Introduction
The prevalence of traumatic brain injury (TBI) has been found to be far higher in people within the justice system in comparison to the general population (Allely, Reference Allely2016). Studies suggest that up to 82% of people in prison report having experienced at least one TBI in their lifetime (Schofield, Butler, Hollis & D’Este, Reference Schofield, Butler, Hollis, Smith, Lee and Kelso2006), with one third experiencing multiple injuries (Mitchell, Theadom & du Preez, Reference Mitchell, Theadom and du Preez2017). Whilst there has been debate regarding the reliability of subjective reporting of TBI in prisoners, evidence suggests subjective accounts are generally accurate when compared to medical records (Schofield et al., Reference Schofield, Butler, Hollis and D’Este2011). Indeed, some authors argue subjective reports may be more accurate than medical records as many people do not seek medical attention following injury for fear of reprisal or lack of awareness (Feigin et al., Reference Feigin, Theadom, Barker-Collo, Starkey, McPherson, Kahan and Ameratunga2013).
The impacts of TBI can last for many years, even following a mild TBI (McMahon et al., Reference McMahon, Hricik, Yue, Puccio, Inoue, Lingsma and Vassar2014; Theadom et al., Reference Theadom, Starkey, Barker-Collo, Jones, Ameratunga, Feigin and Group2018). Studies have shown that people can experience persistent symptoms such as headaches, impaired concentration or memory, that can impact on productivity, social relationships and life satisfaction (McMahon et al., Reference McMahon, Hricik, Yue, Puccio, Inoue, Lingsma and Vassar2014; Theadom et al., Reference Theadom, Starkey, Barker-Collo, Jones, Ameratunga, Feigin and Group2018). A potential link between TBI and antisocial behaviour has been proposed, however, the exact nature of this link remains unclear (Williams et al., Reference Williams, Chitsabesan, Fazel, McMillan, Hughes, Pasrsonage and Tonks2018). One study has shown that adults who experienced a TBI in their childhood had a 1.7 increased risk of imprisonment in comparison to non-injured siblings (McKinlay, Reference McKinlay2014). The relationship may also be bi-directional as engaging in antisocial behaviour may also increase risk of TBI through higher exposure to physical violence. For example, in Australia, it has been revealed that assaults were the second most common cause of TBIs experienced by people within the prison setting (Butler, Kariminia, Bond & Trevathan, Reference Butler, Kariminia, Bond and Trevathan2004). Multiple additional factors are also likely to influence a potential link between TBI and antisocial behaviour (Williams et al., Reference Williams, Chitsabesan, Fazel, McMillan, Hughes, Pasrsonage and Tonks2018). For example, the age the first TBI was sustained, how many injuries have been experienced, the duration between injuries, severity of injuries, as well as pre-injury behaviour and social deprivation (Schofield et al., Reference Schofield, Malacova, Preen, D’Este, Tate, Reekie and Butler2015).
Yet despite the higher prevalence of TBI within the prison population, there is little evidence on the potential impact of TBI history on people within the justice sector. One study conducted with male prisoners with a history of TBI in an Australian prison revealed that inmates experienced a range of neuropsychiatric and social sequelae. Pertinently, one third (33%) of participants reported continuing to experience headaches, with one in five experiencing uncontrollable anger, personality changes and impaired memory (Schofield et al., Reference Schofield, Butler, Hollis, Smith, Lee and Kelso2006). There are several difficulties encountered when exploring the potential longer-term impacts of TBI. Firstly, most post-concussion symptoms are subjective and difficult to assess (Polinder et al., Reference Polinder, Cnossen, Real, Covic, Gorbunova, Voormolen and von Steinbuechel2018). Secondly, many post-concussion symptoms are not TBI specific and can occur due to other illness or injuries (Polinder et al., Reference Polinder, Cnossen, Real, Covic, Gorbunova, Voormolen and von Steinbuechel2018), and in healthy (non-injured) people (Iverson et al., Reference Iverson, Silverberg, Mannix, Maxwell, Atkins, Zafonte and Berkner2015; Voormolen et al., Reference Voormolen, Cnossen, Polinder, Gravesteijn, Steinbuechel, Real and Haagsma2019). Thirdly, the factor structure of symptom report scales such as the Rivermead Post-Concussion Symptom Questionnaire (RPQ) vary depending on the population and time since injury (Potter, Leigh, Wade & Fleminger, Reference Potter, Leigh, Wade and Fleminger2006). There is consequently a need to compare current symptoms between those with and without a TBI history to document any differences between the two groups. This comparison needs to be based on a symptom tool with its underlying factor structure identified specifically for the prison population.
