The Apgar score is an important indicator of a newborn’s health and is established immediately after birth (Apgar, Reference Apgar1953). Apgar scoring is standard in obstetrics and neonatological practice, and the advantage of this screening tool is that it allows for prompt standardized assessment (Apgar, Reference Apgar1953; Committee on Obstetric Practice American Academy of Pediatrics — Committee on Fetus and Newborn, 2015). It has proven its utility as a population-level indicator of outcome risk, with predictive value for neonatal and infant death and post neonatal development (Apgar, Reference Apgar1966; Drage et al., Reference Drage, Kennedy and Schwarz1964; Harrington et al., Reference Harrington, Redman, Moulden and Greenwood2007; Nelson & Ellenberg, Reference Nelson and Ellenberg1981). Its value has been confirmed in national register studies (Casey et al., Reference Casey, McIntire and Leveno2001; Grunebaum et al., Reference Grunebaum, McCullough, Sapra, Brent, Levene, Arabin and Chervenak2013; Iliodromiti et al., Reference Iliodromiti, Mackay, Smith, Pell and Nelson2014; Li et al., Reference Li, Wu, Lei, Zhang, Mao and Zhang2013; Straube et al., Reference Straube, Voigt, Jorch, Hallier, Briese and Borchardt2010; Thorngren-Jerneck & Herbst, Reference Thorngren-Jerneck and Herbst2001) and is recognized by national guidelines and the World Health Organization (Siddiqui et al., Reference Siddiqui, Cuttini, Wood, Velebil, Delnord, Zile and Comm2017; WHO, 2012). However, there is a discussion regarding other, possibly more precise, monitoring tools (O’Donnell et al., Reference O’Donnell, Kamlin, Davis, Carlin and Morley2006; Rudiger et al., Reference Rudiger, Konstantelos and Consortium2015). Apgar scores are evaluated at the first and fifth minutes after birth. While a low first-minute score is often transitory, persistence of poor health, resulting in a low fifth-minute score, often implies complications of clinical importance and indicates that the newborn has not responded to initial intervention (Drage et al., Reference Drage, Kennedy and Schwarz1964; Harrington et al., Reference Harrington, Redman, Moulden and Greenwood2007; Kattwinkel et al., Reference Kattwinkel, Perlman, Aziz, Colby, Fairchild, Gallagher and Zaichkin2010; Siddiqui et al., Reference Siddiqui, Cuttini, Wood, Velebil, Delnord, Zile and Comm2017; Thorngren-Jerneck & Herbst, Reference Thorngren-Jerneck and Herbst2001).
The assessment of Apgar scores in twins was first presented by Virginia Apgar in 1953. For twins, the Apgar score of the first-born twin typically was better than that of the second-born twin (Apgar, Reference Apgar1953). Replication studies of differences among first- and second-born twins (Haest et al., Reference Haest, Roumen and Nijhuis2005; Herbst & Kallen, Reference Herbst and Kallen2008; Morley et al., Reference Morley, Cole, Powell and Lucas1989; Wen et al., Reference Wen, Fung Kee Fung, Oppenheimer, Demissie, Yang and Walker2004), among twins and singletons (Hegyi et al., Reference Hegyi, Carbone, Anwar, Ostfeld, Hiatt, Koons and Paneth1998; Thorngren-Jerneck & Herbst, Reference Thorngren-Jerneck and Herbst2001), among term and preterm newborns (Dolgun et al., Reference Dolgun, Inan, Altintas, Okten and Sayin2016; Morley et al., Reference Morley, Cole, Powell and Lucas1989) and among different presentation and modes of delivery (Rossi et al., Reference Rossi, Mullin and Chmait2011) followed this initial work. Two small studies (Franchi-Pinto et al., Reference Franchi-Pinto, Dal Colletto, Krieger and Beiguelman1999; Riese, Reference Riese1990) suggested that genetic factors also contribute to variation in Apgar scores. However, because of small sample sizes, the results of these studies were inconclusive.
The aim of the present study is twofold. First, we present a literature review of studies of Apgar scores, including singleton and multiple births, to identify pre and perinatal characteristics associated with variation in Apgar scores in population-based studies. Second, in a large sample of Dutch monozygotic (MZ) and dizygotic (DZ) twins enrolled in the Netherlands Twin Register (NTR) (van Beijsterveldt et al., Reference van Beijsterveldt, Groen-Blokhuis, Hottenga, Franic, Hudziak, Lamb and Boomsma2013), we estimated effects of pre and perinatal factors shared and nonshared by twins, including the parameters identified in the literature search and test for a contribution of genetic factors in the classical twin design.
Methods
Literature Review
A review of the literature regarding pre and perinatal characteristics associated with Apgar scores was conducted in PubMed (MEDLINE), Web of Science, Embase and reference lists of retrieved articles. Search terms were ‘Apgar scores’ and ‘heritability,’ ‘genetic effect,’ ‘prenatal factors,’ ‘twins,’ ‘fetal presentation,’ ‘mode of delivery,’ ‘gestational age’ and ‘neonatal outcome’. Studies on specific clinical aspects of pregnancy and neonatology and on mortality and morbidity were excluded. We followed prior research on Apgar scores (Milsom et al., Reference Milsom, Ladfors, Thiringer, Niklasson, Odeback and Thornberg2002; Sibony et al., Reference Sibony, Touitou, Luton, Oury and Blot2006; Straube et al., Reference Straube, Voigt, Jorch, Hallier, Briese and Borchardt2010) and grouped pre and perinatal characteristics in the following categories — biological maternal and paternal factors, socioeconomic factors, mode of conception, gestational age (GA), pregnancy and delivery characteristics and newborn characteristics. The list of characteristics was included in an empirical study in which resemblance in MZ and DZ twins in a bivariate (1- and 5-min Apgar scores) model was evaluated.
Empirical Study in Twins: Data Collection
Data on Apgar scores and pre and perinatal characteristics were obtained from the NTR (van Beijsterveldt et al., Reference van Beijsterveldt, Groen-Blokhuis, Hottenga, Franic, Hudziak, Lamb and Boomsma2013). The NTR recruits families with twins a few weeks to months after birth. Informed consent is obtained from parents. Surveys, including questions on pregnancy, birth and outcomes, were sent to mothers after registration of newborn twins.
Zygosity
For the majority of twin-pairs, genotyping for zygosity was based on a genome-wide single nucleotide polymorphism (SNP) array (Odintsova et al., Reference Odintsova, Willemsen, Dolan, Hottenga, Martin, Slagboom and Boomsma2018), or on genome-wide sets of microsatellites. Zygosity typing in earlier studies was based on smaller numbers of microsatellite markers, blood groups (van Dijk et al., Reference van Dijk, Boomsma and de Man1996) or SNPs (van Beijsterveldt et al., Reference van Beijsterveldt, Groen-Blokhuis, Hottenga, Franic, Hudziak, Lamb and Boomsma2013). For 27% of the same-sex pairs, zygosity was based on items about physical similarity and frequency of confusion of the twins by parents and strangers from later surveys that correctly determine zygosity in 93% of the cases (Rietveld et al., Reference Rietveld, van Der Valk, Bongers, Stroet, Slagboom and Boomsma2000). In 19% of the cases, zygosity was based on a single item, indicating how much the children look alike at age 2, which gives a correct determination of zygosity in 92% of the cases (Groen-Blokhuis et al., Reference Groen-Blokhuis, Middeldorp, van Beijsterveldt and Boomsma2011). For the other same-sex pairs, zygosity was based on a single question from survey 1.
