Chronic fluoride (F) toxicity is caused due to exposure to excess F >1·5 parts per million (ppm)(1), mainly through water and is endemic in twenty-five countries across the globe. In India, F endemicity has been reported in 196 districts of nineteen states and is considered as a public health problem(2). Chronic F toxicity is categorised as dental, skeletal and non-skeletal fluorosis, based on the tissue affected. The very first sign of chronic F toxicity is exhibited by teeth, i.e. dental mottling, called dental fluorosis(Reference Anusuya, Bamji, Rao and Reddy3). Skeletal fluorosis progresses in a slow manner and, therefore, is not clinically visible in its initial phase. Clinical symptoms of skeletal fluorosis include restricted movements of joints, stiffness and deformities of the spine such as kyphosis, bony exostoses and paraplegia due to spinal compression(Reference Krishnamachari4). Non-skeletal fluorosis affects tissues other than the dental and skeletal system such as the gastrointestinal tract, brain, muscle, etc. Any kind of manifestation in these organs of inhabitants of fluorotic areas may indicate non-skeletal fluorosis(Reference Anusuya, Bamji, Rao and Reddy3).
Apart from these well-defined clinical symptoms, reduced food intake and body weight gain (BWG) have been observed in F-fed animals(Reference Khandare, Kumar and Shankar5–Reference Lohakare, Pattanaik and Khan8). However, other studies did not show any difference in BWG(Reference Turner, Garetto and Dunipace9) in F-fed animals. The reason behind this contradiction may be due to different species used, dose of F and duration of the study. Moreover, none of the aforementioned studies have reported food efficiency ratio (FER), which is an important measure of the food converted into BWG.
It has also been reported that chronic exposure to F may disturb Ca homeostasis by producing hypocalcaemia due to its stimulating effect on osteoblastic cell proliferation(Reference Das and Susheela10–Reference Gruber and Baylink12), resulting in an increase in bone formation(Reference Kleerekoper and Mendlovic13–Reference Gupta, Gambhir and Mithal15), which needs extra Ca. This alteration of extracellular Ca level is sensed by Ca-sensing receptor (CASR)(Reference Saidak, Mentaverri and Brown16) and the parathyroid glands are triggered to secrete more parathyroid hormone to combat the Ca imbalance in the body, producing secondary hyperparathyroidism(Reference Krishnamachari14, Reference Koroglu, Ersoy and Koroglu17). Parathyroid hormone triggers the conversion of 25-hydroxy-vitamin D3 (25(OH) vitamin D3) to 1,25-dihydroxy-vitamin D3 (1,25(OH)2 vitamin D3) in the proximal convoluted tubule of the kidneys(Reference Kochupillai18) to maintain plasma ionised Ca concentrations within narrow physiological limits by acting on the intestine, kidneys and bone(Reference Kochupillai18) through its receptor – vitamin D receptor (VDR)(Reference Jones, Strugnell and DeLuca19). 1,25(OH)2 vitamin D3 alters the transcription of S100 Ca-binding protein G (S100G) in the intestine(Reference Bronner20, Reference Christakos, Gill and Elee21) to increase Ca absorption. In a study carried out in rats fed 50 ppm F for 6 weeks, it was found that F exhibited an inhibitory effect on duodenal VDR and S100G gene transcription, thereby reducing Ca absorption(Reference Tiwari, Gupta and Kumar22). However, serum parathyroid hormone was not assessed in the study.
Preliminary research carried out in F-fed rats(Reference Ranganathan23) and kittens(Reference Burkhart and Jowsey24) showed that dietary Ca reduces F toxicity. It was suggested that a possible mechanism of action is the formation of insoluble calcium fluoride, which is excreted through faeces. Further, a Ca balance study carried out in human fluorotic subjects showed increased retention of Ca because of decreased faecal and urinary Ca, suggesting enhanced absorption of Ca at the kidney as well as the intestine(Reference Srikantia and Siddiqui25). Similar observations were reported in F-fed monkeys also(Reference Reddy and Rao26). It was proposed that on a given intake of F, increased intake of Ca might lead to the formation and deposition of calcium fluoride in bone, thereby reducing F in the non-osseous tissues. Furthermore, many other researchers reported that the adverse effects of F could be more aggravated in the presence of a low dietary Ca intake(Reference Khandare, Harikumar and Sivakumar11, Reference Singh and Jolly27, Reference Mithal, Trivedi and Gupta28).
According to the National Nutrition Monitoring Bureau (India) report, the average dietary Ca intake of the Indian population (including fluorotic areas) is 335 mg/d and the average consumption of milk and milk products is 82 g/d(29), contrary to RDA of 600 mg/d and 200 ml/d for Ca and milk, respectively(30). Consumption of a phytate-rich diet worsens the condition of low dietary Ca intake by reducing the absorption of dietary Ca(Reference Harinarayan, Ramalakshmi and Prasad31), which makes the Indian population more vulnerable to fluorosis. Recently, a pilot project on the prevention and control of fluorosis in six districts of the fluorotic areas has been started in India, which targets comprehensive prevention and management of fluorosis by training of health professionals, community nutrition education (especially for intake of dairy products and Ca-rich foods) and providing F-free water (FFW) wherever possible(32). However, rehabilitation of fluorotic patients is still an issue and reversibility of fluorosis has not been studied adequately. In addition, some gaps still exist in the current knowledge. Studies related to chronic effects of F on Ca homeostasis and expression of genes involved in Ca homeostasis are scanty.
Based on previous studies, we hypothesised that low Ca aggravates chronic F toxicity, which can be ameliorated by providing normal Ca and FFW. The objectives were as follows: (1) to study the effect of F on food intake, FER, Ca homeostasis and expression of genes involved in Ca homeostasis under adequate or inadequate Ca and (2) to explore the effect of FFW and adequate Ca on amelioration of chronic F toxicity.