Methods
All men admitted to the South Auckland Correctional Facility (Kohuora) in New Zealand received an initial health assessment by a registered nurse within the first month of their arrival. Flexibility in conducting the assessment was required in order to account for triage priorities following admission, including the management of significant or unstable health issues. Verbal consent was obtained to conduct the initial health assessment including permission for their anonymised data to be used for research purposes. The assessment was conducted in a private interview space located in the accommodation areas. If the prisoner had a low propensity for violence, custodial staff (officers) waited outside the door to allow for privacy. If English was not the prisoner’s primary language, a member of staff who was able to converse in the participant’s primary language administered the health screen, or, alternatively, a telephone interpreting service was utilised. Prisoner engagement in the initial health assessment has previously been demonstrated to be high, with only 0.7% not providing their consent to take part in the assessment (Mitchell et al., Reference Mitchell, Theadom and du Preez2017). As part of the initial health assessment, the men were asked whether they had been involved in an incident where they hit their head and were knocked out or felt dazed and confused afterwards. Examples of playing sport, being in a car accident, falling over or being assaulted were given as potential situations when a TBI may occur. Details for each injury, including age at time of injury, causes and severity of each injury, and if they received medical treatment, were recorded. This self-reported data were used to determine the number of TBIs sustained over the lifetime. Injuries where the description did not meet the World Health Organisation criteria for TBI were excluded (Carroll, Cassidy, Holm, Kraus & Coronado, Reference Carroll, Cassidy, Holm, Kraus and Coronado2004). For example, injuries were not classified as a TBI if there was no evidence of altered consciousness after the injury, or if the injury was the result of an excluded mechanism, such as strangulation.
The TBI screen took between 5 and 15 min to complete, depending on the number of TBI events. The men were then asked to complete the RPQ, a 16-item questionnaire that asks about the presence and severity of symptoms commonly associated with TBI. The men were asked to rate how much they currently experienced each of the symptoms on a scale of 0 (no symptoms), 2 (mild), 3 (moderate) or 4 (severe). Due to the need to protect privacy for this analysis, the team were only able to extract data on whether the person reported a TBI history (defined as experiencing at least one TBI in their lifetime of any severity) or not. Specific details of the injuries were not available for this analysis. Details of TBI histories experienced within the same facility at an earlier time period have previously been described (Mitchell et al., Reference Mitchell, Theadom and du Preez2017). For the purposes of this analysis data on age and ethnicity, whether the person had experienced at least one TBI or not, in addition to symptom responses on the RPQ, were extracted from the prison database by a member of the correctional health care team. The dataset was checked to remove any potentially identifying details to ensure anonymity before being shared with the external research team for analysis. Ethics approval was obtained from the Auckland South Correctional Facility and the Auckland University of Technology ethics committee (Reference: AUTEC 21/160).
To check the underlying factor structure of the RPQ within this population, a principal components analysis (PCA) with varimax rotation was conducted using SPSS version 28. An absolute loading of 0.4 or higher was used as criterion for variables to be loaded on the corresponding factors. Items loading highest onto each factor were summed to yield sub-scores representing the identified factors of the RPQ for this population extracted from the PCA. Mann Whitney U tests were used to determine if there were any differences in symptom reporting on the two subscales of the RPQ by TBI history and ethnicity. Non-parametric tests of difference were used due to a high proportion of participants reporting a low level of symptoms (i.e. data was negatively skewed). As this was an exploratory study a significance value of α = 0.05 was used to determine statistical significance.
