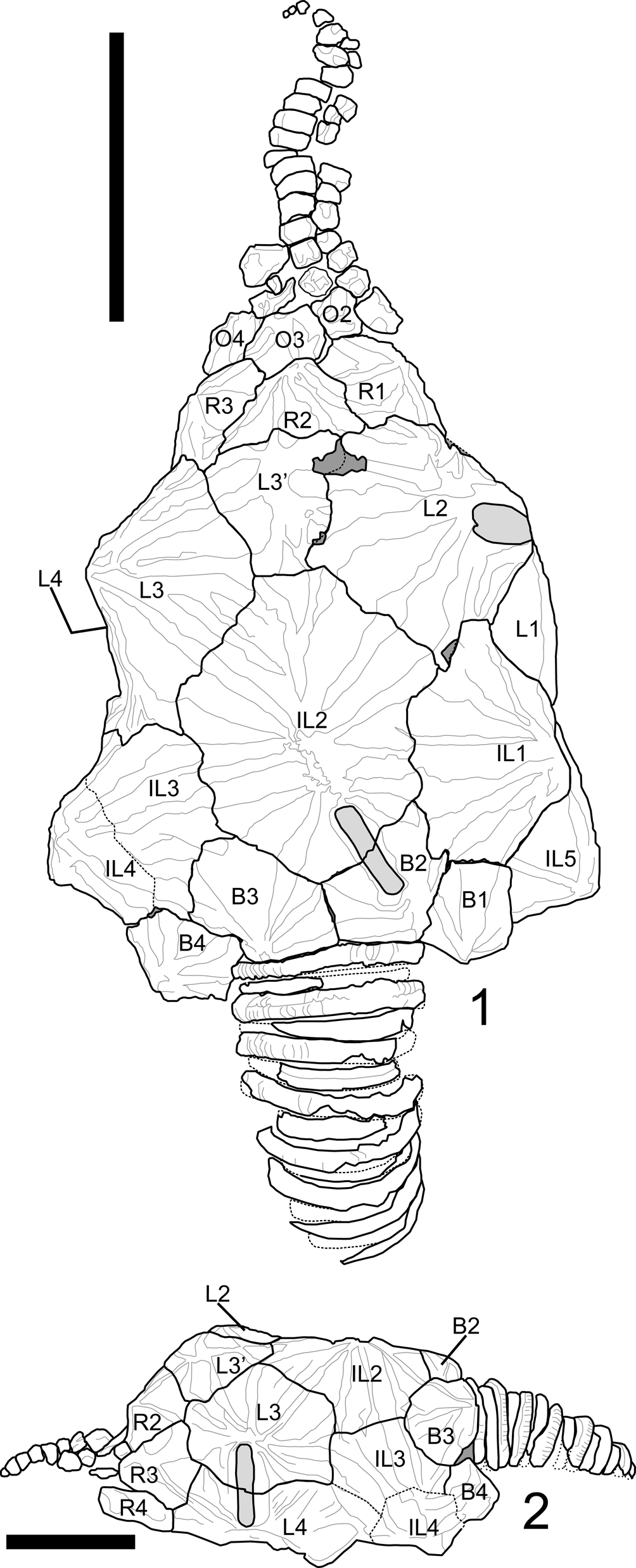
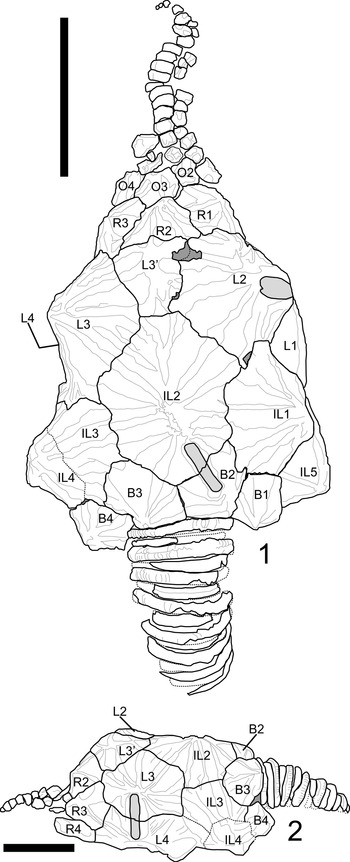
Figure 4. Plate diagram of Pleurocystites? scylla n. sp. from the Benbolt Formation, upper Ordovician, near Thorn Hill, Grainger County, Tennessee. (1) Dorsal view of plating; note thecal shape, ornamentation, and additional plate denoted as L3′. (2) Lateral view of left side; note IL3 does not enter periproctal margin. The L3/L4 pectinirhomb is shown in this view as it cannot be seen in the dorsal aspect. Scale bars = 1 cm. B = basal plate; IL = infralateral plate; L = lateral plate; R = radial plate; O = oral plate.