Materials and methods
Animals and maintenance
A total of seventy-six, Wistar, weanling, male rats (body weight 55 (sem 15) g) were obtained from the National Centre for Laboratory Animal Sciences, National Institute of Nutrition, Hyderabad, India. Rats were housed individually in hanging polyvinyl wire-bottomed cages and maintained at controlled conditions (22 ± 2°C temperature, 55 ± 10 % humidity and 12 h light–12 h dark cycles). Diet and water were provided ad libitum. All experimental protocols and procedures were approved by the Institutional Animal Ethical Committee, the National Institute of Nutrition, Hyderabad, India.
Experimental design
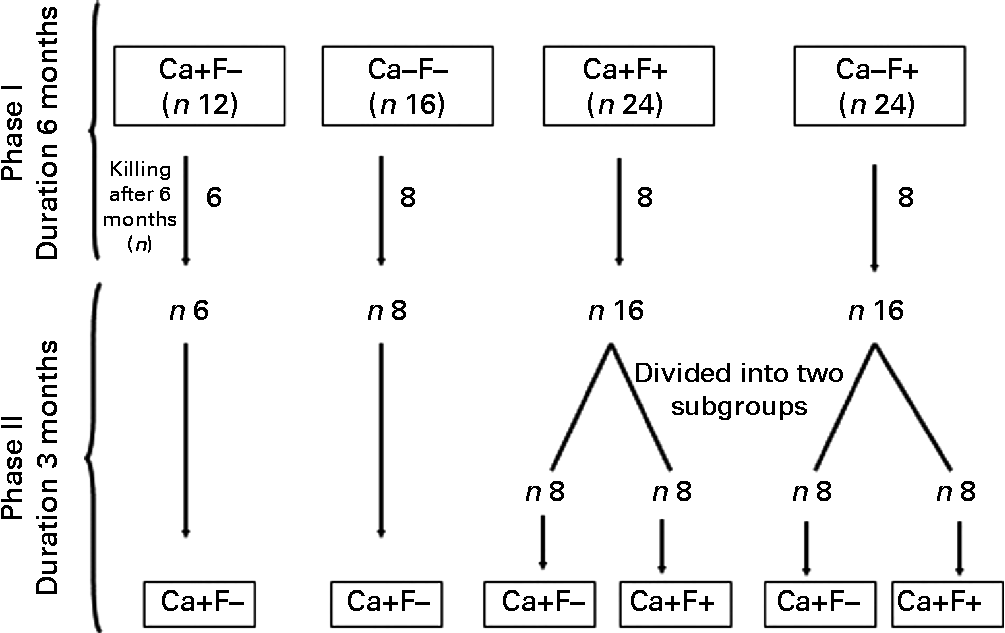
Experimental design is shown in Fig. 1. The present experiment was a completely randomised design, performed in two phases. In phase I (duration 6 months), rats were allocated to four treatments: normal-Ca (0·5 %) diet (NCD)+FFW (Ca+F − /control n 12); low-Ca (0·25 %) diet (LCD)+FFW (Ca − F − , n 16); NCD+100 ppm F water (Ca+F+, n 24); or LCD+100 ppm F water (Ca − F+, n 24). After 6 months of this treatment, six animals from the Ca+F − treatment (control) and eight animals from all other treatments were killed. In phase II (reversal phase), low Ca (Ca − ) was replaced by normal Ca (Ca+). In some of the fluorotic areas, it is not possible to provide FFW and a nutritional approach is the only option left to mitigate the fluorosis. Keeping that in mind in phase II, treatment groups Ca+F+ and Ca − F+ were divided into two subgroups (n 8) to compare the effect of continuation v. discontinuation along with Ca supplementation on reversal of chronic F toxicity. The duration of phase II was 3 months.

Fig. 1 Animal experiment protocol. Ca+, normal-calcium (0·5 %) diet; Ca − , low-calcium (0·25 %) diet; F+, 100 parts per million fluoride; F − , fluoride-free water.
Diets
To simulate the condition of a fluorotic area, cereal–legume-based stock diet (prepared at the National Institute of Nutrition, Hyderabad, India) was fed to rats. The Ca content of the normal-Ca diet (Ca+, 0·5 %) was based on the RDA for rats(Reference Reeves, Nielsen and Fahey33), whereas the Ca content of the low-Ca diet (Ca − , 0·25 %) was selected on the basis of the average Ca intake (335 mg/d) of the Indian population(29), which is almost half of the RDA for Ca (600 mg/d)(30). The basic composition was same for both diets (LCD and NCD), except the mineral mixture composition (Table 1). Dietary Ca content was checked by atomic absorption spectrometer (Avanta AAS, GBC Scientific Equipment Limited) to make sure that the diet contained the desired level of Ca.
Table 1 Basic composition of diet used for feeding of rats

* Fine powder of roasted Bengal gram (Cicer arietinum), a variety of pulse; it provides 22·5 % protein, 5·2 % fat and 58·1 % carbohydrate.
† Fine powder of wheat (Triticum aestivum); it provides 11·8 % protein, 1·5 % fat and 71·2 % carbohydrate.
‡ Commercially available; it provides 38 % protein, 0·1 % fat and 51 % carbohydrate.
§ Milk protein, commercially available.
∥ Vitamin mixture provided per kg of diet: dl-α-tocopherol acetate, 120 mg; menadione, 1·5 mg; thiamine, 12 mg; riboflavin, 5 mg; pyridoxine, 6 mg; niacin, 10 mg; pantothenic acid (Ca salt), 12 mg; cyanocobalamine, 5 mg; folic acid, 1 mg; paraamino benzoic acid, 100 mg; biotin, 400 mg; inositol, 100 mg; choline chloride, 1000 mg.
¶ Mineral mixture provided per kg of diet: NaCl, 3·0 g; magnesium sulphate, 2·3 g; ferrous sulphate, 500 mg; manganous sulphate, 160 mg; potassium iodide, 10 mg; zinc sulphate, 22 mg; copper sulphate, 19 mg; cobalt chloride, 0·12 mg; calcium carbonate, 7·12 g in normal-Ca diet/0·868 g in low-Ca diet; starch, 26·88 g in normal-Ca diet/32·43 g in low-Ca diet.