The sample comprised 5181 twin pairs born between 2005 and 2017. Of these, 1763 were MZ and 3418 were DZ (34% and 66%, respectively, reflecting population prevalence in the Netherlands). The data set includes complete information on zygosity, GA and time between birth of the first and second twins. One-min Apgar scores were available in 4947 pairs and 5-min Apgar were available scores in 4724 pairs. Both Apgar scores were available for 4623 pairs.
The study protocols were approved on March 16, 2004, by the Central Ethics Committee on Research Involving Human Subjects of the VU University Medical Center, Amsterdam; and May 25, 2017 (NTR-25-mei-2007). All participants provided informed consent.
Variables
Apgar scores were analyzed: (a) as a continuous variable (scores between 0 and 10); (b) as conventional categories (ordinal variables): Apgar values of 0–6 (low), 7–9 (intermediate) and 10 (high); a total score of lower than 7 is considered a source of concern (Committee on Obstetric Practice American Academy of Pediatrics — Committee on Fetus and Newborn, 2015).
Based on the literature review, we tested for the effects of birth order (first or second born), zygosity (MZ or DZ), sex (boys or girls), GA, birth weight, mother’s and father’s age at birth, mother’s body mass index (BMI) at birth, fetal presentation (head presentation: cephalic; breech and horizontal presentations: noncephalic), mode of delivery (vaginal and intervention with vacuum extraction, forceps or cesarean section), and intertwin delivery interval. For 1003 MZ twins, we had information on chorionicity (van Beijsterveldt et al., Reference van Beijsterveldt, Overbeek, Rozendaal, McMaster, Glasner, Bartels and Boomsma2016). Of these, 745 were monochorionic (MC) and 258 were dichorionic (DC).
Data Analyses
Frequencies and means
The data were analyzed using SPSS version 25. The frequencies of maternal, delivery and infant characteristics were obtained within each Apgar score category, for first- and second-born twins and for MZ and DZ pairs. Differences between continuous variables in MZ and DZ pairs were tested using ANOVA, comparisons between first and second born with paired t tests.
Fixed effect analysis
The role of maternal, pregnancy, delivery and infant characteristics were analyzed in the first- and second-born twin by ordinal regression. The significant characteristics were included in genetic covariance structure (GCS) analysis of the twin data. Four variables (GA, fetal presentation, mode of delivery and birth weight) were selected for inclusion in the analyses of twin resemblance for 1- and 5-min Apgar scores.
Twin correlations
Twin (polychoric) correlations of ordinal Apgar scores in MZ and DZ twins were estimated in Mplus. MZ twins are genetically identical, while DZ twins share on average 50% of their alleles identical by descent (from their parents). The MZ correlation (rMZ) is expected to be greater than the DZ correlations (rDZ) if the phenotype is influenced by genes (rMZ > rDZ). The presence of shared environmental factors is suggested if the DZ correlation is larger than half the MZ correlation (rDZ > rMZ/2). Unshared environmental influences are present if the MZ correlation is less than 1 (Boomsma et al., Reference Boomsma, Busjahn and Peltonen2002).
GCS modeling
We carried out GCS analyses of polychoric correlation matrices of the ordinal (3-point) 1- and 5-min Apgar scores using Mplus 6 (Muthen & Muthen, Reference Muthen and Muthen2007). The analysis of ordinal data is based on the liability-threshold model (Falconer, Reference Falconer1993), in which the ordinal scores arise by imposing thresholds on a continuous (standard normal) liability dimension. The twin resemblance at the level of this dimension is expressed by the polychoric correlations. The thresholds are a function of frequencies of the ordinal Apgar values. Given the 3-point ordinal Apgar scores, as there are two thresholds in GCS analysis, we first fitted models to estimate the polychoric correlation matrices in the MZ and DZ twins and to analyse the thresholds in the presence of the covariates. Subsequently, we fitted an ACE model (see below), in which the phenotypic polychoric correlations are model in terms of genetic and shared and unshared environmental effects. Parameter estimates were obtained by means of the weighted least squares estimation (the Mplus estimator WLSMV; Muthen & Muthen, Reference Muthen and Muthen2007). Model comparisons were based on the comparison of model chi-square (goodness of fit) statistics, using the Mplus difference test procedure. The main aim of the GCS modeling was to assess the contributions of genetic and environmental influences to the phenotypic (co)variance matrix of the first- and fifth-minute Apgar scores, while correcting for relevant covariates. We fitted a bivariate ACE model, which included additive genetic (A), shared environmental (C) and unshared environmental effects (E). The results of the analyses provide us with the decomposition of the phenotypic variance of the 1- and 5-min Apgar scores and the decomposition of the phenotype covariance (1-min with 5-min Apgar scores) into genetic and environmental components. In fitting the bivariate ACE model, we used a chi-square difference test to test sex differences, zygosity difference and birth-order differences in the covariates. We also tested sex differences in the ACE variance components.
We first fitted a model in which the thresholds differed with respect to birth order (first- vs. second-born twin), sex and zygosity (see Table S2 in the Supplementary material). We tested whether the thresholds were equal in males and females (retaining birth order and zygosity-related differences), which was found to be the case, χ2(16) = 23.8, p = .09. We tested whether the thresholds were equal in MZ and DZ twins, but this was not the case, χ2(8) = 23.4, p = .003. So, in the model of choice, we estimated 16 thresholds: 2 for 1-min Apgar scores and 2 for 5-min Apgar scores, which were different for zygosity and birth order. In the model, the regression coefficients of the covariates differed with respect to birth order.
We fitted the bivariate ACE model next. In this model, we allowed for sex differences in the ACE covariance matrices. As the twin correlations are suggestive of a CE model (i.e., absence of additive genetic effects), we first tested whether we could fix the additive genetic parameters to zero. This was found to be the case: χ2(6) = 10.3, p = .11. In this CE model, we constrained the shared and nonshared parameters to be equal over sex and found sex differences in the shared and nonshared parameters to be absent, χ2(4) = 3.958, p = .41.