Diagnosis
Pleurocystites with coarse thecal ornamentation that consists of strong axial and radial ridging as well as strong concentric growth lines, IL1 and IL3 not in contact with the periproctal border, and conjunct pectinirhombs.
Occurrence
Pleurocystites? scylla n. sp. is known only from the Upper Ordovician (Sandbian) Benbolt Formation of Grainger and Knox Counties, Tennessee, USA.
Description
Description is based on the near-complete holotype CMCIP95726, smaller incomplete paratype CMCIP95727, and two isolated plates CMCIP95728 and CMCIP95729. Theca subtriangular in outline and highly domed with three compressed pectinirhombs. Two stout brachioles present, one preserved proximally and the second preserved as scattered plates (Fig. 3.2). Periproct and ventral surface unknown. Thecal ornamentation ridges radiate from center of each plate from knob-like nodes (Fig. 3.1–3.3). Plate junctures occur in depressions between nodes. Theca asymmetric, exhibiting greater concavity on left than on right. Theca bearing minimal posterior lobes (Fig. 3.2), bearing five circlets, basals (B), infralaterals (IL), laterals (L), radials (R), and orals (O); basal circlet composed of plates B1, B2, B3, and B4 (Fig. 4.1), forming stem facet. B3 largest plate in circlet. Infralateral circlet closed, composed of five plates forming arc at widest point of theca (Fig. 4.1). IL2 largest, placed along thecal midline; IL3 and IL4 smaller compared with IL1 and IL5; IL4 and IL5 forming distal corners of theca; IL1 contains central node, whereas IL3 does not, contributing to overall thecal asymmetry (Fig. 4.2). Lateral circlet closed, contains four visible plates plus presumed anomalous plate denoted as plate L3′ (Figs. 3.9, 4.1). L3′ inferred to be teratogenic and a nondiagnostic feature of this species. L5 not seen, presumed on ventral face as in other pleurocystitids. As with IL circlet, L3 and L4 reduced in size compared with L2 and L1; L3′ smallest plate in circlet, although likely the unobserved L5 plate is smaller (Fig. 4.1). Radial circlet occurs at dramatic narrowing of theca; contains plates R1, R2, R3, and R4. R4 reduced compared with R2 and R3; R1 larger in circlet than other species. L3′ borders R2 and appears to reduce R2 plate size (Fig. 4.1). Complete extent of radial circlet unknown. Oral circlet is known from O2, O3, and O4 (Fig. 4.1). Complete extent of oral circlet unknown. Designations of radial and oral plates made with some uncertainty on the basis of studies of more-completely known taxa due to obscured ventral side of holotype. One stout brachiole present, and second brachiole inferred from presence of plates and fragments above the oral circlet unassociated with known brachiole (Figs. 3.5, 4.1). Brachiole biserially alternate, plates wider than high, oriented with food groove down as in other pleurocystitids, cover plates unknown (Fig. 3.2). Three pectinirhombs present in standard pleurocystitid positions along the L3/L4, L1/L2, and B2/IL2 sutures (Figs. 3.2, 4.1, 4.2); pectinirhomb on B2/IL2 somewhat smaller and eroded in holotype. Pectinirhombs compressed in shape and conjunct with confluent dichopores. Pectinirhomb length much greater than width (6:1), giving an oblong-shaped appearance. Five folds occur in each pectinirhomb (Fig. 3.7). All pectinirhombs elevated above theca with robust rim, slightly depressed along suture. Thecal ornamentation present on all dorsal thecal plates. Dorsal thecal plates exhibit strong and numerous concentric growth lines that cross ridges (Fig. 