Water
A measure of 100 ppm F (2·21 g sodium fluoride/10 litres of deionised water) was given through drinking water. The average F intake in endemic fluorotic areas is 21 mg/d(Reference Teotia, Teotia and Singh34). To correspond to human intake, rats must be given four to five times F(Reference Angmar-Mansson and Whitford35, Reference Dunipace, Brizendine and Zhang36). Therefore, 100 ppm F was used to feed the rats.
Daily food and calcium intake, body weight gain and food efficiency ratio
Daily food intake (food given − (food left+wastage)) was measured at the interval of 15 d for three consecutive days and the mean was taken. Daily Ca intake was calculated from diet intake.
Body weight was monitored weekly using a digital balance (Model BL3, Sartorius, maximum capacity-3000 g) and BWG was calculated by deducting baseline body weight from the body weight taken at the particular time interval.
FER (increase in body weight per g of food intake) was calculated by dividing BWG from food consumed in a particular time period(Reference Arancha, Ana and Sara37).
Blood and urine collection
Blood was collected by retro-orbital sinus puncture after an overnight fast at the end of phases I and II. Serum was separated and stored at − 80°C until further investigation. The 24 h urine was collected under toluene in separate containers by keeping the rats in metabolism cages for three consecutive days at the end of phases I and II and stored at 4°C till further investigation. Urine samples collected at days 1, 2 and 3 were treated as triplicates.
Euthanisation and tissue collection
Rats were killed by CO2 asphyxiation (phase I+phase II = 30+46 = 76). All vital organs were collected, washed with cold saline, blotted dry and snap-frozen under liquid N2 and stored at − 80°C for further use. Femurs were cleaned of adherent tissues and stored at − 80°C. Proximal small intestine (5 cm) was collected and immediately washed with cold saline. Duodenal mucosa was scraped, snap-frozen and preserved at − 80°C for gene expression studies.
Serum and urinary calcium
Total serum and urinary Ca excretion were measured by atomic absorption spectrometry(38).
Serum 25-hydroxy-vitamin D3and 1,25-dihydroxy-vitamin D3
Both parameters were analysed by the RIA kit provided by Immune Diagnostics Systems. The manufacturer's protocol was followed. The sensitivity, intra-assay and inter-assay variation of the 25(OH) vitamin D3 and 1,25(OH)2 vitamin D3 assay were < 3 nmol/l, 5·0 pg/ml; 5·0, 7·7 %; and 8·1, 12·3 %, respectively.
Serum intact parathyroid hormone
Serum intact parathyroid hormone was assessed using a two-site immune radiometric kit (IDS). The sensitivity, intra-assay variation and inter-assay variation of the parathyroid hormone assay were 1·0 pg/ml, < 4·7 and < 4·3 %, respectively.
Fluoride: urinary and faecal excretion and skeletal deposition
Urinary F excretion and faecal F excretion were measured using an ion-selective electrode (Model EA 940 Orion). Bone F was determined as previously described(Reference Singer and Armstrong39).
Real-time PCR
Total RNA was isolated from duodenal mucosa using TRIzol reagent (Invitrogen Life Technologies), according to the manufacturer's protocol. Quantitative analysis of the isolated total RNA sample was done using NanoDrop (Thermo Scientific NanoDrop™ 1000 Spectrophotometer; Thermo Fisher Scientific Inc.). Integrity of RNA was confirmed by running RNA samples on 1·5 % denaturing agarose gel. A measure of 3 μg of total RNA was reverse transcribed into complementary DNA using the Superscript first-strand synthesis system (Invitrogen Life Technologies). Quantitative PCR was carried out for CASR, VDR and S100G using SYBR Premix Ex Taq (Takara Bio, Inc.) in the Stratagene Mx3000P QPCR system (Agilent Technologies). Expressions of VDR and S100G genes were quantified using primer sets and PCR conditions reported by Mendoza et al. (Reference Mendoza, Lopez and Canalejo40) and Charoenphandhu et al. (Reference Charoenphandhu, Nakkrasae and Kraidith41), respectively. For CASR, primers were designed using National center for Biotechnology Information (NCBI) Primer blast. PCR conditions for CASR were as follows: forward primer, 5′-AAGCCATGATATTCGCCATA-3′; reverse primer, 5′-TTCTGGGCAACAAAACTCAA-3′; annealing temperature, 52°C; and the amplicon size was 139 bp. Expression of target genes was normalised to expression levels of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene(Reference Kuroda, Virdi and Dai42).
Statistical analysis
Descriptive statistics were carried out for all parameters. Values are presented as means with their standard errors. For phase I, two-way ANOVA was carried out. P values for the main effect of Ca, F and their interaction effect on different parameters are shown in corresponding tables and figure legends. In phase II, one-way ANOVA was carried out followed by the least significant difference post hoc test. The data that did not meet the assumptions of parametric statistics were log transformed and then subjected to ANOVA. In the present study, data were collected at days 180 (phase I) and 270 (phase II) for every animal (except the animals killed at the end of phase I). To know the significance of the change in the values of different variables at the end of phase II (day 270) with respect to phase I (day 180), or in other words to see the extent of reversibility, the paired t test was applied to the data collected at days 270 and 180 for different treatment groups. All statistical calculations were carried out using SPSS version 16.0 (SPSS, Inc.) and P< 0·05 was considered significant for all tests.
Results
All rats tolerated the experiment without any complications.