Results
Review of the Literature
The review of studies on characteristics associated with Apgar score included population-based and twin studies published from 1981 to 2018, with exclusion of studies that concern specific clinical groups (e.g., preeclampsia, gestational diabetes), mortality and morbidity in newborns, and long-term outcomes associated with Apgar scores. Our literature search identified 63 studies, including studies of twins (see Table 1) and singletons (see Table S1 in the Supplementary material). The characteristics associated with Apgar scores may be summarized as:
Table 1. Review of studies on prenatal characteristics and Apgar scores in twins
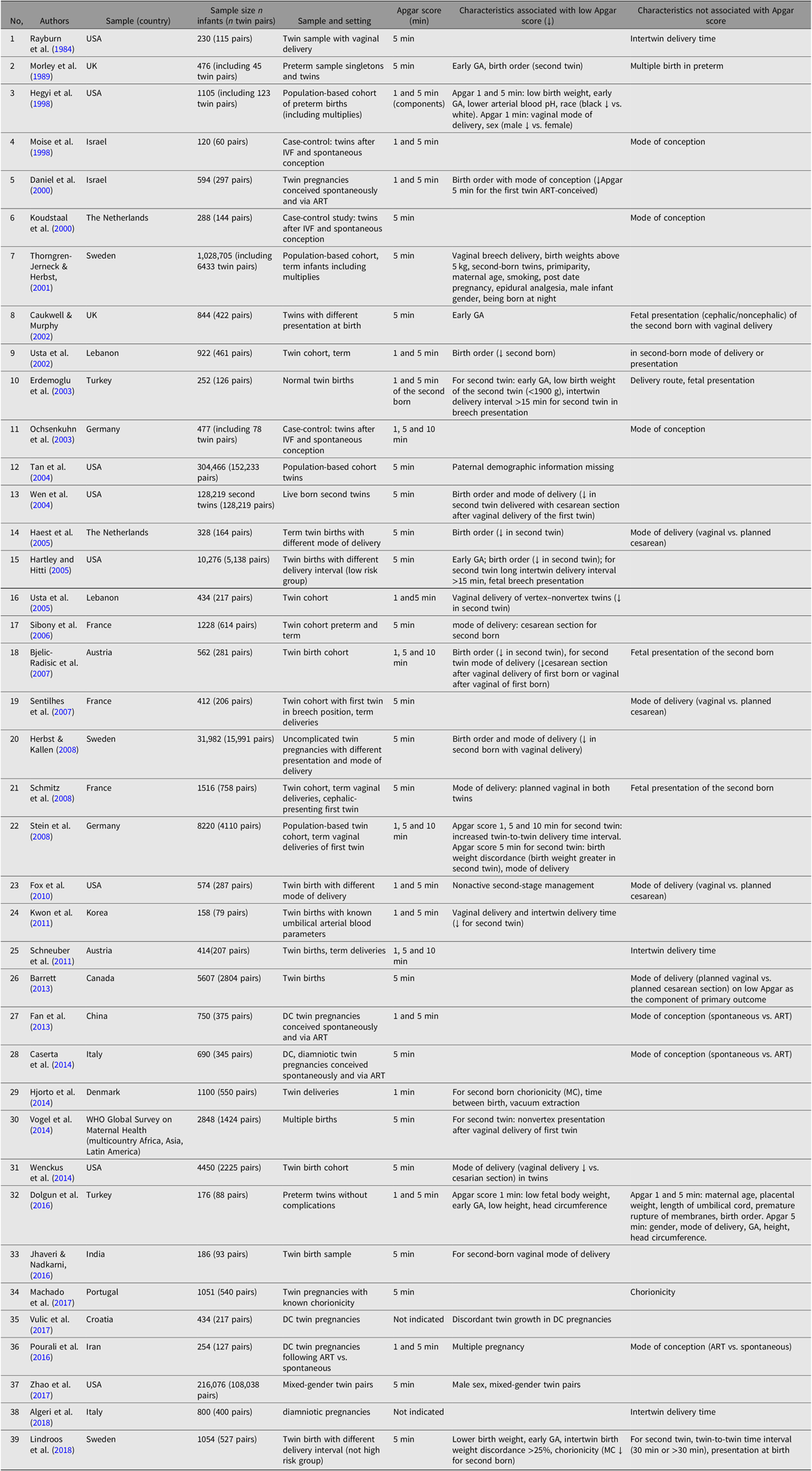
Note: Studies are indicated with ** in the reference list.
Biological maternal and paternal factors
Maternal short stature (Camilleri, Reference Camilleri1981; Svenvik et al., Reference Svenvik, Brudin and Blomberg2015), low maternal age <17 (X. K. Chen et al., Reference Chen, Wen, Fleming, Demissie, Rhoads and Walker2007), high maternal age >40 years (Jahromi & Husseini, Reference Jahromi and Husseini2008; Straube et al., Reference Straube, Voigt, Jorch, Hallier, Briese and Borchardt2010) and high paternal age >55 years (Khandwala et al., Reference Khandwala, Baker, Shaw, Stevenson, Lu and Eisenberg2018) were associated with low Apgar scores. However, Milsom et al. (Reference Milsom, Ladfors, Thiringer, Niklasson, Odeback and Thornberg2002) found no association of Apgar scores with maternal age. Higher mother’s BMI and maternal obesity were associated with low Apgar scores in some (Chen et al., Reference Chen, McNiff, Madan, Goodman, Davis and Dammann2010; Straube et al., Reference Straube, Voigt, Jorch, Hallier, Briese and Borchardt2010; Thorngren-Jerneck & Herbst, Reference Thorngren-Jerneck and Herbst2001), but not all studies (Kiran et al., Reference Kiran, Hemmadi, Bethel and Evans2005; Rode et al., Reference Rode, Nilas, Wojdemann and Tabor2005).
Socioeconomic factors
These were studied in European countries. Maternal occupation and single-parent status did not show an association with Apgar scores in the study of Straube et al. (Reference Straube, Voigt, Jorch, Hallier, Briese and Borchardt2010). No significant association was found with employment status during pregnancy (Marbury et al., Reference Marbury, Linn, Monson, Wegman, Schoenbaum, Stubblefield and Ryan1984; Milsom et al., Reference Milsom, Ladfors, Thiringer, Niklasson, Odeback and Thornberg2002; Straube et al., Reference Straube, Voigt, Jorch, Hallier, Briese and Borchardt2010). Low Apgar scores were associated with single motherhood (Milsom et al., Reference Milsom, Ladfors, Thiringer, Niklasson, Odeback and Thornberg2002), missing paternal demographic information (Tan et al., Reference Tan, Wen, Walker and Demissie2004), low level of mother’s education and manual work (Hemminki et al., Reference Hemminki, Malin and Rahkonen1990; Odd et al., Reference Odd, Doyle, Gunnell, Lewis, Whitelaw and Rasmussen2008) and adverse social circumstances (Kalland et al., Reference Kalland, Sinkkonen, Gissler, Merilainen and Siimes2006).
Mode of conception
Two small studies reported an association of 5-min Apgar score with mode of conception (Daniel et al., Reference Daniel, Ochshorn, Fait, Geva, Bar-Am and Lessing2000; Ramoglu et al., Reference Ramoglu, Kavuncuoglu, Ozbek and Aldemir2014). No significant difference was found in terms of the 1- and 5-min Apgar scores between twins who were conceived naturally and twins who were conceived with the aid of artificial reproductive technologies (Caserta et al., Reference Caserta, Bordi, Stegagno, Filippini, Podagrosi, Roselli and Moscarini2014; Fan et al., Reference Fan, Sun, Yang, Ye and Wang2013; Koudstaal et al., Reference Koudstaal, Bruinse, Helmerhorst, Vermeiden, Willemsen and Visser2000; Moise et al., Reference Moise, Laor, Armon, Gur and Gale1998; Ochsenkuhn et al., Reference Ochsenkuhn, Strowitzki, Gurtner, Strauss, Schulze, Hepp and Hillemanns2003; Pourali et al., Reference Pourali, Ayati, Jelodar, Zarifian and Andalibi2016).