3.2) as well as axial and radial ridging; about 6–10 ridges occur on each dorsal plate. Where ridges join, dorsal thecal plates form nodes on convex surfaces. All three-plate junctions occur in depressions between nodes (Fig. 4.1). Ventral side mostly obscured by substrate, and ornamentation on anal surface unknown. Gonopore and hydropore, not observed; presumably on ventral side as with other pleurocystitids. Anal pyramid unknown. Anal surface mostly obscured and bounded by basals B1 and B4, infralaterals IL5 and IL4, and laterals L1 and L4. L5 may or may not be in contact with periproctal border but is not observed in holotype. IL1 and IL3 precluded from periproctal border (Figs. 3.4, 4.2). Overall periproctal border shape not known, extends to edge of IL5 and L1. The periproctal margin appears mostly flat, with bordering plate extending only slightly onto the surface, forming tight marginal rim around periproctal membrane. Posterior lobe minimal. Periproct inferred to consist of many small platelets, a few of which are observed in the holotype. Proximal stem consists of alternating inner and outer columnals. Outer columnals thin with large lumen, ornamented with longitudinal ridges; inner columnals smaller, with large lumen, unornamented (Fig. 3.2). Present portion of proximal stem displays little tapering; distal end of stem unknown (Fig. 3.2, 3.6).
Etymology
Scylla is a Greek mythical monster representing dangerous current systems in the Strait of Messina (Alpers and Salusti, Reference Alpers and Salusti1983). Scylla does not have a consistent form across the mythos but always combines aspects of a woman, a dog, and the sea (Hopman, Reference Hopman2013).
Materials
The holotype CMCIP95726 is a mostly complete theca with a proximal stem and one brachiole known, somewhat disrupted and incomplete. The paratype CMCIP95727 consists of plates B1, B2, and B3 and the proximal stem (Fig. 3.6). One isolated plate CMCIP95728 is included in this description. The plates have concentric growth lines and axial/radial ridges that end in a node (Fig. 3.5). This plate is provisionally assigned to P? scylla as the only other known pleurocystitid from this locality, Coopericystis pyriformis (Parsley, Reference Parsley1970), has stronger growth lines and weaker ridging on its thecal plates than P? scylla.
Remarks
Pleurocystites? scylla, n. sp. is like Pleurocystites squamosus, the type species of Pleurocystites, in the plating of the periproctal margin, absence of posterior lobes, and three conjunct pectinirhombs on the L3/L4, B2/IL2, and L1/L2 sutures. It differs by having a tumid theca, a highly ridged ornamentation, and oblong-shaped pectinirhombs. There is much variability in the outline and periproctal plating of P. squamosus. While P? scylla does align in these characters, some specimens of P. squamosus are inconsistent in these features. It is for this reason that characters that normally would not be heavily weighted are considered in this diagnosis.
Pleurocystites beckeri bears the greatest inflation of a Pleurocystites before P? scylla. The tumidity shown in P. beckeri is less than that of any Praepleurocystis and that of P? scylla (Foerste, Reference Foerste1921). The thecal ornamentation of P. beckeri lacks the concentric growth lines observed in P? scylla and has accessory ridging, giving a pseudo-rhomb appearance (Parsley, Reference Parsley1970). Some specimens of P. beckeri exhibit a similar thecal outline to P? scylla, but variation in thecal outline of this species is known.