Daily food intake, food efficiency ratio and body weight gain
Daily food intake of groups receiving different treatments was comparable in both phases of the study (Tables 2 and 3). The main effects of Ca, F and their interaction effect were not significant for food intake in phase I (Table 2). FER of treatment group Ca − F+ was significantly lower, as compared with groups receiving Ca+F − and Ca − F − in phase I, and only F had a significant effect on FER (Table 2). In phase II, FER of treatment groups Ca − F+/Ca+F − and Ca − F+/Ca+F+ was significantly lower, as compared with Ca+F − /Ca+F − (P= 0·010), Ca − F − /Ca+F − (P= 0·036) and Ca+F − /Ca+F − (P= 0·003), Ca − F − /Ca+F − (P= 0·002), Ca+F+/Ca+F − (P= 0·044), Ca+F+/Ca+F+ (P= 0·021) and Ca − F+/Ca+F − (P= 0·029), respectively (Table 3). BWG of treatment group Ca − F+ was significantly lower, as compared with all groups in phase I. The main effect of F as well as the interaction effect of Ca and F affected BWG (Table 2). In phase II also, groups receiving Ca − F+/Ca+F − and Ca − F+/Ca+F+ had significantly lower BWG, as compared with groups Ca+F − /Ca+F − (P= 0·001) and Ca − F − /Ca+F − (P= 0·001) (Table 3).
Table 2 Daily food intake, food efficiency ratio (FER) and body weight gain (BWG) in rats fed a normal-calcium (0·5 %) diet (Ca+F−), a low-calcium (0·25 %) diet (Ca−F−), the normal-calcium (0·5 %) diet+100 parts per million (ppm) fluoride (Ca+F+) or the low-calcium (0·25 %) diet+100 ppm fluoride (Ca−F+) for 6 months in phase I of the study (Mean values with their standard errors)

a,b,cMean values within a row with unlike superscript letters are significantly different (P< 0·05).
Table 3 Daily food intake, food efficiency ratio (FER) and body weight gain (BWG) in rats after providing a normal-calcium (0·5 %) diet (Ca+) and fluoride-free water (F−) in phase II (reversal phase, duration 3 months) of the study (Mean values with their standard errors)

Ca − , low-Ca (0·25 %) diet; F+, 100 parts per million F.
a,b,c,dMean values within a row with unlike superscript letters are significantly different (P< 0·05).
* The characters preceding the ‘/’ refer to the dietary treatment in phase I and those following the ‘/’ refer to the dietary treatment in phase II.
Fluoride: urinary and faecal excretion and skeletal deposition
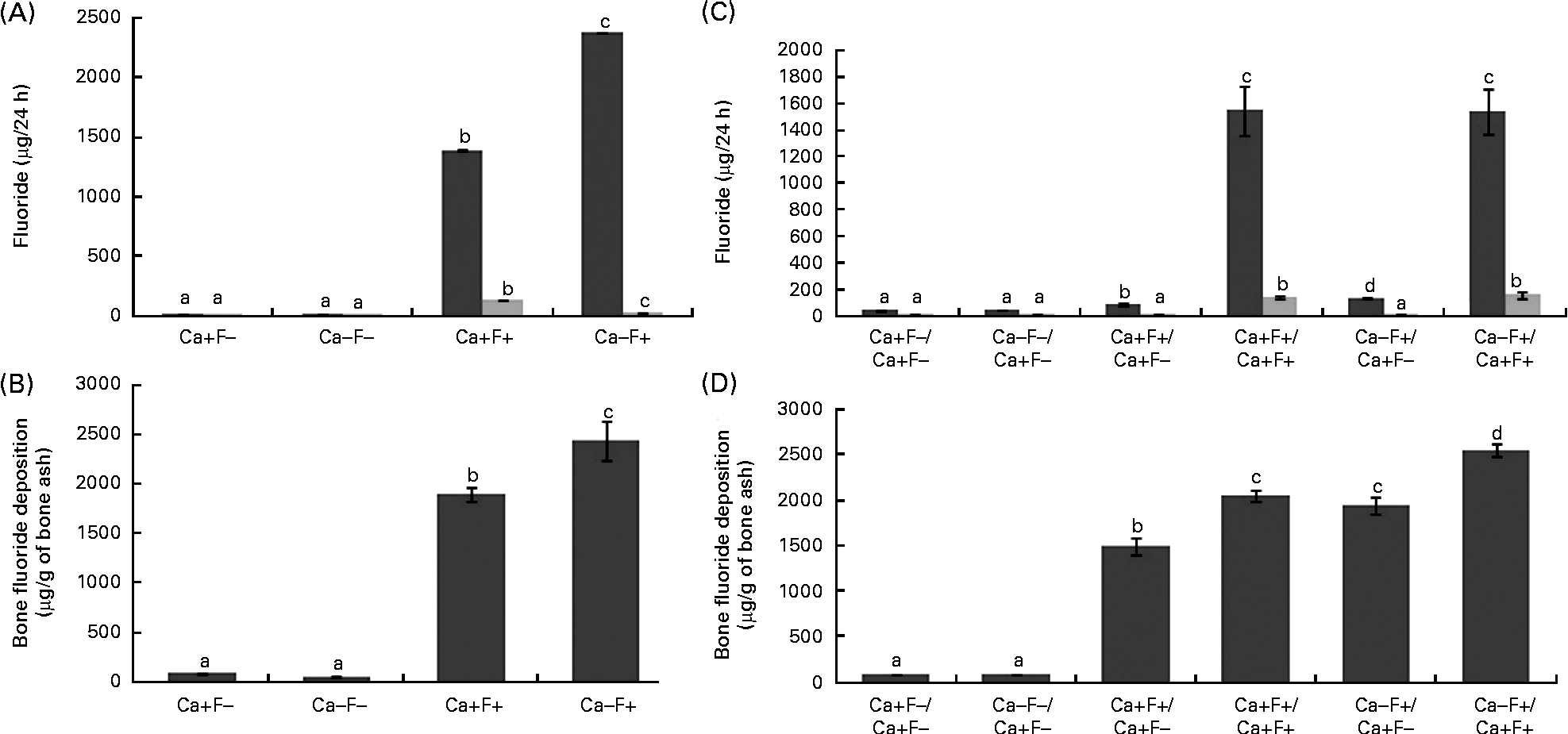
In phase I, urinary F excretion of treatment group Ca − F+ was significantly higher and faecal F excretion was significantly lower, as compared with the Ca+F+ treatment group (Fig. 2(A)). Bone F deposition was significantly higher in treatment group Ca − F+, as compared with the Ca+F+ treatment group (Fig. 2(B)). The main effects of Ca and F and their interaction effect were significant for all the three parameters. In phase II, urinary F excretion of the Ca − F+/Ca+F − group was significantly higher (P= 0·002), as compared with group Ca+F+/Ca+F − (Fig. 2(C)). Groups receiving Ca+F+/Ca+F+ and Ca − F+/Ca+F+ treatments showed significantly higher bone F deposition, as compared with treatment groups Ca+F+/Ca+F − (P= 0·001) and Ca − F+/Ca+F − (P= 0·002), respectively. Further, bone F deposition in treatment group Ca − F+/Ca+F − was significantly higher (P= 0·001) than in the Ca+F+/Ca+F − group (Fig. 2(D)).