Gestational age
Apgar scores were associated with both low and high GA. For results on low GA, see Caukwell and Murphy (Reference Caukwell and Murphy2002), Dolgun et al. (Reference Dolgun, Inan, Altintas, Okten and Sayin2016), Erdemoglu et al. (Reference Erdemoglu, Mungan, Tapisiz, Ustunyurt and Caglar2003), Hartley & Hitti (Reference Hartley and Hitti2005), Hegyi et al. (Reference Hegyi, Carbone, Anwar, Ostfeld, Hiatt, Koons and Paneth1998), Iliodromiti et al. (Reference Iliodromiti, Mackay, Smith, Pell and Nelson2014), Lindroos et al. (Reference Lindroos, Elfvin, Ladfors and Wennerholm2018), Morley et al. (Reference Morley, Cole, Powell and Lucas1989), Svenvik et al. (Reference Svenvik, Brudin and Blomberg2015) and van der Ven et al (Reference van der Ven, Schaaf, van Os, de Groot, Haak, Pajkrt and Mol2014). For results on high GA, see Svenvik et al. (Reference Svenvik, Brudin and Blomberg2015) and Thorngren-Jerneck & Herbst (Reference Thorngren-Jerneck and Herbst2001).
Pregnancy characteristics
A multiple pregnancy is a risk factor for adverse outcomes, including low Apgar scores (Morley et al., Reference Morley, Cole, Powell and Lucas1989; Pourali et al., Reference Pourali, Ayati, Jelodar, Zarifian and Andalibi2016; Svenvik et al., Reference Svenvik, Brudin and Blomberg2015; Thorngren-Jerneck & Herbst, Reference Thorngren-Jerneck and Herbst2001). This holds specifically for MC pregnancies (Hjorto et al., Reference Hjorto, Nickelsen, Petersen and Secher2014; Lindroos et al., Reference Lindroos, Elfvin, Ladfors and Wennerholm2018) and DC pregnancies with discordant fetal weight (Vulic et al., Reference Vulic, Lalic, Vulic, Roje, Benzon and Mestrovic2017). Again, some studies failed to find an association with chorionicity (Machado et al., Reference Machado, Lima Teixeira, Ferreira, Rodrigues, Henriques and Afonso2017). Even though maternal smoking is generally associated with negative outcomes in neonates, most studies found no significant association between Apgar scores and prenatal maternal smoking after accounting for other confounders (Gilman et al., Reference Gilman, Gardener and Buka2008; Kalland et al., Reference Kalland, Sinkkonen, Gissler, Merilainen and Siimes2006; Milsom et al., Reference Milsom, Ladfors, Thiringer, Niklasson, Odeback and Thornberg2002; Straube et al., Reference Straube, Voigt, Jorch, Hallier, Briese and Borchardt2010), although an association between mother’s smoking during the first trimester and low Apgar score was seen by Kallen (Reference Kallen2001).
Delivery characteristics
Multiple deliveries were associated with adverse outcomes in the second-born twin (Haest et al., Reference Haest, Roumen and Nijhuis2005; Herbst & Kallen, Reference Herbst and Kallen2008; Kwon et al., Reference Kwon, Yoon, Lee, Kim, Shin and Park2011; Morley et al., Reference Morley, Cole, Powell and Lucas1989; Thorngren-Jerneck & Herbst, Reference Thorngren-Jerneck and Herbst2001; Usta et al., Reference Usta, Nassar, Awwad, Nakad, Khalil and Karam2002; Wen et al., Reference Wen, Fung Kee Fung, Oppenheimer, Demissie, Yang and Walker2004). The intertwin delivery interval is an important determinant of the adverse effects on the second born (Erdemoglu et al., Reference Erdemoglu, Mungan, Tapisiz, Ustunyurt and Caglar2003; Hartley & Hitti, Reference Hartley and Hitti2005; Hjorto et al., Reference Hjorto, Nickelsen, Petersen and Secher2014; Kwon et al., Reference Kwon, Yoon, Lee, Kim, Shin and Park2011; Stein et al., Reference Stein, Misselwitz and Schmidt2008) as this interval is related to the risk of hypoxia, due to decreasing pH in the umbilical arterial blood. However, other studies showed that even a relatively long intertwin delivery interval was not associated with unfavorable Apgar scores (Algeri et al., Reference Algeri, Callegari, Mastrolia, Brienza, Vaglio Tessitore and Paterlini2018; Lindroos et al., Reference Lindroos, Elfvin, Ladfors and Wennerholm2018; Milsom et al., Reference Milsom, Ladfors, Thiringer, Niklasson, Odeback and Thornberg2002; Rayburn et al., Reference Rayburn, Lavin, Miodovnik and Varner1984; Schneuber et al., Reference Schneuber, Magnet, Haas, Giuliani, Freidl, Lang and Bjelic-Radisic2011).
Noncephalic (breech and horizontal) presentation at birth is associated with low Apgar score in singletons (Krebs & Langhoff-Roos, Reference Krebs and Langhoff-Roos1999; Krebs et al., Reference Krebs, Langhoff-Roos and Thorngren-Jerneck2001; Vogel et al., Reference Vogel, Holloway, Cuesta, Carroli, Souza and Barrett2014) and twins (Hartley & Hitti, Reference Hartley and Hitti2005). After a vaginal delivery of a vertex first twin, nonvertex presentation of the second twin was associated with increased odds of a low 5-min Apgar score (Vogel et al., Reference Vogel, Holloway, Cuesta, Carroli, Souza and Barrett2014). However, other studies found no support for an effect of fetal presentation on the Apgar score of the second twin (Bjelic-Radisic et al., Reference Bjelic-Radisic, Pristauz, Haas, Giuliani, Tamussino, Bader and Schlembach2007; Caukwell & Murphy, Reference Caukwell and Murphy2002; Lindroos et al., Reference Lindroos, Elfvin, Ladfors and Wennerholm2018; Schmitz et al., Reference Schmitz, Carnavalet Cde, Azria, Lopez, Cabrol and Goffinet2008; Usta et al., Reference Usta, Nassar, Awwad, Nakad, Khalil and Karam2002). Emergency interventions (vacuum extraction, forceps and urgent operative delivery) were associated with adverse outcomes, including low Apgar scores (Hjorto et al., Reference Hjorto, Nickelsen, Petersen and Secher2014; Milsom et al., Reference Milsom, Ladfors, Thiringer, Niklasson, Odeback and Thornberg2002; Rode et al., Reference Rode, Nilas, Wojdemann and Tabor2005). The risk of a low Apgar score given at planned vaginal delivery was much higher than the risk associated with a selective cesarean section in singletons (Hegyi et al., Reference Hegyi, Carbone, Anwar, Ostfeld, Hiatt, Koons and Paneth1998; Krebs & Langhoff-Roos, Reference Krebs and Langhoff-Roos1999) and twins (Schmitz et al., Reference Schmitz, Carnavalet Cde, Azria, Lopez, Cabrol and Goffinet2008), especially in the second-born twin (Herbst & Kallen, Reference Herbst and Kallen2008; Jhaveri & Nadkarni, Reference Jhaveri and Nadkarni2016; Kwon et al., Reference Kwon, Yoon, Lee, Kim, Shin and Park2011; Usta et al., Reference Usta, Rechdan, Khalil and Nassar2005; Wenckus et al., Reference Wenckus, Gao, Kominiarek and Wilkins2014). The effect of mode of delivery in twins was not supported by some studies analyzing different fetal presentation deliveries (Barrett, Reference Barrett2013; Fox et al., Reference Fox, Silverstein, Bender, Klauser, Saltzman and Rebarber2010; Sentilhes et al., Reference Sentilhes, Goffinet, Talbot, Diguet, Verspyck, Cabrol and Marpeau2007). Cesarean section was associated with low Apgar in the second twin following vaginal delivery of first twin (Sibony et al., Reference Sibony, Touitou, Luton, Oury and Blot2006; Wen et al., Reference Wen, Fung Kee Fung, Oppenheimer, Demissie, Yang and Walker2004).
