Introduction
Schizophrenia spectrum disorder is a group of severe mental illnesses with multidimensional psychopathology, including maladaptive stress coping (Norman & Malla, Reference Norman and Malla1993). Psychological and physiological stress reactivity are related to the severity of psychotic symptoms and influence the quality of life in schizophrenia patients (Borges, Gayer-Anderson, & Mondelli, Reference Borges, Gayer-Anderson and Mondelli2013; Brenner, St-Hilaire, Liu, Laplante, & King, Reference Brenner, St-Hilaire, Liu, Laplante and King2011; van Venrooij et al., Reference van Venrooij, Fluitman, Lijmer, Kavelaars, Heijnen, Westenberg and Gispen-de Wied2012). The stress-diathesis model of schizophrenia (Nuechterlein & Dawson, Reference Nuechterlein and Dawson1984; Pruessner, Cullen, Aas, & Walker, Reference Pruessner, Cullen, Aas and Walker2017; Walker & Diforio, Reference Walker and Diforio1997) proposes that psychiatric symptoms emerge when cumulative stressors exceed the individual's vulnerability threshold. Compared to controls, patients with schizophrenia reported feelings of lack of control over stressful experiences (Horan et al., Reference Horan, Ventura, Nuechterlein, Subotnik, Hwang and Mintz2005). Perceived stress contributes to the onset and exacerbation of psychotic symptoms in vulnerable individuals (Parmigiani et al., Reference Parmigiani, Mandarelli, Tarsitani, Roselli, Gaviano, Buscajoni and Ferracuti2021). Moreover, an early study demonstrated that subjective experiences of stress predicted poor clinical outcomes among patients with schizophrenia (Malla & Norman, Reference Malla and Norman1992). Recent research has also demonstrated a relationship between higher perceived stress with depression and the severity of positive symptoms in first-episode psychosis patients (Lataster, Valmaggia, Lardinois, van Os, & Myin-Germeys, Reference Lataster, Valmaggia, Lardinois, van Os and Myin-Germeys2013; Raune, Bebbington, Dunn, & Kuipers, Reference Raune, Bebbington, Dunn and Kuipers2006), and their first-degree relatives (Myin-Germeys, van Os, Schwartz, Stone, & Delespaul, Reference Myin-Germeys, van Os, Schwartz, Stone and Delespaul2001). Moreover, higher levels of familial risk for psychosis were associated with higher emotional reactivity to daily life stress in a dose-response fashion (Myin-Germeys et al., Reference Myin-Germeys, van Os, Schwartz, Stone and Delespaul2001). Studies have also indicated that acute onset of schizophrenia could directly follow stressful life events (Brown & Birley, Reference Brown and Birley1968; Norman & Malla, Reference Norman and Malla1993), with more severe symptoms accompanying greater recent life stress (Norman & Malla, Reference Norman and Malla1993).
Early life stressors, including emotional and physical abuse, sexual abuse, and emotional and physical neglect, among others, may have particularly strong influences on the pathophysiological processes of psychosis (Alameda et al., Reference Alameda, Ferrari, Baumann, Gholam-Rezaee, Do and Conus2015; Nugent, Chiappelli, Rowland, & Hong, Reference Nugent, Chiappelli, Rowland and Hong2015). Childhood trauma appeared prevalent among these stressors (Larsson et al., Reference Larsson, Andreassen, Aas, Rossberg, Mork, Steen and Lorentzen2013; Ucok & Bikmaz, Reference Ucok and Bikmaz2007) and represented a risk factor of schizophrenia (Varese et al., Reference Varese, Smeets, Drukker, Lieverse, Lataster, Viechtbauer and Bentall2012). Individuals with a history of childhood trauma are at high risk for psychosis (Kraan et al., Reference Kraan, van Dam, Velthorst, de Ruigh, Nieman, Durston and de Haan2015). Childhood trauma affects the onset (De-Nardin, Muratori, Ribeiro, Huguete, & Salgado, Reference De-Nardin, Muratori, Ribeiro, Huguete and Salgado2022) and severity of positive and dysthymia symptoms (Ruby, Rothman, Corcoran, Goetz, & Malaspina, Reference Ruby, Rothman, Corcoran, Goetz and Malaspina2017) as well as cognitive functions (Dauvermann & Donohoe, Reference Dauvermann and Donohoe2019) and treatment outcomes (Kilian et al., Reference Kilian, Asmal, Phahladira, Plessis, Luckhoff, Scheffler and Emsley2020) in schizophrenia. For instance, patients with a history of childhood trauma demonstrated delayed symptom remission (Aas et al., Reference Aas, Andreassen, Aminoff, Faerden, Romm, Nesvag and Melle2016). Both early trauma and current perceived stress showed significant correlations with the clinical severity in schizophrenia (Ruby et al., Reference Ruby, Rothman, Corcoran, Goetz and Malaspina2017). On the other hand, the neural processes underlying the association between early trauma and current perceived stress in schizophrenia remains poorly understood.