Fig. 2 (A) Urinary (■) and faecal (![]() ) fluoride excretion in rats fed a normal-calcium (0·5 %) diet (Ca+F − ), a low-calcium (0·25 %) diet (Ca − F − ), the normal-calcium (0·5 %) diet+100 parts per million (ppm) fluoride (Ca+F+) or the low-calcium (0·25 %) diet+100 ppm fluoride (Ca − F+) for 6 months in phase I. P for main effect of calcium, main effect of fluoride and calcium × fluoride on urinary fluoride excretion and faecal fluoride excretion = 0·001. (B) Bone fluoride deposition in rats fed the normal-calcium (0·5 %) diet (Ca+F − ), the low-calcium (0·25 %) diet (Ca − F − ), the normal calcium (0·5 %) diet+100 ppm fluoride (Ca+F+) or the low-calcium (0·25 %) diet+100 ppm fluoride (Ca − F+) for 6 months in phase I. There were significant main effects for calcium (P= 0·028), fluoride (P= 0·001) and calcium × fluoride (P= 0·014). (C) Urinary and faecal fluoride excretion and (D) bone fluoride deposition in rats after providing the normal-calcium (0·5 %) diet and fluoride-free water treatment in phase II (reversal phase, duration 3 months) of the study. In (C) and (D), the characters preceding the ‘/’ on the x axis refer to the dietary treatment in phase I and the characters following the ‘/’ refer to the dietary treatment in phase II. Values are means, with their standard errors represented by vertical bars. Phase I, Ca+F − (n 12), Ca − F − (n 16), Ca+F+ and Ca − F+ (n 24); phase II, n 8 except Ca+F − /Ca+F − treatment group (n 6). a,b,c,dMean values with unlike letters are significantly different (P< 0·05).
) fluoride excretion in rats fed a normal-calcium (0·5 %) diet (Ca+F − ), a low-calcium (0·25 %) diet (Ca − F − ), the normal-calcium (0·5 %) diet+100 parts per million (ppm) fluoride (Ca+F+) or the low-calcium (0·25 %) diet+100 ppm fluoride (Ca − F+) for 6 months in phase I. P for main effect of calcium, main effect of fluoride and calcium × fluoride on urinary fluoride excretion and faecal fluoride excretion = 0·001. (B) Bone fluoride deposition in rats fed the normal-calcium (0·5 %) diet (Ca+F − ), the low-calcium (0·25 %) diet (Ca − F − ), the normal calcium (0·5 %) diet+100 ppm fluoride (Ca+F+) or the low-calcium (0·25 %) diet+100 ppm fluoride (Ca − F+) for 6 months in phase I. There were significant main effects for calcium (P= 0·028), fluoride (P= 0·001) and calcium × fluoride (P= 0·014). (C) Urinary and faecal fluoride excretion and (D) bone fluoride deposition in rats after providing the normal-calcium (0·5 %) diet and fluoride-free water treatment in phase II (reversal phase, duration 3 months) of the study. In (C) and (D), the characters preceding the ‘/’ on the x axis refer to the dietary treatment in phase I and the characters following the ‘/’ refer to the dietary treatment in phase II. Values are means, with their standard errors represented by vertical bars. Phase I, Ca+F − (n 12), Ca − F − (n 16), Ca+F+ and Ca − F+ (n 24); phase II, n 8 except Ca+F − /Ca+F − treatment group (n 6). a,b,c,dMean values with unlike letters are significantly different (P< 0·05).
Calcium homeostasis
Daily calcium intake, serum calcium and urinary calcium excretion
In phase I, daily Ca intake of treatment groups Ca − F − and Ca − F+ was significantly lower, as compared with the treatment groups Ca+F − and Ca+F+ (Table 4). Serum Ca of treatment groups Ca − F − and Ca − F+ was significantly lower, as compared with the treatment groups Ca+F − and Ca+F+. Treatment group Ca+F+ also showed lower serum Ca, as compared with the treatment group Ca+F − (Table 4). Urinary Ca excretion of the treatment group Ca − F+ was significantly lower, as compared with the treatment group Ca+F − (Table 4). For daily Ca intake and urinary Ca excretion, only the main effect of Ca was significant. However, main as well as interaction effects of both factors significantly affected serum Ca (Table 4). In phase II, treatment groups Ca − F+/Ca+F − and Ca − F+/Ca+F+ showed significantly lower serum Ca, as compared with the treatment groups Ca+F − /Ca+F − (P= 0·002) and Ca+F − /Ca+F − (P= 0·016), Ca − F − /Ca+F − (P= 0·004), Ca+F+/Ca+F − (P= 0·004), Ca+F+/Ca+F+ (P= 0·004) and Ca − F+/Ca+F − (P= 0·004), respectively (Table 5).
Table 4 Daily calcium intake, serum calcium and urinary calcium excretion in rats fed a normal-calcium (0·5 %) diet (Ca+F−) a low-calcium (0·25 %) diet (Ca−F−), the normal-calcium (0·5 %) diet+100 parts per million (ppm) fluoride (Ca+F+) or the low-calcium (0·25 %) diet+100 ppm fluoride (Ca−F+) for 6 months in phase I of the study (Mean values with their standard errors)

a,b,c,dMean values within a row with unlike superscript letters are significantly different (P< 0·05).