Results
Data were extracted for 363 men who completed the TBI screen and RPQ over an 8-month period (03/06/2020 and 18/03/2021). The sample ranged in age between 19 and 74 years of age. The average time from admission to the health screen assessment was 15.1 days. Of the total sample, 66.1% stated that they had experienced at least one TBI in their lifetime. The sample had been detained for offences ranging from violence, dishonesty, sexual and drug-related offences, with sentence length ranging between two months and life sentences. There were no differences in age (t(361) = -1.61, p = 0.11) between the TBI (M age = 34.8 years) and the no TBI (M age = 36.8 years) group, respectively. There was also no difference in ethnicity (X 2(3) = 4.58, p = 0.21) between the two groups, with Māori and Pasifika (the indigenous populations of NZ) comprising 74.6% and 75.6% between the TBI group and no TBI group, respectively.
Figure 1 shows responses to each symptom on the RPQ scale. A higher proportion of men with a TBI history reported experiencing each symptom more than those with no TBI history, with Chi-Square statistics being significant (p < 0.001) for all symptoms. The most commonly reported symptoms were sleep disturbance, headache, forgetfulness and feeling frustrated.
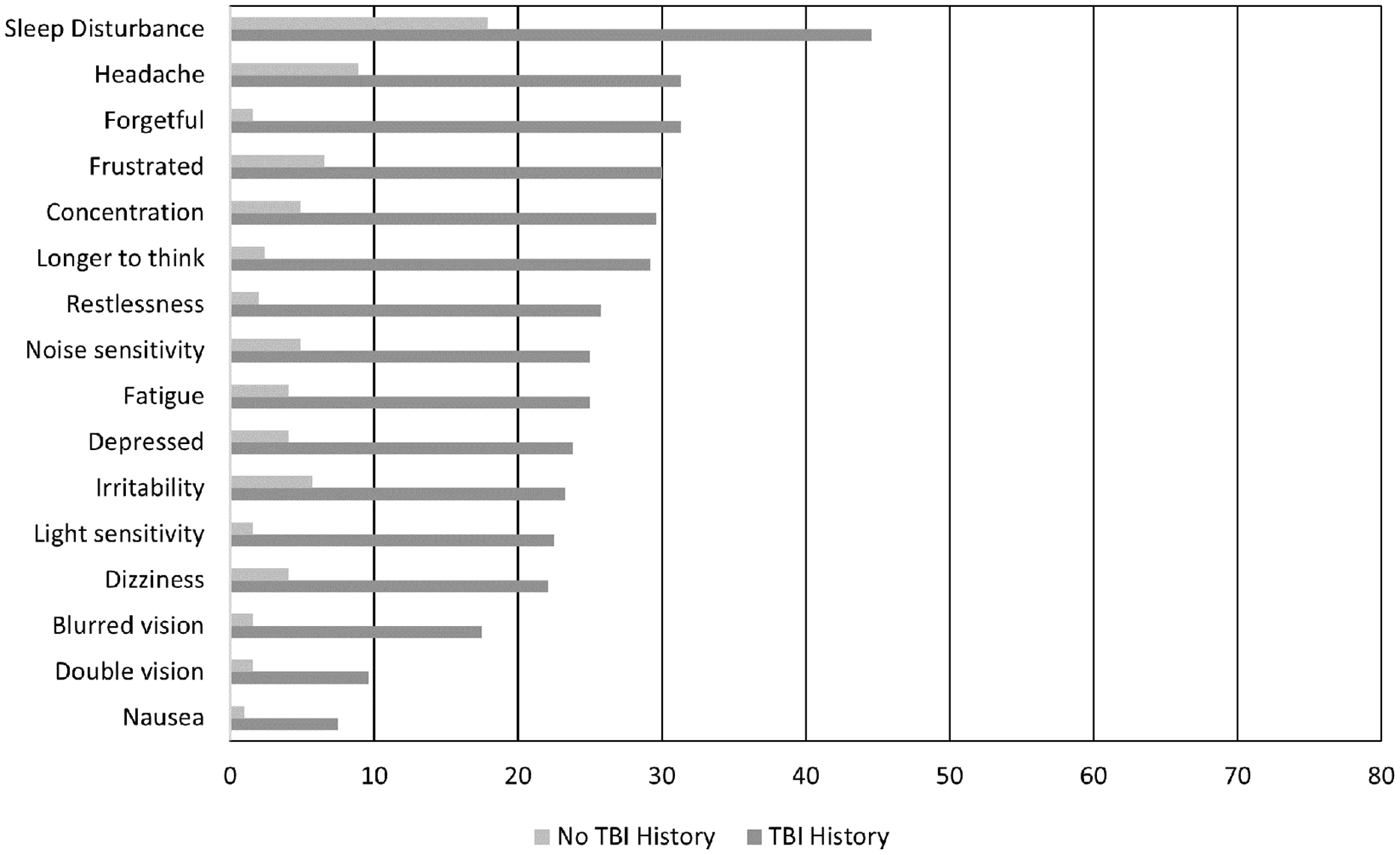
Figure 1. Comparison of the percentage of men reporting experiencing each symptom (score of 2 or more) between the two groups.
To derive the factor structure of the RPQ within this population, a PCA was conducted, using varimax rotation. The data were found to be suitable for factor analysis with a Kaiser–Meyer–Olkin measure of sampling adequacy of 0.94. The data revealed a two-factor structure accounting for 60.2% of the variance. Highest loadings were used to determine final factor fit (Table 1). All items loaded onto one of the two factors, (cognitive, emotional, behavioural or visuo-ocular subscales) with evidence of cross-loading with the forgetfulness symptom.
Table 1. Factor loadings for the Rivermead Post-Concussion Symptom Questionnaire (RPQ) for the male prison sample population
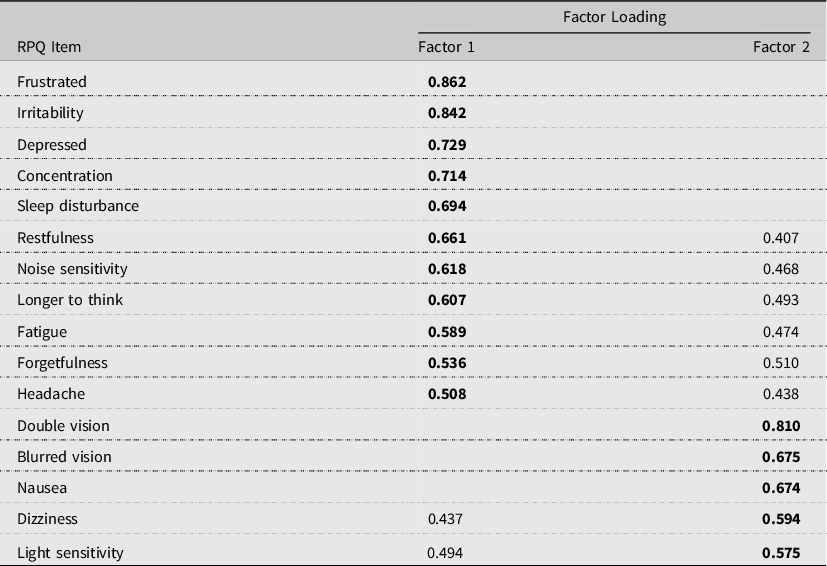
Note. Highest loadings indicated in bold type. Loadings of <0.3 have been deleted.