Pleurocystites toddi is like P? scylla in thecal outline and pectinirhomb type and shape. It differs in having a less-tumid theca with thecal plates that are more planar with less-robust ridging than is seen in P? scylla (Sprinkle et al., Reference Sprinkle, Henry, Zimmer, Kelley and Whiteley1985). Like Pleurocystites mitratus (Regnéll and Paul, Reference Regnéll and Paul1981), P? scylla displays vaulted thecal plates with axial and radial ridging and concentric growth lines (Regnéll and Paul, Reference Regnéll and Paul1981). Both species have incomplete holotypes, but the preserved distal surfaces differ. In P. mitratus, IL1 plate lacks the knob-like structure observed in P? Scylla; the shapes of B2 and B3 form periproctal lobes in P. mitratus. Overall, the thecal outline is far more rounded in P. mitratus than in P? scylla. Pleurocystites rugeri differs from P? scylla and all other members of Pleurocystites through the absence of the B2/IL2 pectinirhomb (Paul, Reference Paul1984). Pleurocystites? scylla differs from Pleurocystites strimplei (Brower, Reference Brower1999), Pleurocystites anglicus, Pleurocystites cristatus, Pleurocystites foriolus (Paul, Reference Paul1984), Pleurocystites filitexitus (Billings, Reference Billings1854), and Pleurocystites distans (Parsley, Reference Parsley1970) in robust thecal ornamentation, tumidity, and the shape of the pectinirhombs.
Comparing P? scylla with members of Praepleurocystis, there is the uniform difference of all members having disjunct pectinirhombs. Pleurocystites? scylla is similar in thecal outline, ornamentation, raised pectinirhombs with strong rims, and tumidity to Praepleurocystis watkinsi (Parsley, Reference Parsley and Sprinkle1982). Apart from the pectinirhomb type, they differ in that P. watkinsi has IL4 and IL5 sharing the periproctal margin. Praepleurocystis nodosus (Westphal, Reference Westphal1974) and Praepleurocystis ranaformis (Guensburg, Reference Guensburg1982) both have a more rounded thecal outline than P? scylla although they are similar in their tumidity and lack of a periproctal lobe. As with P. watkinsi, IL4/IL5 attain the margin, and the pectinirhombs differ in being disjunct. Despite concern regarding characters, it is for these reasons that P? scylla is placed in Pleurocystites despite sharing some characters with Praepleurocystis.
Discussion
Teratologies in fossil echinoderms
Many early echinoderms are noted for having irregular thecal plating, in which plates are continuously added through ontogeny (Ubaghs, Reference Ubaghs1975). In most edrioasteroid- and eocrinoid-grade taxa, this growth pattern led to an irregular mosaic of plates (Guensburg and Sprinkle, Reference Guensburg and Sprinkle2001; Nardin et al., Reference Nardin, Lefebvre, Fatka, Nohejlová, Kašička, Šinágl and Szabad2017). Even in mosaic plating styles, there are patterns followed by echinoderms. Interambulacral plates are added along the edges of the ambulacra in edrioasteroids (Zamora et al., Reference Zamora, Sumrall and Vizcaïno2012), and new plates are inserted at three plate junctions in some blastozoans. In other cases, there is a regular order to plate additions, such as in echinoids, where patterns of plate insertion can be derived from the characteristics of their development (Zachos and Sprinkle, Reference Zachos, Sprinkle and Elewa2011). In many derived taxa, however, plating of the theca becomes stabilized around a stereotypical bauplan where individual plates of the theca can be recognized among all taxa within a clade like blastoids, hemicosmitoids, and glyptocystitoids (Sumrall and Waters, Reference Sumrall and Waters2012). Such fossil taxa offer an opportunity to investigate deviations from standardized plate arrangements and their potential causes.
Consistency is the norm in echinoderms bearing standardized thecal plating, but exceptions to normal plating arrangements are also documented (Kesling, Reference Kesling and Moore1967). For example, among Crinoidea, specimens with additional arms (Ausich and Kammer, Reference Ausich and Kammer1989); addition, reduction, or fusion of infrabasal plates (Peter, Reference Peter2019); and reduced rays (Webster and Donovan, Reference Webster and Donovan2012) are observed. Anomalous morphologies among blastoids have been documented as well, with the most common anomalies occurring in the AB–D plane (Macurda, Reference Macurda1980). In general, anomalies are observed in the fusion or division of a thecal plate (Kesling, Reference Kesling and Moore1967).