Table 5 Daily calcium intake, serum calcium and urinary calcium excretion in rats after providing a normal-calcium (0·5 %) diet (Ca+) and fluoride-free water (F−) in phase II (reversal phase, duration 3 months) of the study (Mean values with their standard errors)

Ca − , low Ca (0·25 %) diet; F+, 100 parts per million F.
a,b,c,dMean values within a row with unlike superscript letters are significantly different (P< 005).
* The characters preceding the ‘/’ refer to the dietary treatment in phase I and those following the ‘/’ refer to the dietary treatment in phase II.
Serum parathyroid hormone, 25-hydroxy-vitamin D3 and 1,25-dihydroxy-vitamin D3
In phase I, serum 25(OH) vitamin D3 of treatment group Ca − F+ was significantly lower, as compared with the treatment groups Ca+F − and Ca+F+, whereas serum 25(OH) vitamin D3 of treatment group Ca − F − was significantly lower, as compared with the treatment group Ca+F+ only (Table 6). As expected, treatment groups Ca − F − and Ca − F+ had significantly higher serum 1,25(OH)2 vitamin D3, as compared with the treatment groups Ca+F − and Ca+F+. Further, treatment group Ca − F+ showed significantly increased 1,25(OH)2 vitamin D3, as compared with the treatment group Ca − F − (Table 6). Ca treatment significantly affected serum 25(OH) vitamin D3. For serum 1,25(OH)2 vitamin D3, the main as well as interaction effects of both factors were significant (Table 6). In phase II of the study, treatment group Ca − F+/Ca+F+ showed significantly higher (P= 0·043) serum parathyroid hormone, as compared with the group receiving Ca+F+/Ca+F+ treatment (Table 7).
Table 6 Serum 25-hydroxy-vitamin D3, parathyroid hormone and 1,25-dihydroxy-vitamin D3 in rats fed a normal-calcium (0·5 %) diet (Ca+F−), a low-calcium (0·25 %) diet (Ca−F−), the normal-calcium (0·5 %) diet+100 parts per million (ppm) fluoride (Ca+F+) or the low-calcium (0·25 %) diet+100 ppm fluoride (Ca−F+) for 6 months in phase I of the study (Mean values with their standard errors)

a,b,cMean values within a row with unlike superscript letters are significantly different (P< 0·05).
Table 7 Serum 25-hydroxy-vitamin D3, parathyroid hormone and 1,25-dihydroxy-vitamin D3 in rats after providing a normal-calcium (0·5 %) diet (Ca+) and fluoride-free water (F−) in phase II (reversal phase, duration 3 months) of the study (Mean values with their standard errors)

Ca − , low Ca (0·25 %) diet; F+, 100 parts per million F.
a,b,cMean values within a row with unlike superscript letters are significantly different (P< 0·05).
* The characters preceding the ‘/’ refer to the dietary treatment in phase I and those following the ‘/’ refer to the dietary treatment in phase II.
Gene expression studies
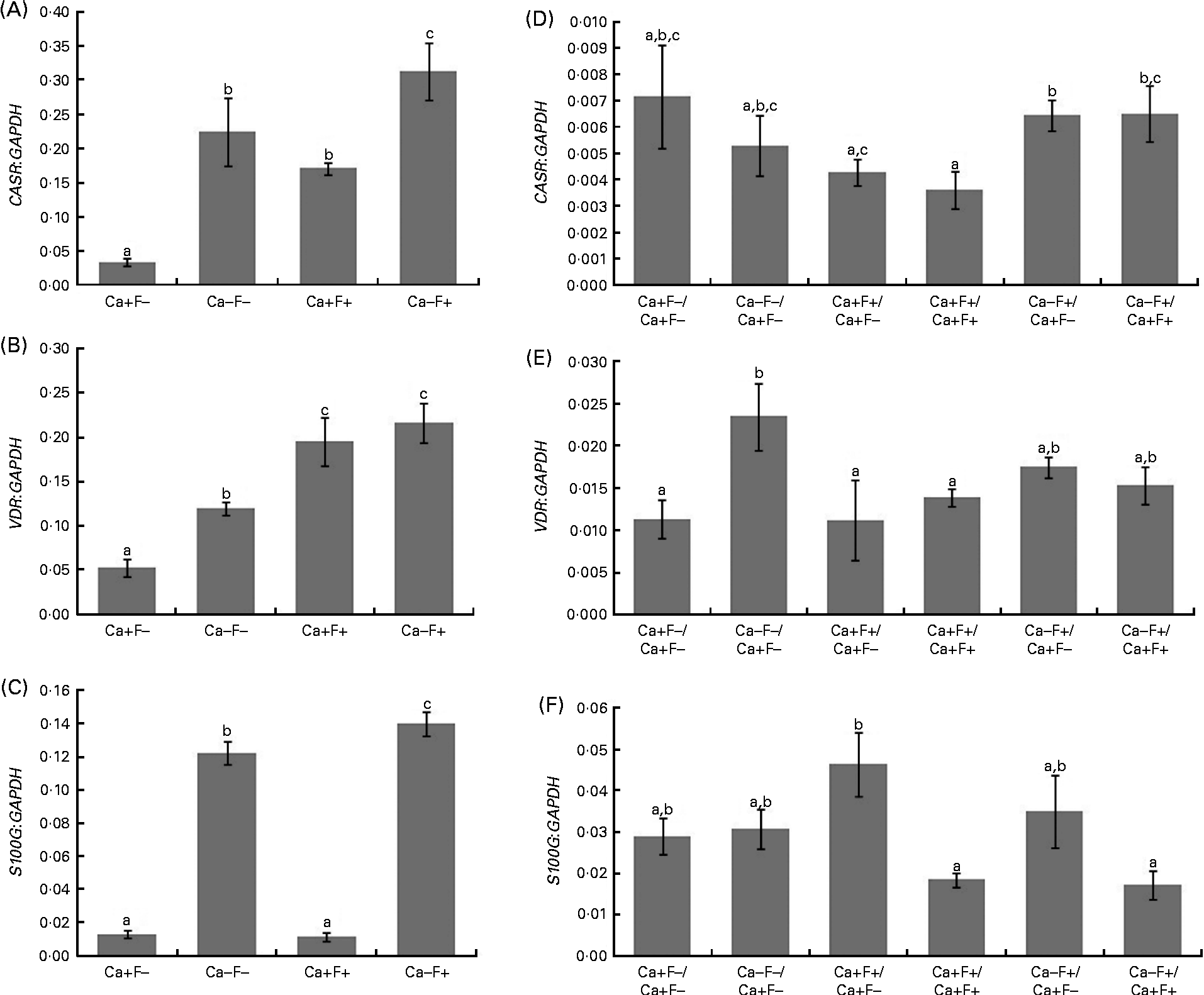
In phase I of the study, expression levels of CASR and VDR genes showed a significant increase in treatment groups Ca − F − , Ca+F+ and Ca − F+, as compared with the treatment group Ca+F − (Fig. 3(A) and (B)). Further, treatment group Ca − F+ showed a significantly higher expression of CASR, as compared with groups receiving Ca − F − and Ca+F+. F-treated groups (Ca+F+ and Ca − F+) exhibited significantly higher VDR expression, as compared with the group receiving Ca − F − . Expression of S100G was significantly higher in treatment group Ca − F − , as compared with Ca+F − and Ca+F+. Treatment group Ca − F+ showed significantly higher expression of S100G, as compared with all other groups (Fig. 3(C)). The main effects of both factors (Ca and F) were significant for expression of CASR and VDR; however, their interaction effect was not significant. For S100G expression, F was not a significant factor, though the main effect of Ca and the interaction effect of Ca and F were significant.