Neuroimaging studies have examined the neural correlates of childhood trauma and perceived stress (Cancel, Dallel, Zine, El-Hage, & Fakra, Reference Cancel, Dallel, Zine, El-Hage and Fakra2019; Heany et al., Reference Heany, Groenewold, Uhlmann, Dalvie, Stein and Brooks2018). For instance, childhood maltreatment was associated with a reduced total gray matter volume (GMV) and hippocampal GMV (Cancel et al., Reference Cancel, Comte, Truillet, Boukezzi, Rousseau, Zendjidjian and Fakra2015; Frissen et al., Reference Frissen, van Os, Peeters, Gronenschild, Marcelis, for Genetic and Outcome in2018; Lim et al., Reference Lim, Hart, Mehta, Worker, Simmons, Mirza and Rubia2018). Childhood maltreatment was also associated with amygdala hyper-reactivity in maltreated individuals (Teicher & Samson, Reference Teicher and Samson2013). The hippocampus and amygdala exhibited the most extensive volumetric reductions among all subcortical brain regions in schizophrenia (Okada et al., Reference Okada, Fukunaga, Yamashita, Koshiyama, Yamamori, Ohi and Hashimoto2016; van Erp et al., Reference van Erp, Hibar, Rasmussen, Glahn, Pearlson, Andreassen and Turner2016), and these two subcortical regions were linked to childhood trauma (du Plessis et al., Reference du Plessis, Scheffler, Luckhoff, Asmal, Kilian, Phahladira and Emsley2020; Rokita et al., Reference Rokita, Holleran, Dauvermann, Mothersill, Holland, Costello and Donohoe2020). Moreover, one recent study, which employed 28 patients, showed the influence of childhood trauma on the clinical features and neurobiology of schizophrenia (Ruby et al., Reference Ruby, Rothman, Corcoran, Goetz and Malaspina2017). However, the volumetric correlates inter-linking early trauma and clinical symptoms, including depression and perceived stress, remain unclear.
In this study, we proposed to examine how childhood trauma influences clinical symptoms, including perceived stress and depression, and the subcortical volumetric correlates of this relationship in patients with schizophrenia. We hypothesized that subcortical volumetrics would mediate the association between childhood trauma and perceived stress.
Materials and methods
Clinical characteristics
This study included 127 patients with schizophrenia (73 men; age 40.8 ± 13.1 years, mean ± s.d.) and 83 healthy controls (44 men; age 38.6 ± 12.2 years). The inclusion criteria for patients included (1) DSM-IV (American Psychiatric Association, Reference American Psychiatric Association1994) diagnostic criteria for schizophrenia; (2) right-handedness as confirmed by the short version of the Edinburgh Handedness Scale; and (3) age between 15 and 65 years. The exclusion criteria included (1) a history of head trauma; (2) current or previous substance or alcohol (other than nicotine) use disorders; (3) an organic brain disease as confirmed by MRI; (4) symptoms of significant involuntary movement; and (5) learning disability or mental retardation. Healthy volunteers had no family history of psychotic illnesses, according to the Family History Research Diagnostic Criteria. All participants had no current or past neurological conditions or substance (except nicotine) dependence. This study was conducted according to the Declaration of Helsinki, and all participants signed consent forms per a protocol approved by the Ethics Committee of the Beijing Huilongguan Hospital.
Demographic details are presented in Table 1. Five patients were medication-free, seven patients were on first-generation antipsychotic medications, and the remaining patients were on risperidone (n = 42); clozapine (n = 32); olanzapine (n = 38); aripiprazole (n = 19); paliperidone (n = 8); or amisulpride, iloperisone, lurazidone, or quetiapine (n = 9), with a total of 45 patients on more than one antipsychotic medication. The dosage of chlorpromazine was calculated as described in previous literature (Woods, Reference Woods2003).