Newborn characteristics
Several studies found a positive association between birth weight and Apgar scores (Dolgun et al., Reference Dolgun, Inan, Altintas, Okten and Sayin2016; Erdemoglu et al., Reference Erdemoglu, Mungan, Tapisiz, Ustunyurt and Caglar2003; Hegyi et al., Reference Hegyi, Carbone, Anwar, Ostfeld, Hiatt, Koons and Paneth1998; Ladehoff et al., Reference Ladehoff, Pedersen and Sorensen1986; Lindroos et al., Reference Lindroos, Elfvin, Ladfors and Wennerholm2018; Stein et al., Reference Stein, Misselwitz and Schmidt2008; Thorngren-Jerneck & Herbst, Reference Thorngren-Jerneck and Herbst2001). However, Iliodromiti et al. (Reference Iliodromiti, Mackay, Smith, Pell and Nelson2014) found no association of birth weight and Apgar scores in the large population-based sample of more than 1 million births in Scotland. Birth weight discordance in twins is associated with low Apgar scores for the second born (Lindroos et al., Reference Lindroos, Elfvin, Ladfors and Wennerholm2018; Stein et al., Reference Stein, Misselwitz and Schmidt2008). On average, girls have higher Apgar scores than boys (Dolgun et al., Reference Dolgun, Inan, Altintas, Okten and Sayin2016; Hegyi et al., Reference Hegyi, Carbone, Anwar, Ostfeld, Hiatt, Koons and Paneth1998; Stevenson et al., Reference Stevenson, Verter, Fanaroff, Oh, Ehrenkranz, Shankaran and Papile2000; Zhao et al., Reference Zhao, Zou, Lei and Zhang2017).
Based on our literature review, we included the following risk factors in our analyses of the twin data: zygosity, chorionicity, birth order, GA, birth weight, sex, mother’s age and father’s age at birth, mother’s BMI at birth, mode of delivery and fetal presentation.
Descriptives and Apgar Scores in Twins
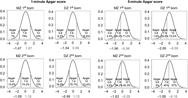
Proportions of newborn NTR twins in three categories of 1- and 5- min Apgar scores (low, intermediate and high) for first- and second born in MZ and DZ twin pairs are presented in Figure 1 (distribution in pairs, see Table S3 in the Supplementary material). In terms of the proportions, the first- and second-born twins do not differ greatly with respect to the 1-min Apgar scores. For instance, the proportion of intermediate 1-min Apgar score (7–9) is about 79%, 74%, 77% and 71% (MZ first born, MZ second born, DZ first born and DZ second born, respectively). There are, however, appreciable differences between the first- and second-born twins in the 5-min Apgar scores. For instance, the proportion of a high 1-min Apgar score (10) is about 71%, 40%, 72%, and 57% (MZ first born, MZ second born, DZ first born and DZ second born, respectively).
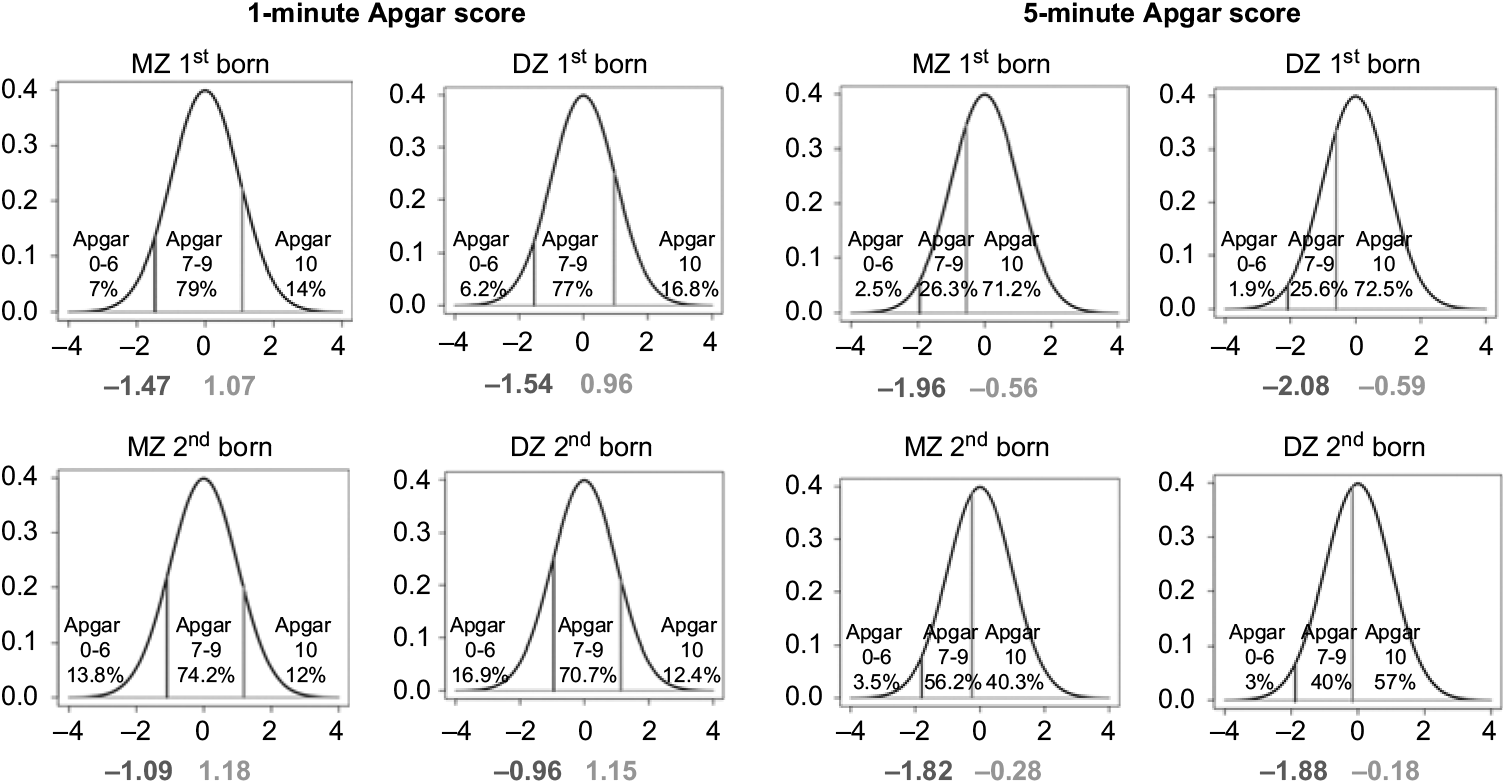
Fig. 1. Proportions of MZ and DZ newborn twins in different categories of 1- and 5-min Apgar score.
We compared the means of Apgar scores in MZ and DZ twin pairs between first- and second-born twins. Mean continuous Apgar scores were higher in the first born than in second-born twins in both MZ and DZ pairs: in MZ at first minute, 8.45 versus 8.1 (p < .0001), at fifth minute, 9.36 versus 9.22 (p < .0001); in DZ at first minute, 8.61 versus 8.03 (p < .0001), at fifth minute, 9.51 versus 9.26 (p < .0001). MZ first-born twins had lower Apgar scores than DZ first-born twins: at first minute, 8.43 versus 8.6 in DZ, at fifth minute, 9.36 versus 9.51 in DZ (p < .0001). In second-born twins there was no effect of zygosity (see Table S4 in the Supplementary material).