Among glyptocystitoid rhombiferans, several specimens exhibit additional plate insertions into the thecal circlets. The type specimen of Hadrocystis pauli (Sprinkle, Reference Sprinkle1974) bears two additional plates: one in the infralateral and one in the lateral circlet. Paratypes confirm that this arrangement is an anomaly within the holotype (Sprinkle, Reference Sprinkle1974). In Callocystitidae, the holotype of Tyrridiocystis chelyon (Broadhead and Strimple, Reference Broadhead and Strimple1978) exhibits an additional basal plate not shown in the paratype (Broadhead and Strimple, Reference Broadhead and Strimple1978). A specimen of Callocystites canadensis (Billings, Reference Billings1866) with a division of the L4 plate into two plates (Fig. 1.2) also exists. Within Glyptocystitidae, some specimens of Glyptocystella loeblichi (Sprinkle, Reference Sprinkle and Sprinkle1982) exhibit reductions in the ambulacral system, one specimen of Pirocystella strimplei (Sprinkle, Reference Sprinkle and Sprinkle1982) is missing a basal plate and includes an additional lateral plate, and the holotype of Pirocystella cooki (Sprinkle, Reference Sprinkle and Sprinkle1982) has an extra plate between B3 and IL2 (Sprinkle, Reference Sprinkle and Sprinkle1982). In these cases, the overall shape of the theca and the arrangement of the ornamentation remain unchanged except for minor variation in the ridge networks to accommodate the position of the affected sutures.
Most of these deviations from consistent plating can be attributed to teratogenesis—the presence of an abnormality in an organisms’ morphology, behavior, cognitive function, or biochemistry caused by an agent that disrupts signaling pathways during development (Conley and Richards, Reference Conley, Richards, Jorgensen and Fath2008). In the preceding examples of anomalous echinoderm specimens, the overall shape of the organism is generally undistorted by the presence, absence, or fusion of individual skeletal elements. Instead, the interplate sutural contacts are shifted to accommodate the anomalies, and subtle changes to the linkages in the plate ridge network may occur. As demonstrated by the holotypes of H. pauli, T. chelyon, and P. cooki, a species diagnosis can still be made from an anomalous holotype (Sprinkle, Reference Sprinkle1974; Sprinkle, Reference Sprinkle and Sprinkle1982; Broadhead and Strimple, Reference Broadhead and Strimple1978). We then interpret the additional plate in P? scylla as a nondiagnostic teratologic feature and base the diagnosis on other morphologic characters.
Anomalous adult organisms are known from both modern and fossil collections, suggesting that the physiological differences these anomalies instilled did not significantly affect longevity. The large size and apparent maturity of all documented fossil specimens further support that, while recognizable, these differences are not detrimental to the organism's survival. Furthermore, anomalous organisms represent a small proportion of the total population given the causal mechanism of teratogenesis (Hengsbach, Reference Hengsbach, Landman, Tanabe and Davis1996). Anomalous or teratologic individuals are a normal, but small, part of populations through time.
Teratology in Pleurocystites? scylla
Before this description, there was not a published record of a teratological specimen within the Pleurocystitidae. Difficulties in identifying the specimen to a species level and issues with preservation are noted (Nardin and Bohatý, Reference Nardin and Bohatý2013) and are potential barriers to prior identification of teratological specimens. Furthermore, this description is complicated by an anomaly wherein the only complete specimen of Pleurocystites? scylla n. sp. has an additional plate in the lateral circlet. However, as discussed in the preceding section, there is precedence for species designations made from teratologic holotypes.