Table 1. Demographic and clinical characteristics of patients with schizophrenia and healthy controls

PANSS, Positive and Negative Syndrome Scale; CDSS, Calgary Depression Scale for Schizophrenia; PSS, Perceived Stress Scale.
Clinical symptoms, childhood trauma, and perceived stress
Patients were assessed using the Structured Clinical Interview for DSM-IV (SCID), the Positive and Negative Syndrome Scale (PANSS) and the Calgary Depression Scale for Schizophrenia (CDSS-C) by one of three attending psychiatrists who had extensive experience in the evaluation and treatment of schizophrenia patients. The inter-rater reliabilities were above 0.80.
We used the Childhood Trauma Questionnaire (CTQ) to assess traumatic experiences during childhood up to the age of 16 (Bernstein et al., Reference Bernstein, Stein, Newcomb, Walker, Pogge, Ahluvalia and Zule2003). The CTQ is a 28-item self-report questionnaire with five subscales: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect, each consisting of five items. Individuals were requested to answer whether they had experienced the event on a Likert scale ranging from 1 (never true) to 5 (very often true). Higher scores indicated more traumatic experiences.
The Perceived Stress Scale (PSS) (Cohen, Kamarck, & Mermelstein, Reference Cohen, Kamarck and Mermelstein1983; Katsarou et al., Reference Katsarou, Panagiotakos, Zafeiropoulou, Vryonis, Skoularigis, Tryposkiadis and Papageorgiou2012) is a 14-item self-report questionnaire used to evaluate perceived stress in the last month, with each item ranging from 0 (never) to 4 (almost always). Seven of the 14 items assess the subjects' perception of stress, which are negatively stated (e.g. unable to control things, felt difficulties were piling up), with higher scores indicating stronger senses of stress. The other seven items assess the ability to cope with stress, which are positively worded items (e.g. felt confident in handling problems, been able to control irritations), with higher scores indicating a better ability of coping with stress. The Chinese version of the PSS has shown good reliability and validity (Yang & Huang, Reference Yang and Huang2003).
MRI protocol and data processing
Imaging data were collected on a Siemens Prisma 3 T MRI scanner with a 64-channel head coil. Head motions were minimized by foam pads. Parameters for structural MRI were acquired by covering the whole brain with a sagittal 3D-magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence: echo time (TE) = 2.98 ms, inversion time (TI) = 1100 ms, repetition time (TR) = 2530 ms, flip angle (FA) = 7°, field of view (FOV) = 256 × 224 mm2, matrix size = 256 × 224, thickness/gap = 1/0 mm.
For each participant, structural T1 images were preprocessed using the Freesurfer software version 6.0 (Fischl, Reference Fischl2012; Fischl et al., Reference Fischl, Salat, Busa, Albert, Dieterich, Haselgrove and Dale2002) (http://surfer.nmr.mgh.harvard.edu). GMV of the left and right thalamus, caudate, putamen, pallidum, nucleus accumbens, hippocampus, and amygdala, total cortical GMVs, and intracranial volume (ICV) as well as ventricular volumes were computed. The GMVs of the eight subcortical structures were summed across hemispheres and considered as the primary volumes of interest. The ICV was used as a covariate in all analyses to account for differences in head size. For quality control, we followed the ENIGMA pipeline (http://enigma.usc.edu/protocols/imaging-protocols): all regions of interest (ROIs) with a volume >1.5 or <1.5 times the interquartile range were identified and visually inspected by overlaying the segmentations on the subjects' anatomical images. Only ROI data for which segmentation was judged to be accurate upon visual inspection were included for statistical analyses. No subject was excluded.
Statistical analysis
Statistical analyses were conducted with the Statistical Package for Social Sciences (SPSS, Version 23) software. Log transformations were applied to reduce the skewness of the childhood trauma variables that were not normally distributed. Group comparisons of subcortical volumes were performed using a general linear model controlling for age, sex, and ICV. We evaluated the imaging results with a p value of 0.0056 (i.e. 0.05/9) for Bonferroni correction for multiple comparisons. Finally, we performed mediation analysis using the PROCESS tool (Version 2.16.3) (www.afhayes.com) to test whether subcortical GMVs that showed significant differences between patients and controls mediated the association between childhood trauma and perceived stress. Furthermore, we tested the antipsychotic medication effect on subcortical volumes and the above mediation analysis.