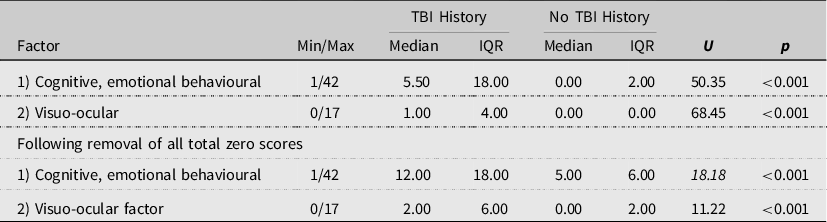
The scale reliability of the two extracted factors was supported, with a Cronbach’s alpha of αc = 0.94 for the cognitive, emotional, behavioural factor and αc = 0.81 for the visuo-ocular factor. Men reporting a history of TBI had significantly higher symptoms compared to those with no TBI history on both RPQ factors as shown in Table 2. It was observed that some participants reported experiencing no symptoms at all across both groups. This would be considered unusual given many symptoms are experienced within the general population. Sensitivity analyses were conducted excluding these cases to check if there was any influence on the findings. This yielded a sample of N = 216. Despite exclusion of these cases, there was still a significant difference in total symptom reporting between the two groups on both factors.
Table 2. Comparisons on Rivermead Post-Concussion Symptom Questionnaire (RPQ) factor-based scores between those reporting a traumatic brain injury (TBI) history and those with no TBI history
Tests of difference revealed that there were no significant differences in symptom reporting on either of the two subscales by ethnicity, as shown in Table 3.
Table 3. Comparison of symptom presentation by ethnicity

Discussion
This study aimed to identify the extent to which men within the prison population with a history of TBI reported experiencing TBI symptoms compared to those with no TBI history. The RPQ assessment was found to have a two-factor structure within this population (cognitive, emotional, behavioural and visuo-ocular). It was found that those with a history of TBI reported significantly higher symptom burden on all 16 symptoms items of the RPQ and on both factor scores, than those reporting no history of TBI.
The level of symptom reporting in those with a TBI history was slightly lower than findings from a TBI sample of the NZ general population who had experienced a mild TBI one year previously (e.g. 36.1% of adults post-mild TBI reported headaches compared to 31.3% of the current sample) (Theadom et al., Reference Theadom, Parag, Dowell, McPherson, Starkey, Barker-Collo and Feigin2016). The slightly lower percentage of people affected in the current sample is likely to reflect that participants would have experienced their most recent injury many years ago, and that the sample did not include females. This suggests that the prison environment may not necessarily lead to over- or under-reporting of symptoms.
Symptom burden was significantly higher in the TBI history group across all 16 symptoms of the RPQ assessment than those with no reported history of TBI. Whilst the increased symptom burden cannot be directly attributed to TBI based on the study design, the findings indicate an increased health need for those with a history of TBI that needs to be addressed within correctional services. The findings of this study support findings in Australia that highlighted the need for screening in prisons for potential TBI sequelae (Schofield et al., Reference Schofield, Butler, Hollis, Smith, Lee and Kelso2006). One of the challenges in exploring TBI symptoms is that they are not specific to TBI. Indeed, some participants in the no TBI history group reported experiencing some symptoms, particularly sleep disturbance, headache, frustration and irritability. This highlights the utility of having a no-TBI prison control group for comparison. There were a small proportion of men across both groups who reported experiencing no symptoms at all. It was not clear whether these men were indeed symptom free or whether this reflected a reluctance to engage in the health screen. Trust has been identified as a significant issue for prisoners and the assessment being conducted within the first month of arrival may impact on this (Liebling & Arnold, Reference Liebling and Arnold2013). Sensitivity analyses were conducted to determine the impact of these cases on the results and significant differences between the groups remained.