Next, we tested pre and perinatal characteristics for the first- and second-born twins. The distribution of perinatal and delivery characteristics of NTR twins are presented in Table 2 (for information on characteristics of low, intermediate and high Apgar score groups, see Table S5 in the Supplementary material). Given alpha of .05, mother’s age, father’s age, and mother’s BMI at birth did not predict Apgar scores (Table S6 in the Supplementary material). The intertwin delivery time was significant for Apgar scores of the second-born twin. There was no significant effect of chorionicity on Apgar score in MZ twins with chorionicity data, taking into account GA, birth weight, sex, mode of delivery and fetal presentation (Table S7 in the Supplementary material). Characteristics without significant effects for both twins were excluded from further analysis.
Table 2. Prenatal, delivery and infant characteristics of MZ and DZ twin Pairs
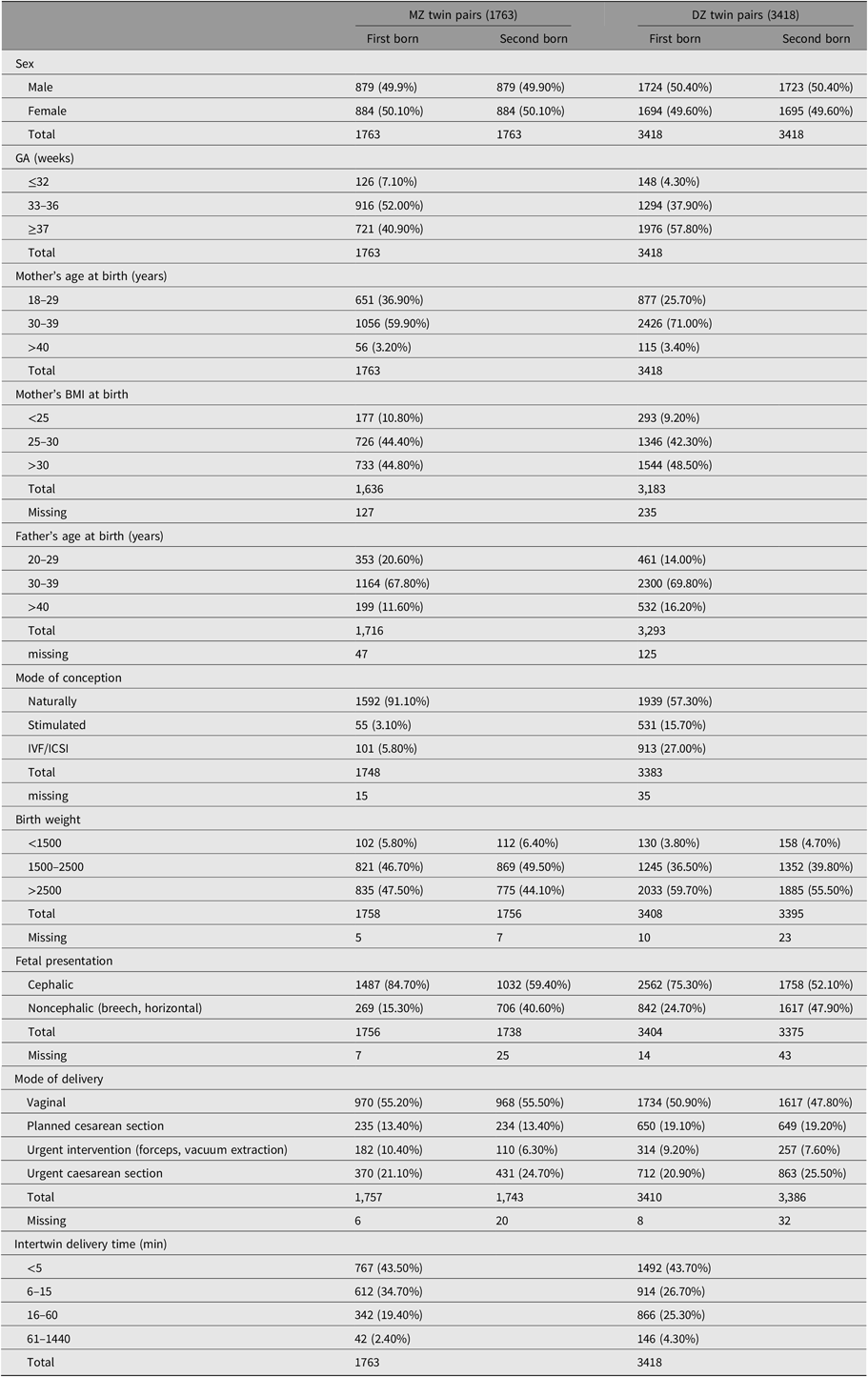
Note: Values are n (%).
Multigroup genetic covariance structural equation modeling of the ordinal 1- and 5-min Apgar scores included monozygotic male (MZM) and female (MZF), dizygotic male (DZM) and female (DZF), dizygotic male–female (DZMF) and dizygotic female–male (DZFM) pairs. We included GA (a characteristic of twin pairs) and fetal presentation, birth weight and mode of delivery (characteristics of individual twins) as covariates. We estimated the effects of covariates and the polychoric twin correlations (Table S2 in the Supplementary material).
In multigroup genetic analyses, the effects of covariates did not differ with respect to sex, χ2 (16) = 8.88, p = .91. GA had a positive effect on both Apgar measurements in both twins (p < .0001; Table 3). Birth weight had a positive effect on 5-min Apgar score of the first born (ß1min =.09, p = .002). The effects of delivery characteristics, such as mode of delivery and fetal presentation at birth, were different for first- and second-born twins. Noncephalic presentation at birth of the first-born twin had a positive effect on Apgar scores of the first born (ß1min = .11, p = .02; ß5min = .19, p < .0001) and noncephalic presentation of the second-born twin have negative effect on Apgar scores (ß1min = −.23, p < .0001; ß5min = −.16, p < .0001). First-born twins delivered vaginally were more likely to have higher Apgar scores at both points (ß1min = .26, ß5min = .42, p < .0001). Second-born twins delivered vaginally were more likely to have lower 1-min Apgar scores (ß1min = −.14, p < .0001), and the effect was not significant for the 5-min Apgar score (ß5min = −0.002, ns).