Fig. 3 (A) Expression of calcium-sensing receptor (CASR), there were significant main effects for calcium (P= 0·001), fluoride (P= 0·003) and calcium × fluoride (P= 0·503); (B) vitamin D receptor (VDR), there were significant main effects for calcium (P= 0·040), fluoride (P= 0·001) and calcium × fluoride (P= 0·265); (C) S100 calcium-binding protein G (S100G), there were significant main effects for calcium (P= 0·001), fluoride (P= 0·069) and calcium × fluoride (P= 0·029) normalised to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the duodenal mucosa of rats fed a normal-calcium (0·5 %) diet (Ca+F − ), a low-calcium (0·25 %) diet (Ca − F − ), the normal-calcium (0·5 %) diet+100 parts per million (ppm) fluoride (Ca+F+), or the low-calcium (0·25 %) diet+100 ppm fluoride (Ca − F+) for 6 months in phase I. Expression of (D) CASR, (E) VDR and (F) S100G normalised to GAPDH in duodenal mucosa of rats after providing the normal-calcium (0·5 %) diet and fluoride-free water treatment in phase II (reversal phase, duration 3 months) of the study. In (D), (E) and (F), the characters preceding the ‘/’ on the x the axis refer to the dietary treatment in phase I and the characters following the ‘/’ refer to the dietary treatment in phase II. Values are means, with their standard errors represented by vertical bars. Phase I, Ca+F − (n 12), Ca − F − (n 16), Ca+F+ and Ca − F+ (n 24); phase II, n 8 except Ca+F − /Ca+F − treatment group (n 6). a,b,cMean values with unlike letters are significantly different (P< 0·05).
In phase II, treatment groups Ca − F+/Ca+F − and Ca − F+/Ca+F+ showed significantly higher CASR expression, as compared with treatment groups Ca+F+/Ca+F − (P= 0·020), Ca+F+/Ca+F+ (P= 0·012) and Ca+F+/Ca+F+ (P= 0·030), respectively (Fig. 3(D)). VDR expression of all groups was comparable except for treatment group Ca − F − /Ca+F − , which was significantly higher, as compared with groups receiving Ca+F − /Ca+F − (P= 0·018), Ca+F+/Ca+F − (P= 0·006) and Ca+F+/Ca+F+ (P= 0·043) (Fig. 3(E)). Treatment groups Ca+F+/Ca+F+ and Ca − F+/Ca+F+ showed significantly lower expression of S100G, as compared with Ca+F+/Ca+F − (P= 0·002 for Ca+F+/Ca+F+ and P= 0·018 for Ca − F+/Ca+F+) (Fig. 3(F)).
Discussion
Fluorosis becomes aggravated in the presence of inadequate Ca nutrition(Reference Khandare, Harikumar and Sivakumar11, Reference Singh and Jolly27, Reference Mithal, Trivedi and Gupta28). The present study explored adverse effects of chronic F toxicity on FER, BWG, body composition, Ca homeostasis and expression of genes involved in Ca homeostasis in the presence of adequate or inadequate Ca as well as amelioration of the same by providing FFW and adequate Ca. Data collected in the study indicated that F exhibited a negative impact on BWG in the presence of inadequate Ca. Reduced FER in the treatment group Ca − F+ indicated interference of F with digestion and proper utilisation of food. Decrease in BWG due to F toxicity in the presence of Ca malnutrition has also been reported previously(Reference Dunipace, Edward and Wilson7, Reference Xu, Liu and Zhang43). In phase II, no effect of providing FFW or NCD was observed on BWG. Significantly higher FER of the treatment group Ca − F+/Ca+F − , as compared with group Ca − F+/Ca+F+ (difference of means, 0·011; sem, 0·0016; P= 0·001), indicated towards the role of FFW for efficient utilisation of food.