Results
Demographic and clinical characteristics
The demographic and clinical characteristics of the participants are shown in Table 1. Patients with schizophrenia and healthy controls were age- and sex-matched. Patients relative to controls had fewer years of education (p < 0.05). The age of illness onset for the patients was 25.05 ± 7.83 (mean ± s.d.) years, and the duration of illness was 15.16 ± 14.01 years.
Group differences in childhood trauma and perceived stress
Compared to controls, patients with schizophrenia showed higher scores in total and three domains of childhood trauma, including emotional abuse, emotional neglect, and physical neglect (p < 0.05, Bonferroni corrected, Table 1). Patients relative to controls showed higher perceived stress (PSS perception) and poorer copying (PSS coping; both p's <0.05, Table 1).
Subcortical volumes
Patients showed significantly smaller total cortical GMVs and amygdala and hippocampus GMVs, as compared to controls in a covariance analysis with age, sex, and ICV as covariates (all p values <0.05, Bonferroni corrected, Fig. 1, Table 2). The findings remained the same when the left and right hemispheric volumes were examined separately (online Supplementary Table S1).
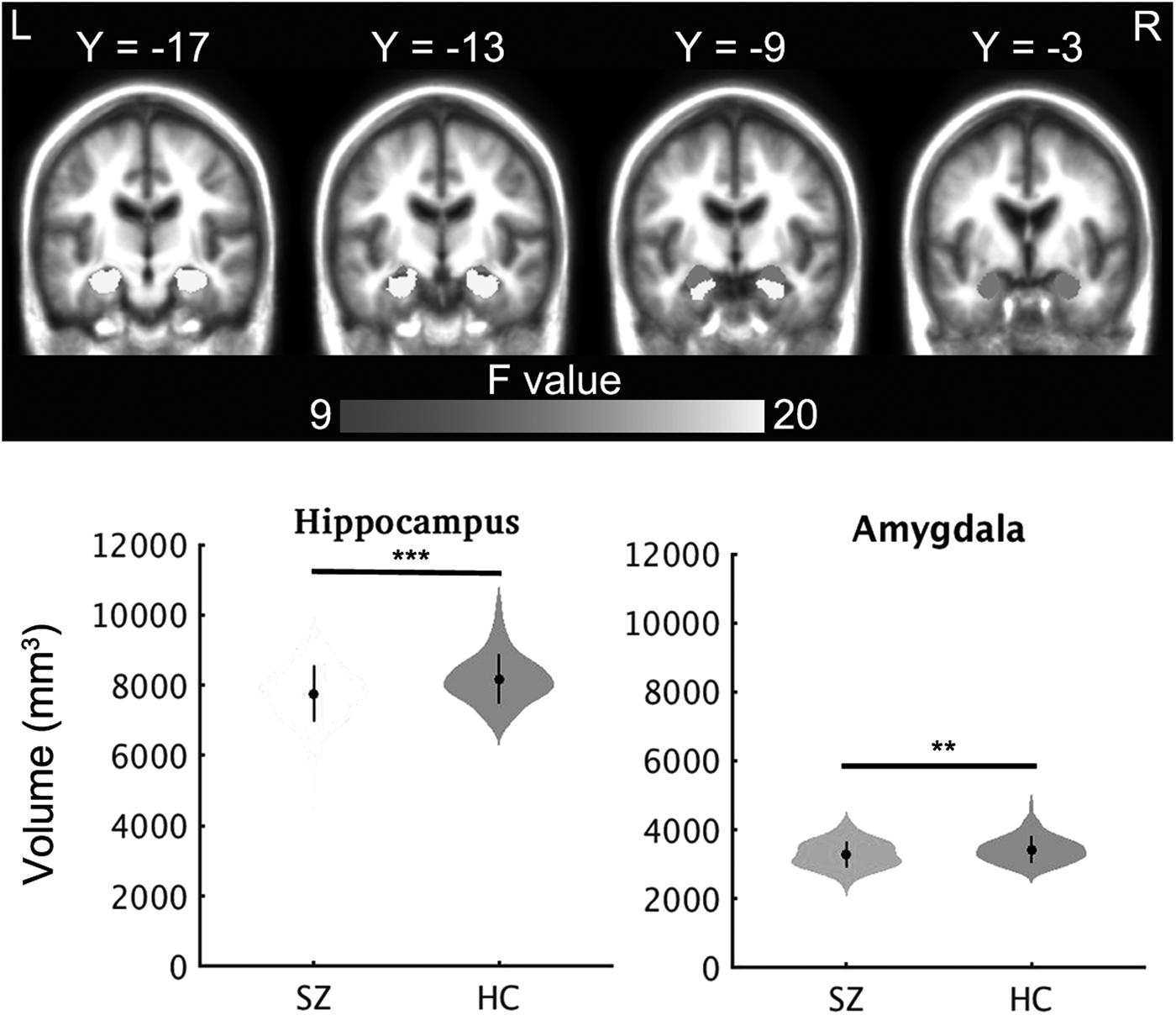
Fig. 1. Group differences in volumes of subcortical structures. ** indicates p < 0.01, *** indicates p < 0.001, the dot and bar indicate mean ± s.d. HC, Healthy control; SZ, schizophrenia.