Table 3. Average for continuous Apgar scores and effect of GA, birth weight, fetal presentation and mode of delivery on ordinal 1- and 5-min Apgar score in first- and second-born twins
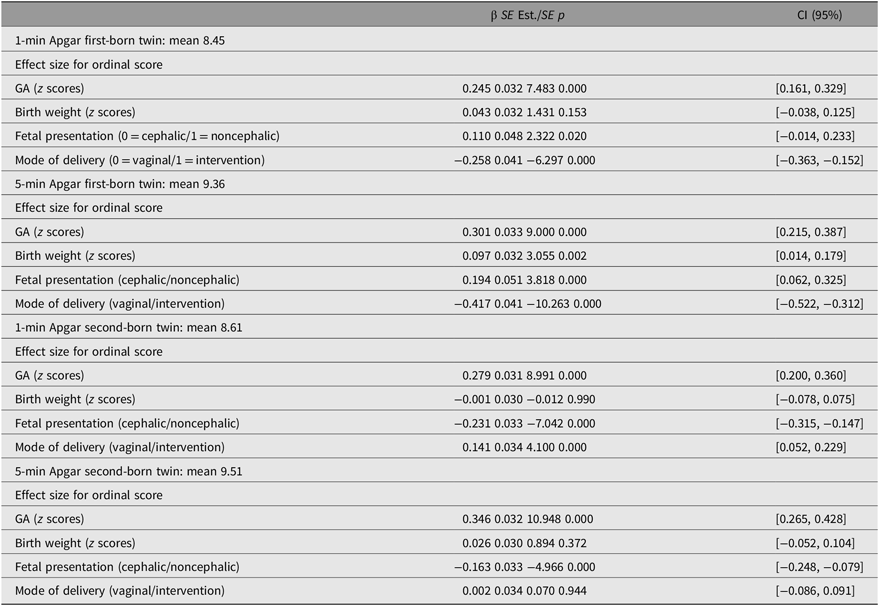
Note: SE = standard error, Est. = estimates and CI = confidence interval.
Table 4 summarizes the twin correlations with and without the correction for covariates (see also Table S8 in the Supplementary material). Overall, the correlations, which varied between .43 and .55, did not differ greatly between zygosity, which suggests the absence of genetic effects. Shared environmental influences accounted for 52.6% (95% confidence interval (CI) [0.51, 0.54]) and 50.2% (95% CI [0.48, 0.52]) of the variance of the 1- and 5-min Apgar scores, respectively. The remainder of the variance was explained by nonshared environmental effects: 47.4% (95% CI [0.46, 0.49]) and 49.8% (95% CI [0.48, 0.52]) of the variance of the 1- and 5-min Apgar scores, respectively. The correlation between the 1- and 5-min Apgar scores was .70 (95% CI [ 0.68, 0.72]), which is consistent with the correlations shown in Table 4. This correlation is decomposed into .33 (95% CI [0.30, 0.35]) due to shared prenatal environmental factors, and .37 (95% CI [0 .39, 0.40]) due to unshared prenatal environmental factors (see Table S9 and Figure S1 in the Supplementary material).
Table 4. Twin correlations for 1- and 5-min Apgar score (ordinal variables) noncorrected and corrected for GA, birth weight, mode of delivery and fetal presentation
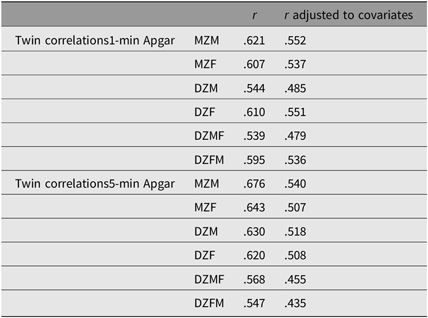
Note: MZM = monozygotic male, MZF = monozygotic female, DZM = dizygotic male, DZF = dizygotic female, DZMF = dizygotic male–female and DZFM = dizygotic female–male.
Discussion
Our literature review identified characteristics that were significantly associated with Apgar scores in population-based and twin studies. These included GA, birth weight, sex, mother’s and father’s age at birth, mother’s BMI, mode of delivery, and fetal presentation, specifically for twins’ zygosity, chorionicity, birth order and intertwin delivery time. In the current analyses of twins, birth order, zygosity, GA, birth weight, fetal presentation at birth and mode of delivery contributed to Apgar scores.
In our empirical study, parental characteristics were not associated with Apgar scores. The effects of mother’s and father’s age on Apgar scores as established in other studies may be explained by families included in these other studies, such as teenage mothers (X. K. Chen et al., Reference Chen, Wen, Fleming, Demissie, Rhoads and Walker2007), mothers over age 40 (Jahromi & Husseini, Reference Jahromi and Husseini2008), and older fathers (Khandwala et al., Reference Khandwala, Baker, Shaw, Stevenson, Lu and Eisenberg2018). In our sample, only 171 women were above 40 years (3.2% MZ and 3.4% DZ of mothers), and there were no mothers younger than 18. An effect of a maternal BMI on Apgar score was found in some studies of singletons (M. Chen et al., Reference Chen, McNiff, Madan, Goodman, Davis and Dammann2010; Straube et al., Reference Straube, Voigt, Jorch, Hallier, Briese and Borchardt2010; Thorngren-Jerneck & Herbst, Reference Thorngren-Jerneck and Herbst2001). Multiple pregnancies are generally accompanied by greater maternal BMI than in singleton pregnancies due to gestational weight gain. This may explain the nonsignificance of maternal BMI effect on Apgar score in our study (near 45% of mothers in our sample had BMI >30). Optimal gestational weight gain in twin pregnancy is unclear (Bodnar et al., Reference Bodnar, Pugh, Abrams, Himes and Hutcheon2014). However, some studies also support our findings that maternal age (Milsom et al., Reference Milsom, Ladfors, Thiringer, Niklasson, Odeback and Thornberg2002) and BMI at birth (Kiran et al., Reference Kiran, Hemmadi, Bethel and Evans2005; Rode et al., Reference Rode, Nilas, Wojdemann and Tabor2005) are not associated with Apgar score. Monochorionicity was an important risk factor for adverse perinatal outcomes in twins (Dube et al., Reference Dube, Dodds and Armson2002; Hjorto et al., Reference Hjorto, Nickelsen, Petersen and Secher2014; Lindroos et al., Reference Lindroos, Elfvin, Ladfors and Wennerholm2018; van Beijsterveldt et al., Reference van Beijsterveldt, Overbeek, Rozendaal, McMaster, Glasner, Bartels and Boomsma2016), but was not associated with Apgar scores in our study.
In accordance with many singleton (Hegyi et al., Reference Hegyi, Carbone, Anwar, Ostfeld, Hiatt, Koons and Paneth1998; Iliodromiti et al., Reference Iliodromiti, Mackay, Smith, Pell and Nelson2014; Svenvik et al., Reference Svenvik, Brudin and Blomberg2015; van der Ven et al., Reference van der Ven, Schaaf, van Os, de Groot, Haak, Pajkrt and Mol2014) and twin studies (Caukwell & Murphy, Reference Caukwell and Murphy2002; Dolgun et al., Reference Dolgun, Inan, Altintas, Okten and Sayin2016; Erdemoglu et al., Reference Erdemoglu, Mungan, Tapisiz, Ustunyurt and Caglar2003; Hartley & Hitti, Reference Hartley and Hitti2005; Lindroos et al., Reference Lindroos, Elfvin, Ladfors and Wennerholm2018; Morley et al., Reference Morley, Cole, Powell and Lucas1989), we found a large effect of GA. In premature newborns, a low Apgar score may indicate intrinsic physiological immaturity and inadequate capacity for response rather than abnormal physiological functions (Iliodromiti et al., Reference Iliodromiti, Mackay, Smith, Pell and Nelson2014). Preterm twins have the same prognosis as preterm singletons (Morley et al., Reference Morley, Cole, Powell and Lucas1989). The effect of GA was stronger than birth weight. We found effects of birth weight on Apgar scores in first-born twins, but not in the second born.