In phase I, higher urinary F excretion and bone F deposition in treatment group Ca − F+ with respect to treatment group Ca+F+ shows that F toxicity was worse in the presence of inadequate Ca. However, excretion of F through faeces was higher in the presence of adequate Ca, due to the removal of F in the form of calcium fluoride from the body(Reference Ranganathan23, Reference Burkhart and Jowsey24). All the findings of the present study are in agreement with an earlier study(Reference Dunipace, Edward and Wilson7) carried out in rats (fed 0·125 or 0·25 % Ca and 5, 15 or 50 ppm F), which showed that with reduction in dietary Ca, faecal F excretion and the absorption as well as tissue retention of F increased. In phase II, after receiving NCD+FFW, treatment group Ca − F+/Ca+F − exhibited higher urinary clearance of F, as compared with treatment group Ca+F+/Ca+F − , which suggests that feeding an adequate-Ca diet with FFW can mitigate the additive adverse effects of chronic F and LCD treatment. However, still higher bone F deposition in treatment group Ca − F+/Ca+F − , as compared with the treatment group Ca+F+/Ca+F − , implies that a longer duration might be needed for complete reversal.
Consumption of the LCD produced hypocalcaemia in treatment groups Ca − F − and Ca − F+, as shown by serum Ca levels and further supported by low urinary Ca excretion (only in treatment group Ca − F+). In addition, decreased serum Ca in treatment group Ca+F+, as compared with Ca+F − (FFW-treated counterpart), was also observed. Due to the increased demand of Ca for new bone formation in fluorosis, lowering of serum Ca has been reported earlier also(Reference Das and Susheela10, Reference Khandare, Harikumar and Sivakumar11). In phase II, after replacing the LCD with the NCD, treatment groups Ca − F+/Ca+F − and Ca − F+/Ca+F+ could not show significant recovery in serum Ca. The group receiving Ca+F+ in phase I was divided into two subgroups to compare the effects of continuation v. discontinuation of F, and it was found that the serum Ca of the treatment group Ca+F+/Ca+F − improved (P= 0·48).
Serum 25(OH) vitamin D3 in treatment groups Ca − F − and Ca − F+ was low, whereas serum 1,25(OH)2 vitamin D3 was high due to increased enzymatic conversion of 1,25(OH)2 vitamin D3 from 25(OH) vitamin D3 to promote intestinal absorption of Ca(Reference Kochupillai18). In phase II, after receiving the NCD, serum 25(OH) vitamin D3 and 1,25(OH)2 vitamin D3 of treatment groups Ca − F − /Ca+F − , Ca − F+/Ca+F − and Ca − F+/Ca+F+ were comparable with other groups. A significant increase in expression of CASR levels in treatment groups Ca − F − (6-fold), Ca+F+(4·5-fold) and Ca − F+(9-fold), as compared with Ca+F − , was observed as reported previously also(Reference Tiwari, Gupta and Kumar22). The reason behind significant increases of VDR and S100G expression levels in LCD-treated groups in phase I was Ca insufficiency, in which expression of VDR is up-regulated(Reference McDonnell, Mangelsdorf and Pike44). VDR is known to induce S100G transcription in the intestine(Reference Theofan, Nguyen and Norman45, Reference Dupret, Brun and Perret46) and facilitates the intracellular diffusion of Ca(Reference Wasserman and Fullmer47) to maintain Ca homeostasis. There was significant increase in the VDR expression levels of the treatment group Ca+F+, but without any significant increase in either serum 1,25(OH)2 vitamin D3 levels or S100G expression. Studies(Reference Strom, Sandgren and Brown48, Reference Goff, Reinhardt and Beckman49) have suggested that the increase in VDR expression may also be due to post-translational events, resulting in increased receptor stability(Reference Favus, Mangelsdorf and Timbe50).
In phase II, after providing the NCD, CASR (except treatment groups Ca − F+/Ca+F − and Ca − F+/Ca+F+) and VDR (except treatment group Ca − F − /Ca+F − ) expressions of all other groups were comparable. Down-regulation of any of the receptors studied was not observed in the presence of F in phase I. However, in phase II, S100G expression of F-treated groups Ca+F+/Ca+F+(2·5-fold) and Ca − F+/Ca+F+(2-fold) was down-regulated (as compared with treatment group Ca+F+/Ca+F − ), as reported previously also(Reference Tiwari, Gupta and Kumar22). This observation suggests that in the long term, irrespective of nutritional background, chronic F toxicity may reduce Ca absorption by inhibiting the expression of S100G, a key molecule involved in active Ca transport in the intestine(Reference Bronner20, Reference Christakos, Gill and Elee21), resulting in adverse effects on Ca homeostasis. A protein expression study could not be carried out, which is the limitation of the present study.
In summary, chronic F toxicity along with inadequate Ca showed adverse effects on BWG, FER and Ca homeostasis in phase I. However, irrespective of Ca nutrition, F toxicity down-regulated S100G expression in phase II. A NCD treatment improved BWG irrespective of the type of water administered, and there was better improvement in FER of the LCD+F-treated group after receiving the NCD and FFW for 3 months. Ca homeostasis appeared to be normalised at the end of phase II. It is concluded that adverse effects of chronic F toxicity are more pronounced in the presence of Ca deficiency. These effects may be ameliorated with adequate Ca nutrition and safe drinking water. Chronic F toxicity may affect Ca absorption in the duodenum due to the inhibitory effect on the expression of S100G – a key molecule involved in Ca absorption. Further studies are required to study the protein content of CASR, VDR and S100G. In addition, human studies are needed to explore the reversibility of fluorosis.
Acknowledgements
The present study was supported by the Indian Council of Medical Research, India. We thank N. Hari Shankar, for providing facilities for animal experimentation and acknowledge expert technical assistance of G. Shanker Rao and P. Krishna Swamy (atomic absorption spectrometer). We also thank Bharti Kulkarni, for critical review of the manuscript. None of the authors had a personal or financial conflict of interest. A. L. K. designed the study; P. S. conducted the research and laboratory analysis; S. G. supervised molecular biology work; K. B. guided the analysis of Ca; K. V. guided the statistical analysis of data; and P. S. and A. L. K. wrote the paper and had primary responsibility for the final content. All authors read and approved the final manuscript.