Table 2. Volume of subcortical structure in schizophrenia

Note: Unit of volume is mm3; considering age, sex and intracranial volume as covariates.
Childhood trauma and clinical characteristics
To assess the relationship between childhood trauma and clinical symptoms, we performed pairwise Pearson's correlations of PANSS, PSS, and CTQ scores (Fig. 2). The total CTQ score was positively correlated with the PANSS negative symptom (r = 0.31, p = 0.001), and negatively correlated with the PSS coping score (r = −0.41, p < 0.001). Emotional neglect score was positively correlated with the PANSS negative symptom (r = 0.26, p = 0.003), and negatively correlated with the PSS coping score (r = −0.45, p < 0.001). PANSS general psychosis score was positively correlated with CDSS (r = 0.40, p < 0.001) and PSS perception (r = 0.30, p < 0.001) score. CDSS was positively correlated with PSS perception (r = 0.41, p < 0.001) and negatively correlated with PSS coping (r = −0.31, p < 0.001) score. The above results were statistically significant after Bonferroni correction (p < 0.05/11 = 0.0045).
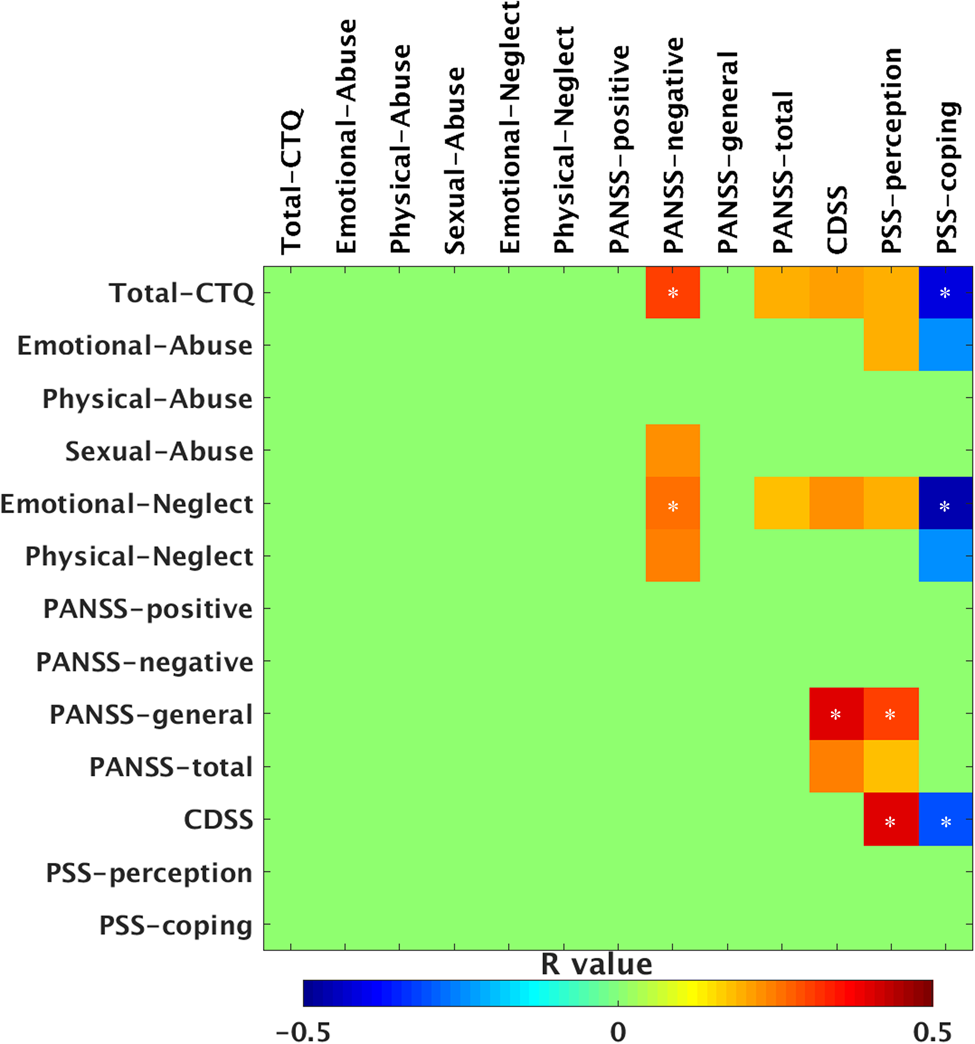
Fig. 2. Correlational analyses in all patients. Note that only significant correlations are shown with warm and cold colors, the other correlational coefficients are set to green in the figure. Warm and cool color with * indicate statistically significant p values after Bonferroni correction for associations between the childhood trauma and clinical characteristics (11 domains; p < 0.05/11 = 0.0045). The other warm and cool colors indicate nominally significant values (uncorrected p < 0.05). CTQ, Childhood Trauma Questionnaire; PANSS, Positive and Negative Syndrome Scale; CDSS, Calgary Depression Scale for Schizophrenia; PSS, Perceived Stress Scale.