Our findings on birth order agree with previous findings. The first twin is in better clinical condition (Franchi-Pinto et al., Reference Franchi-Pinto, Dal Colletto, Krieger and Beiguelman1999; Haest et al., Reference Haest, Roumen and Nijhuis2005; Herbst & Kallen, Reference Herbst and Kallen2008; Kwon et al., Reference Kwon, Yoon, Lee, Kim, Shin and Park2011; Morley et al., Reference Morley, Cole, Powell and Lucas1989; Thorngren-Jerneck & Herbst, Reference Thorngren-Jerneck and Herbst2001; Usta et al., Reference Usta, Nassar, Awwad, Nakad, Khalil and Karam2002; Wen et al., Reference Wen, Fung Kee Fung, Oppenheimer, Demissie, Yang and Walker2004). The second twin is at greater risk of lower scores, which can be due to longer delivery time, risk of hypoxia, nondefinable fetal presentation before birth to decide the better tactics or complications during delivery. The proportion of low Apgar score in first- and second born reported by Franchi-Pinto et al. (Reference Franchi-Pinto, Dal Colletto, Krieger and Beiguelman1999) corresponds with our findings.
Fetal presentation at birth showed opposite effects in first- and second-born twins and should be further investigated, together with mode of delivery and in application of horizontal/nonhorizontal classification of presentation. The positive effect of noncephalic presentation in first-born twins in our study is in contrast with other studies (Hartley & Hitti, Reference Hartley and Hitti2005) and could be associated with tactics of delivery that can be planned in comparison with delivery of the second born. Delivery practice in the case of noncephalic presentation of the first born can increase the probability of high Apgar scores in the newborns. The previous studies have shown that the effect of fetal presentation on Apgar scores in the second born is associated with fetal presentation and mode of delivery of the first born (Bjelic-Radisic et al., Reference Bjelic-Radisic, Pristauz, Haas, Giuliani, Tamussino, Bader and Schlembach2007; Caukwell & Murphy, Reference Caukwell and Murphy2002; Lindroos et al., Reference Lindroos, Elfvin, Ladfors and Wennerholm2018; Schmitz et al., Reference Schmitz, Carnavalet Cde, Azria, Lopez, Cabrol and Goffinet2008; Usta et al., Reference Usta, Nassar, Awwad, Nakad, Khalil and Karam2002; Vogel et al., Reference Vogel, Holloway, Cuesta, Carroli, Souza and Barrett2014). Cephalic presentation of the second twin is associated with higher Apgar scores in our study, in line with previous studies.
Physicians have gained a clear understanding of how to deliver twins with regard to their presentation and gestation. Some retrospective analyses and meta-analyses reported that the prognosis of twins was not different according to delivery mode (Hogle et al., Reference Hogle, Hutton, McBrien, Barrett and Hannah2003; Sibony et al., Reference Sibony, Touitou, Luton, Oury and Blot2006; Usta et al., Reference Usta, Nassar, Awwad, Nakad, Khalil and Karam2002), but population-based studies reported that the mortality rate or complications in second twins were higher in vaginal deliveries (Herbst & Kallen, Reference Herbst and Kallen2008; Jhaveri & Nadkarni, Reference Jhaveri and Nadkarni2016; Kwon et al., Reference Kwon, Yoon, Lee, Kim, Shin and Park2011; Rossi et al., Reference Rossi, Mullin and Chmait2011; Schmitz et al., Reference Schmitz, Carnavalet Cde, Azria, Lopez, Cabrol and Goffinet2008; Smith et al., Reference Smith, Pell and Dobbie2002; Usta et al., Reference Usta, Rechdan, Khalil and Nassar2005; Wen et al., Reference Wen, Fung Kee Fung, Oppenheimer, Demissie, Yang and Walker2004; Wenckus et al., Reference Wenckus, Gao, Kominiarek and Wilkins2014). We found better outcomes, in terms of Apgar score, for vaginal delivery in first-born twins and intervention delivery in the second born. We did not confirm that cesarean section is associated with low Apgar in second-born twins as shown by Wen et al. (Reference Wen, Fung Kee Fung, Oppenheimer, Demissie, Yang and Walker2004). The mode of twin delivery should be considered on the basis of information on fetal presentation of both twins: the mode of delivery of the second born should take in account the mode of delivery of the first born.
To our knowledge, ours was the first large study evaluating genetic influences on 1- and 5-min Apgar scores. In contrast to a smaller twin study done without precise zygosity definition (Franchi-Pinto et al., Reference Franchi-Pinto, Dal Colletto, Krieger and Beiguelman1999), we did not find evidence for genetic influences on Apgar scores. The slightly higher correlations in MZ twins in our study partly correspond with the intraclass correlation coefficients reported by Riese (Reference Riese1990) for 1-min Apgar scores in a small sample of MZ and DZ twins. We did observe large influences of nongenetic factors shared by twins from the same pairs. We acknowledge that a shared environmental component could reflect to some extent a shared measurement bias (e.g., if both twins are rated at the same time by one nurse). Apgar scores represent routine clinical practice, but some of the variability could reflect heterogeneity in clinical scoring practices as opposed to true differences in biomedical outcomes (Siddiqui et al., Reference Siddiqui, Cuttini, Wood, Velebil, Delnord, Zile and Comm2017). Also, the genotype of the mother in part creates the prenatal environment of both twins and thus is part of the ‘shared environment’.
Our data do not reflect the whole population as it does not include cases with infant death. If the individual components of Apgar score (skin color or appearance, pulse rate, reflex, activity and respiratory effort) were available for analysis, it is possible that the contribution of shared and nonshared environment and genetic influence would differ across components. Twin-specific in utero environment and epigenetic factors are also of interest for future studies to examine the sources of unique environment. For understanding the variance of shared and nonshared perinatal environment, further analysis of mother’s health status and early medical support is needed.
Conclusions
We have found that for both MZ and DZ pairs, second-born twins have lower Apgar scores in comparison with first-born twins. There are different effects of pre and perinatal characteristics on 1- and 5-min Apgar score in first- and second-born twins. Based on twin analyses, a genetic component was not significant for Apgar scores. For 1- and 5-min Apgar score, about half of the variation was explained by shared and half by nonshared environmental factors. It is possible that some of the shared environment is due to the same rate scoring both twins. The most important factors for Apgar scores are GA, birth weight, birth order, zygosity, fetal presentation and mode of delivery.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/thg.2019.24.
Acknowledgments
The authors would like to acknowledge the Netherlands Organization for Scientific Research (NWO) and the Netherlands Organization for Health Research and Development (ZonMW) grants: Twin family database for behavior genomics studies (NWO 480-04-004); Twin research focusing on behavior (NWO 400-05-717); Genotype/phenotype database for behavior genetic and genetic epidemiological studies (ZonMw Middelgroot 911-09-032); ‘Why some children thrive’ (OCW Gravity program NWO 024.001.003); Netherlands Twin Registry Repository: researching the interplay between genome and environment (NWO-Groot 480-15-001/674); Spinozapremie (NWO 56-464-14192) and KNAW Academy Professor Award (PAH/6635) to DIB ; Amsterdam Public Health (APH) and Amsterdam Reproduction & Development (AR&D).
Author contributions
VVO performed the analysis and wrote the manuscript; CVD designed the statistical analysis, performed the analysis and interpreted data; CvB prepared data set for analysis and commented on the results; JVD and EZ commented on results; DIB supervised the project, designed the manuscript and interpreted the data. All the authors interpreted the results, contributed to writing the manuscript and gave their consensus for submission.
Conflict of interest
The authors have no conflict of interest to declare.