CASE HISTORY
A 26-year-old healthy, athletic male presented to an acute care setting with three months of worsening right calf pain. Pain began while marching with 30 pounds on his back. He was seen by his primary care provider and diagnosed with an exercise-induced muscle strain. The patient took ibuprofen, iced the leg, and avoided exertion for six weeks, noting some improvement. When he resumed normal activity, the pain returned and increased with running. He noticed swelling of the right calf compared to the left, worsening with exercise, and a mass on the medial aspect of his right calf. He denied recent travel, recent surgeries, personal or family history of clotting disorders, and hormone use. The patient was afebrile with pulse of 70 bpm and a blood pressure of 126/73 mm Hg. On examination, the right calf was 4 cm larger in circumference than the left calf with pain upon calf palpation and foot dorsiflexion. He was neurovascularly intact distally with normal strength. There was a firm, mildly tender, mobile 1 cm mass on the medial aspect of his right calf. An ultrasound was ordered to evaluate for deep venous thrombosis (Figure 1).
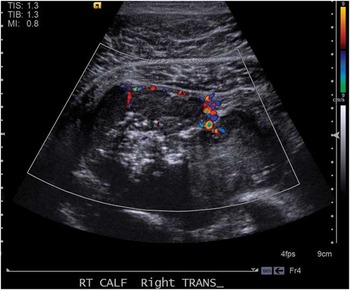
Figure 1 Doppler ultrasonography of large soft tissue mass of right calf measuring 8 cm in diameter.
QUESTION
What is the next appropriate step for this patient?
a) Begin broad-spectrum antibiotics and consult surgery
b) Measure compartment pressures
c)Obtain urgent orthopedics consultation and advanced imaging with CT or MRI
d) Begin low-molecular weight heparin
e) Consult vascular surgery
ANSWER
The correct answer is c) obtain urgent orthopedics consultation and advanced imaging with CT or MRI. An ultrasound negative for deep venous thrombosis noted a deep 8.0 cm posterior soft tissue mass. Magnetic resonance imaging (Figure 2) identified a hemorrhagic multi-lobulated mass infiltrating musculature and neurovascular structures. The patient was urgently referred to the orthopedics department which ordered plain film radiographs and computerized tomography (CT) scans that identified enlarged pulmonary hilar lymph nodes and a left adrenal mass. Biopsies confirmed Stage 4 Ewing sarcoma. Initial radiation and chemotherapy resulted in necrosis of the adrenal tumor but without change in size of the right leg mass. Surgery was deemed futile given the leg mass’s invasive nature and presence of metastasis. After more rounds of chemotherapy, scans demonstrated diffuse disease including a cardiac metastasis, and he opted for home hospice care.

Figure 2 T2 weighted magnetic resonance imaging of bilateral lower extremities demonstrating 8.0×5.3×9.7 cm soft tissue mass of right lower extremity (red arrow). Abnormal marrow enhancing and marrow edema noted within the fibula at the same level (green arrow).
Ewing sarcoma, the second most common childhood malignant bone tumor,Reference Traki, Rkain and Aktaou 1 commonly affects 10-20 year old malesReference Mankin 2 , Reference Ludwig 3 , and most commonly arises in the axial skeleton. More rarely, Ewing sarcoma may originate from soft tissue (40%), also referred to as extraskeletal Ewing sarcoma (EES).Reference Ludwig 3 The typical presentation involves localized swelling and tenderness. Patients are often initially diagnosed with sports injuries potentially delaying diagnosis and contributing to the high metastatic rate (95%).Reference Ludwig 3 Interestingly, while over 70% of patients present with clinically localized disease, over 80% of patients who have only local resection will experience recurrence, indicating that microscopic metastasis at time of presentation is the rule rather than the exception. Radiographs may show lyticReference Bullough 4 or periosteal lesions; ultrasound has previously been noted to help identification of soft-tissue masses in Ewing sarcoma.Reference Hwang and Panicek 5 One study by Traki et al., reported a case in which initial radiographs were non-diagnostic and ultrasound identified irregularities of the cortical bone suggesting possible malignant origin and triggering further work-up.Reference Traki, Rkain and Aktaou 1 Treatment of Ewing sarcoma includes chemotherapy, possible radiation, and surgical debulking or amputation.Reference Mankin 2 There are no major differences in survival or response to treatment in skeletal vs. extraskeletal Ewing sarcoma. The 5-year survival for metastatic disease is 25%-30%.Reference Ludwig 3 , Reference Applebaum, Worch and Matthay 6
Ultrasound is used commonly in the Emergency Department (ED) to assist with diagnosis of numerous diseases. The differential diagnosis for the ultrasound images include abscess (answer a), exercise induced compartment syndrome with muscle necrosis (answer b), deep venous thrombosis (answer d), and vascular malformation (answer e).
Abscesses are a common ED diagnosis, requiring incision, drainage, and potential antibiotics depending on co-incident cellulitis or patient/wound risk factors. This patient’s examination lacked the typical fluctuance and ultrasound findings of an abscess. An abscess should appear on ultrasound as a roughly spherical collection of anechoic or hypoechoic fluid, possibly swirling with compression.Reference Frazee and Dewitz 7 “Cobblestoning” of the tissue would suggest cellulitis.Reference Frazee and Dewitz 7 Treatment of an abscess as extensive as this patient’s lesion would likely involve broad-spectrum antibiotics and surgical consult for operative management.
Chronic exertional compartment syndrome (CECS) is a non-traumatic process most commonly seen in athletes.Reference Barnes 8 In contrast to our patient, however, the typical CECS patient’s symptoms should resolve completely 10-20 minutes after exercise cessation, and the non-exertional examination should be normal. Middle-aged patients are at higher risk, with patients over 40 having a 90-fold higher incidence than patients under 20 years of age.Reference Waterman, Liu and Newcomb 9 Acute-on-chronic compartment syndrome can lead to muscle necrosis, in which case pain and tenderness of the compartment would still be present at rest without the focal findings described here.Reference Goldfarb and Kaeding 10
Deep venous thrombosis (DVT) was the original concern triggering an ultrasound in this patient. DVT most often occurs in patients with risk factors such as hormonal therapy, recent trauma, hypercoagulable states, and inactivity/immobilization. While this patient did not have any of these classic risk factors, he did have a palpable cord on examination and pain on palpation of the posterior calf.Reference Urbano 11 Emergency physicians are generally trained to scan for lower extremity DVT down to the popliteal. Some centers lack experienced technicians to be able to perform ultrasound for calf DVTs, but a technically adequate study has a sensitivity and specificity above 90% for calf clots.Reference Rose, Zwiebel and Nelson 12 D-dimer testing can be helpful in patients with low pretest probability for venous thromboembolism (VTE), with a negative predictive value of 99%.Reference Sartori, Cosmi and Legnani 13 The only findings on ultrasound positive for a DVT should be intravascular clot with unaffected surrounding tissues. If diagnosed, anticoagulation would likely be initiated.
Finally, a vascular malformation would also be on the differential for a patient with the ultrasound shown. Diagnosis would require further imaging, usually magnetic resonance imaging with contrast. High flow vascular lesions (arteriovenous malformation (AVM), arteriovenous fistulas, and hemangiomas) encapsulated by muscle fascia can be difficult to differentiate from a tumor.Reference Konez 14 AVMs can remain asymptomatic until trauma or iatrogenic insults such as biopsies, and an exertional component typically isn’t expected.Reference Jackson 15
Competing Interest: None reported.
Keywords: Ewing, sarcoma, ultrasound