A limitation of the study was that we were not able to access individual social or medical records to determine whether participants in the study had comorbidities, substance use or other sociodemographic factors that may also contribute to symptom experience. However, comparison to a control group of men in the prison population with no history of TBI aimed to control for some of the influence of these factors on outcome. A further limitation was that the details of TBI history, acute injuries sustained within the prison environment, sentence length and offence type were not available to the research team as a method of protecting the privacy of participants. Consequently, the time since last injury for participants remains unclear. Previous studies providing details of the number and type of TBIs within a population of men in this correctional facility indicated that one in five male prisoners sustained their first injury before the age of 15 years, with the most recent injury sustained within the last 10 years (Mitchell et al., Reference Mitchell, Theadom and du Preez2017). Further research is needed to explore links between number, severity, time since injury and acute symptom burden in this population and determine if trends mirror that of mild TBI studies within the general population or whether there are unique needs for this population.
The factor structure of the RPQ identified in this study differed from that found in studies of the mild TBI population outside the justice sector. Studies have previously identified two, three and four underlying factors of the RPQ (Barker-Collo et al., Reference Barker-Collo, Theadom, Starkey, Kahan, Jones and Feigin2018; Lannsjo, Geijerstam, Johansson, Bring & Borg, Reference Lannsjo, Geijerstam, Johansson, Bring and Borg2009; Potter et al., Reference Potter, Leigh, Wade and Fleminger2006; Thomas, Skilbeck, Cannan & Slatyer, Reference Thomas, Skilbeck, Cannan and Slatyer2018). However, factor structure has also been found to vary according to time since injury (Barker-Collo et al., Reference Barker-Collo, Theadom, Starkey, Kahan, Jones and Feigin2018), with data from a longitudinal study finding a three-factor structure at baseline and 1 month post-injury, and a two-factor structure at both 6 and 12 months post-injury. This inconsistency in factor structure across time was explained to be a product of differential recovery rates across the 16 symptoms. With reference to the factor structure reported in Table 1 of this study, we note close concordance to both the 6 and 12 months epochs reported in another NZ mTBI sample (Barker-Collo et al., Reference Barker-Collo, Theadom, Starkey, Kahan, Jones and Feigin2018). Thus, the factor structure found in the current study is likely to reflect the differences in elapsed time since brain injury event across the participants.
It is acknowledged that the findings of this study were based on self-reported TBI history. Recall of TBIs may be less accurate in early life or for injuries sustained several decades prior, when TBI (particularly mild TBI) was less well-recognised. Consequently, it is likely that early life injuries may be underestimated. However, previous research has shown that self-reported TBI history in the prison population links well with medical records (Schofield et al., Reference Schofield, Butler, Hollis and D’Este2011). Self-reported TBI also has the advantage of capturing injuries that may not have been reported to medical services for fear of repercussions or due to financial or other resource restrictions that make seeking health care difficult. The prevalence of TBI in this study of 66% within the range identified in previous systematic reviews of TBI prison prevalence 60–70% (Allely, Reference Allely2016).
In conclusion, higher symptom reporting in male prisoners with a history of TBI reflects those reported by TBI patients in the general population. This finding suggests that symptom over-reporting or under-reporting is not occurring in prisoners with a history of TBI and, given the difference between the TBI and non-TBI samples in the current study, nor is there evidence of over-reporting in those prisoners without a history of TBI. Screening for TBI history and current symptom experience may facilitate access to rehabilitation services for those within the justice sector who may be experiencing previously undetected longer-term effects from TBI.
Acknowledgements
The authors would like to thank the South Auckland Correctional Facility for their support with this study.
Author contribution statements
AT was involved in study design, ethics submissions, data analysis and drafting of the manuscript.
DS was involved in the study design, ethics submissions, data analysis and contributed to the writing of the manuscript.
TM was involved in the study design, data collection, ethics submissions and extraction and contributed to the writing of the manuscript.
Financial support
Alice Theadom is supported by a Rutherford Discovery Fellowship administrated by The Royal Society Te Apārangi.
Conflicts of interest
Alice Theadom has no conflicts of interest to disclose. Tracey Mitchell has no conflicts of interest to disclose. Daniel Shepherd has no conflicts of interest to disclose.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.