Furthermore, we also reported nominally significant correlations with uncorrected p < 0.05 (Fig. 2). The total CTQ score was positively correlated with the PANSS total score (r = 0.20, p = 0.03), CDSS (r = 0.21, p = 0.02) and PSS perception score (r = 0.22, p = 0.02). Emotional abuse score was positively correlated with PSS perception (r = 0.22, p = 0.02) and negatively correlated with PSS coping (r = −0.24, p = 0.009). Sexual abuse was correlated with a PANSS negative symptom score (r = 0.23, p = 0.01). Emotional neglect score was positively correlated with the PANSS total score (r = 0.18, p = 0.04), CDSS (r = 0.23, p = 0.009) and PSS perception score (r = 0.23, p = 0.01). Physical neglect score was positively correlated with the PANSS negative symptom score (r = 0.24, p = 0.009) and negatively correlated with the PSS coping score (r = −0.24, p = 0.008). The PANSS total score was positively correlated with CDSS (r = 0.24, p = 0.008) and PSS perception score (r = 0.18, p = 0.04).
Relationship between subcortical volumes and clinical symptoms, childhood trauma, perceived stress
We examined the correlation between PANSS symptoms, CDSS, CTQ, PSS scores and volumes of brain regions showing significant patient-control differences, i.e., the amygdala, hippocampus, and total cortical GMVs. The volume of the hippocampus was nominally significantly correlated with the PANSS negative symptom (r = −0.26, p = 0.003) and PSS coping (r = 0.21, p = 0.03) in patients (Fig. 3). The volume of the amygdala was nominally significantly correlated with emotional neglect (r = −0.20, p = 0.03) and PSS coping (r = 0.24, p = 0.008) in patients (Fig. 3). Medication dose was nominally significantly correlated with the volume of the hippocampus (r = −0.22, p = 0.01), amygdala (r = −0.21, p = 0.02), total cortical GMV (r = −0.23, p = 0.01), and volume of the lateral ventricle (r = 0.22, p = 0.01) (online Supplementary Fig. S1).

Fig. 3. Correlation between subcortical volumes and clinical symptoms, childhood trauma, perceived stress. PANSS, Positive and Negative Syndrome Scale; PSS, Perceived Stress Scale.
Mediation analyses
As mentioned above, there were significant correlations between the volume of the hippocampus/amygdala, childhood trauma and perceived stress. Thus, to understand the inter-relationship between subcortical GMVs, childhood trauma and perceived stress, we assessed whether the hippocampus and/or amygdala GMVs mediate the association between childhood trauma and clinical characteristics (PANSS symptoms, PSS, and CDSS). The emotional neglect score served as an independent predictor variable; and the PSS coping score was selected as the outcome (dependent) variable; age, sex, ICV and medication dose were included as covariates. The results showed that the effects of childhood trauma (emotional neglect) on the PSS coping score were partially mediated by the amygdala [β = −0.98 (95% CI −2.81 to −0.04)], but not significantly by the hippocampus [β = −0.74 (95% CI −2.77 to 0.15)] (Fig. 4). Moreover, the effects of medication dose on the above mediation analysis were not significant (p > 0.05).