Suitable sample population size, knowledge of developmental pathways, and understanding of normal morphology are requirements for correct identification of a teratological specimen (Macurda, Reference Macurda1980; Diogo et al., Reference Diogo, Guinard and Diaz2016; Diaz, Reference Diaz2020). Having only a few specimens may result in a teratology not being sampled, or in the case of P? scylla, having a single complete specimen that is abnormal can make the anomalous specimen appear as the norm. This may also result in individuals of the same species being erroneously separated into different species in the absence of contextual information. It is highly unlikely that the additional plate is a normal feature of this species or an artifact of an ancestral state with additional plates given what is known of pleurocystitid evolution. Glyptocystitids are thought to be ancestral to pleurocystitids, wherein the plating of the glyptocystitids can be directly applied to the flattened pleurocystitids (Sprinkle, Reference Sprinkle1974). Whether this ancestral organism was a pentaradial or bilateral glyptocystitid is of some debate, but a glyptocystitid ancestor is well accepted (Parsley, Reference Parsley and Sprinkle1982). While derived taxa in echinoderms exhibit reductions in thecal plating, the thecal plates of glyptocystitoid rhombiferans are already standardized into the five circlets with five laterals exhibited in all known pleurocystitids (Sumrall and Waters, Reference Sumrall and Waters2012). It is highly unlikely that a form with standardized plating derived from a form with standardized plating would exhibit a plesiomorphic multiplated state given that the last multiplated ancestor was likely a stem blastozoan (Guensburg and Sprinkle, Reference Guensburg and Sprinkle2001). The L3′ plate is thus most likely not a plesiomorphic character.
The additional plate is not found in other described members of this family that, as mentioned, has a very conservative thecal-plating morphology. The additional plate in the holotype of P? scylla is smaller than other plates in the circlet. The specimen is at a post-larval stage and is either adult or subadult judging from its size and plate relationships consistent with other mature specimens. It is articulated and shows minimal evidence of wear and breakage along the right dorsal side across the nodes. Small holes with raised lips do occur along the IL1/IL2 plate boundary (Fig. 3.8). These holes are interpreted as possibly parasitic with low reliability and a Boucot class of six (Boucot, Reference Boucot1990).
Lateral 3′ is not believed to be a result of the possible parasitism as evidenced by the plate formation sequence. Plates grow concentrically through time, as recorded by growth lines on the plate surface, and plate additions occur through holoperipheral growth along the outer edges of the plate as the organism develops (Kesling, Reference Kesling and Moore1967; Sprinkle, Reference Sprinkle1973). An additional plate interrupting a circlet does not support a calcifying pathological response to a wound or parasite as the cause of additional plate formation. If this were the case, plate fusion or addition near the thecal edge would be expected. Thus, although there is speculative evidence of parasitism, teratogenesis is the most likely reason for an additional lateral plate in the holotype.
Although only one complete theca is known, plating consistency among members of the family is such that L3′ is most parsimoniously considered supernumerous and not an artifact of life stage. A false positive designation of a teratological feature is still possible but unlikely. A false negative is not applicable to this specimen as the species description excludes this feature as nondiagnostic. Additional specimens could relieve any remaining questions regarding the nature of L3′ in the holotype, but given the context of the organism, there is some amount of confidence that the type specimen is teratologic. The designation as a teratology is made with the following evidence: (1) the additional plate is unlikely to be a result of parasitism or wound healing because it is a fully formed plate with consistent growth lines, (2) the plate disrupts the circlet and possibly lends itself to the thecal asymmetry, and (3) the presence of an extra lateral plate as observed does not match any specimen described in the family. For these reasons, the holotype of P? scylla is an example of a teratologic specimen that does not diminish the diagnostic properties of the specimen even though it contains anomalous elements.
Acknowledgments
The land comprising both collection sites was formerly the territory of the Tsalaguwetiyi (Cherokee, East), Shawandasee Tula (Shawanwaki/Shawnee), and S'atsoyaha (Yuchi) tribes. Thanks are given to B. Hunda and C. Schwalbach at the Cincinnati Museum Center for help with specimens. Gratitude is extended to S. Zamora, B. Lefebvre, E. Nardin, J. Sprinkle, and an anonymous reviewer for improvements to this manuscript.
Declaration of competing interests
The authors declare none.