Fig. 4. Amygdala mediated the childhood trauma effect on perceived stress, controlling for age, sex, intracranial volume and medication dose. Path AB (indirect effect) is the mediation effect, and it is significant based on the confidence interval. CTQ, Childhood Trauma Questionnaire; EN, Emotional neglect; PSS, Perceived Stress Scale.
We further tested the effect of hemispheric lateralization on the amygdala's role in the mediation analysis. The results showed that the effects of childhood trauma (emotional neglect) on the PSS coping score were partially mediated by the right amygdala [β = −1.78 (95% CI −4.38 to −0.37)], but not significant by the left amygdala [β = −0.19 (95% CI −1.45 to 0.26)] (online Supplementary Fig. S2).
Discussion
In this study, we explored the relationship between childhood trauma, perceived stress, clinical symptoms, and subcortical areal volumes in patients with schizophrenia. Higher levels of childhood trauma were associated with higher perceived stress and lower stress coping in patients. Patients with schizophrenia showed significantly smaller amygdala and hippocampus GMVs, compared to age-matched controls. Further, mediation analysis showed that the association between childhood trauma exposure (emotional neglect) and lower stress coping was mediated by reduced right amygdala GMV in patients.
Trauma exposure was associated with the severity of clinical symptoms, which is consistent with previous studies (Ruby et al., Reference Ruby, Rothman, Corcoran, Goetz and Malaspina2017). Trauma exposure and perceived stress also predicted higher depression scores, as measured by the CDSS. Among childhood adversities, trauma is particularly associated with psychoses (Heins et al., Reference Heins, Simons, Lataster, Pfeifer, Versmissen, Lardinois and Myin-Germeys2011; Read, van Os, Morrison, & Ross, Reference Read, van Os, Morrison and Ross2005), including schizophrenia (Taylor et al., Reference Taylor, Klein, Lewis, Gruenewald, Gurung and Updegraff2000) at a later developmental period.
We found reduced volume of the hippocampus and amygdala in patients with schizophrenia, which is consistent with our previous findings in a cohort of first-episode schizophrenia patients (Fan et al., Reference Fan, Xiang, Tan, Yang, Fan, Guo and Tan2019). When examining the relationship between childhood trauma and the GMVs of subcortical structure, we observed significant negative associations between emotional neglect and the amygdala volume. In support of this finding, a previous study showed that the amygdala GMV is likely influenced by the type and timing of exposure to childhood trauma (Berens, Jensen, & Nelson, Reference Berens, Jensen and Nelson2017; McCrory, De Brito, & Viding, Reference McCrory, De Brito and Viding2011). The amygdala plays a central role in emotional processing, fear conditioning, and memory of emotional or other salient events (Phelps & LeDoux, Reference Phelps and LeDoux2005). Using functional MRI, investigators found that adults who experienced childhood maltreatment showed hyperactivity of the amygdala in response to negative facial affect (Maheu et al., Reference Maheu, Dozier, Guyer, Mandell, Peloso, Poeth and Ernst2010; Tottenham et al., Reference Tottenham, Hare, Millner, Gilhooly, Zevin and Casey2011). In rats, chronic stress increased dendritic arborization in the central and extended amygdala (Vyas, Bernal, & Chattarji, Reference Vyas, Bernal and Chattarji2003). Early stress and negative emotionality are associated with greater positive amygdala-posterior cingulate cortical functional connectivity during infancy (Graham, Pfeifer, Fisher, Carpenter, & Fair, Reference Graham, Pfeifer, Fisher, Carpenter and Fair2015). The current findings, as well as these earlier findings, implicate the amygdala in childhood trauma and emotional processing dysfunction.
We showed that the effects of childhood trauma on stress coping were partially mediated by amygdala GMV. The role of the amygdala as a potential mediator in the relationship between childhood trauma and stress coping is novel, highlighting the importance of this subcortical brain region in perceived stress and potentially other affective psychopathologies. It is possible that the volumetric development of the amygdala is particularly vulnerable during trauma-sensitive critical periods (Graham et al., Reference Graham, Pfeifer, Fisher, Carpenter and Fair2015; Tottenham et al., Reference Tottenham, Hare, Millner, Gilhooly, Zevin and Casey2011). The developing brain during childhood is marked by high plasticity, which can lead to vulnerability to early life stressors. Previous studies indicate that childhood maltreatment (including early life stress, neglect, emotional ill-treatment, and trauma) is associated with structural aberrations across several brain regions (Dannlowski et al., Reference Dannlowski, Stuhrmann, Beutelmann, Zwanzger, Lenzen, Grotegerd and Kugel2012; Lim, Radua, & Rubia, Reference Lim, Radua and Rubia2014; van Harmelen et al., Reference van Harmelen, van Tol, van der Wee, Veltman, Aleman, Spinhoven and Elzinga2010). Jeong and colleagues found that trauma exposure was associated with smaller GMV in the right amygdala and right putamen (Jeong et al., Reference Jeong, Durham, Moore, Dupont, Mcdowell, Cardenas-Iniguez and Kaczkurkin2021), by using a large sample of children (N = 9270) from the Adolescent Brain Cognitive Development Study (ABCD Study). These studies suggest that childhood trauma may be an important risk factor for structural aberrations, which may further have implications for the manifestation of psychopathology symptoms later in life (McLaughlin et al., Reference Mclaughlin, Green, Gruber, Sampson, Zaslavsky and Kessler2010). Exposure to childhood adversities was significantly associated with the persistence of mood and anxiety disorders, which remained statistically significant throughout the patient's life course (McLaughlin et al., Reference Mclaughlin, Green, Gruber, Sampson, Zaslavsky and Kessler2010).
Several limitations need to be considered for future studies. First, childhood trauma was assessed using the CTQ, a self-report questionnaire. Although widely used, such self-reported measures may involve recall bias, which is especially concerning in studies of schizophrenia patients (Fisher et al., Reference Fisher, Craig, Fearon, Morgan, Dazzan, Lappin and Morgan2011; McKinney, Harris, & Caetano, Reference McKinney, Harris and Caetano2009). Family interviews may help in improving the accuracy of childhood maltreatment measures. Second, most of the patients took antipsychotic medications. Previous studies have shown that antipsychotic medications are associated with volumetric changes in subcortical structures (Huhtaniska et al., Reference Huhtaniska, Jaaskelainen, Heikka, Moilanen, Lehtiniemi, Tohka and Miettunen2017). Therefore, the current findings need to be replicated in first-episode patients with schizophrenia. Third, we investigated the GMVs of subcortical structures. Function MRI studies of the amygdala circuit may provide a more thorough picture of the amygdala's role in relating childhood trauma to perceived stress. Finally, in this study, we focused on the association between subcortical structures, childhood trauma and perceived stress in schizophrenia. Childhood trauma has been reported in many mental disorders (Sivolap & Portnova, Reference Sivolap and Portnova2016), including depression (Aghamohammadi-Sereshki et al., Reference Aghamohammadi-Sereshki, Coupland, Silverstone, Huang, Hegadoren, Carter and Malykhin2021), bipolar disorders (Janiri et al., Reference Janiri, Sani, Rossi, Piras, Iorio, Banaj and Spalletta2017), as well as alcohol use (Phillips et al., Reference Phillips, De Bellis, Brumback, Clausen, Clarke-Rubright, Haswell and Morey2021). It is worth exploring in future studies whether the effect of trauma is independent of diagnosis or if diagnosis specificity is present.
Conclusion
Compared to age-matched controls, patients with schizophrenia showed higher levels of childhood trauma and perceived stress and lower capability in stress coping. Childhood trauma is associated with smaller GMV of the amygdala. The association between early-life trauma exposure and current stress is mediated via the reduced amygdala volume in patients with schizophrenia.
Data
Fengmei Fan has full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291722002860.
Acknowledgements
We thank Wei Feng, Ping Zhang for their assistance in collecting data, Ting Xie and Leilei Wang from Beijing Huilongguan Hospital for their comments in statistical analysis for this manuscript.
Author contribution
Conceived and designed the study: Yunlong Tan, Shuping Tan, Zhiren Wang; Collected the data: Yunlong Tan, Shuping Tan, Shibo Liu, Song Chen, Junchao Huang, Fude Yang; Analyzed the data: Fengmei Fan; Wrote the paper: Fengmei Fan; Chiang-Shan R. Li.
Financial support
Support was received from the National Natural Science Foundation of China (No. 81761128021, 81401115, 31671145), Beijing Municipal Administration of Hospitals' Youth Program (QML20172001), and Capital's Funds for Health Improvement and Research (No.2018-4-2133).
Conflicting interests
All authors have declared no conflicting interests.









