Introduction
Covert (or “silent”) brain infarcts (CBIs) are commonly identified on brain imaging in older persons without a clinically recognized history of symptomatic stroke. They can be subtyped based on location and appearance as lacunes, recent small subcortical infarcts, microinfarcts, or less often, larger subcortical infarcts or cortical infarcts; however, the majority of CBIs are small.Reference Sacco, Kasner and Broderick1,Reference Smith, Saposnik and Biessels2 In the general elderly population, 80–90% of CBIs are subcortical, and >90% of these subcortical infarcts are lacunes (Figure 1).Reference Smith, Saposnik and Biessels2 The pathophysiology of lacunes is complex, and it is a matter of ongoing debate whether some lacunes may be caused by processes other than thrombotic or embolic occlusion with subsequent infarctionReference Wardlaw, Smith and Dichgans3; however, for the purpose of this review, we include all lacunes under the umbrella term of CBI.

Figure 1: Lacune. Lesion of 8–9 mm in the right subcortical white matter, with low signal intensity on T1-weighted sequence and peripheral hyperintense rim with central hyposignal intensity on FLAIR sequence. FLAIR=fluid-attenuated inversion recovery.
CBIs are five times more prevalent than symptomatic brain infarcts. In the USA, as many as 11 million individuals may suffer from CBI every year.Reference Vermeer, Koudstaal, Oudkerk, Hofman and Breteler4–Reference Vermeer, Longstreth and Koudstaal6 Although CBIs do not exhibit acute overt clinical symptoms, they are associated with subtle deficits in physical, neurological, and cognitive function that usually go undiagnosed.Reference Sacco, Kasner and Broderick1,Reference Vermeer, Longstreth and Koudstaal6 Community-based autopsy investigations show that most of the vascular contributions to dementia risk are due to small infarcts without clinical stroke, mostly caused by cerebral small vessel disease (CSVD).Reference Schneider, Arvanitakis and Bang7 Despite their prevalence and association with diverse clinical impairments, there have been few clinical trials for CBI.
Preventing CBI may therefore be a potentially powerful way to reduce risk for cognitive decline and dementia. In this article, we briefly discuss what is known about CBI epidemiology, pathophysiology, and cognitive consequences, followed by review of risk factors for CBI (as it is logical that treating these risk factors should reduce the incidence of CBI and thus preserve cognition), enhancing cognitive reserve (as this is a potential strategy to sustain cognitive function despite CBI), and the current clinical trial literature and its limitations.
Prevalence and Incidence of CBIs
Autopsy studies are the gold standard for identifying brain infarcts. In life, CBI can be identified on computed tomography or magnetic resonance imaging (MRI), with the latter being more sensitive. CBIs are a frequent incidental MRI finding.Reference Smith, Saposnik and Biessels2,Reference Vernooij, Ikram and Tanghe8 The STandards for ReportIng Vascular Changes on NEuroimaging provides criteria for radiological identification of lacunes and recent small subcortical infarcts, appropriate for use in clinical care and research practice.Reference Wardlaw, Smith, Biessels and Bennett9
The prevalence and incidence of CBI are higher than symptomatic stroke.Reference Vermeer, Longstreth and Koudstaal6 The MRI-defined prevalence of CBI in the healthy general population is approximately 11% for ages 55–64, 22% for ages 65–69, 28% for ages 70–74, 32% for ages 75–79, 40% for ages 80–85, and 43% for ages ≥85.Reference Roger, Go and Lloyd-Jones10–Reference Bryan, Wells and Miller12 One study reported a 30–40% higher prevalence of CBI in women compared to men, but this finding has not been replicated in other studies.Reference Vermeer, Koudstaal, Oudkerk, Hofman and Breteler4,Reference Vermeer, Longstreth and Koudstaal6,Reference Howard, Wagenknecht, Cai, Cooper, Kraut and Toole11,Reference Longstreth, Bernick, Manolio, Bryan, Jungreis and Price13 Autopsy studies are concordant with neuroimaging studies in showing a high prevalence of CBI. For example, a Japanese population-based autopsy study found an overall CBI prevalence of 12.9% in persons with a mean age at death of 78.3 ± 9.5.Reference Shinkawa, Ueda and Kiyohara14 In addition to older age, other high-risk groups for CBI and CBI-associated cognitive impairment include patients undergoing cardiovascular and neurovascular surgery, and patients with sickle cell disease.Reference Vermeer, Longstreth and Koudstaal6
Pathophysiology of CBIs
The dominant view is that, like symptomatic acute ischemic stroke, most CBIs result from thrombotic or embolic arterial occlusionReference Smith, Saposnik and Biessels2 (Figure 2). In the case of lacunes and microinfarcts, thrombosis or hypoperfusion may result from arteriolar wall thickening, hyalinization, and endothelial dysfunction. Additionally, branch occlusive disease, atheroembolism from proximal arterial sources, or cardioembolism from atrial fibrillation or other cardiac diseases can occur. However, it is difficult to prove that lacunes and microinfarcts are always caused by vascular occlusion because the involved arteries are too small to visualize by conventional neuroimaging. Even at autopsy, the involved vessels may be too small to identify or may have recanalized or otherwise changed in the interval between CBI and death. High-field-strength MRI (7 T) is beginning to demonstrate acute occlusions in some patients.Reference Miyazawa, Natori and Kameda15 Other mechanisms are also probably at play. A recent comprehensive review summarizes strong evidence that blood-brain barrier dysfunction, impaired vascular reactivity, and vessel wall damage occur in patients with diffuse CSVD, and some of these mechanisms may lead to ischemia or damage the brain independent of ischemia.Reference Wardlaw, Smith and Dichgans3 Another review also suggests that ischemia is one of the mechanisms involved in lacunes.Reference Regenhardt, Das, Lo and Caplan16 A better understanding of these mechanisms may lead to complementary novel therapeutic approaches for preventing lacunar disease that are distinct from the conventional approach of blood pressure control, statins, diabetes prevention and treatment, and antithrombotic medications that have proved successful in preventing atherosclerotic stroke.Reference Bath and Wardlaw17
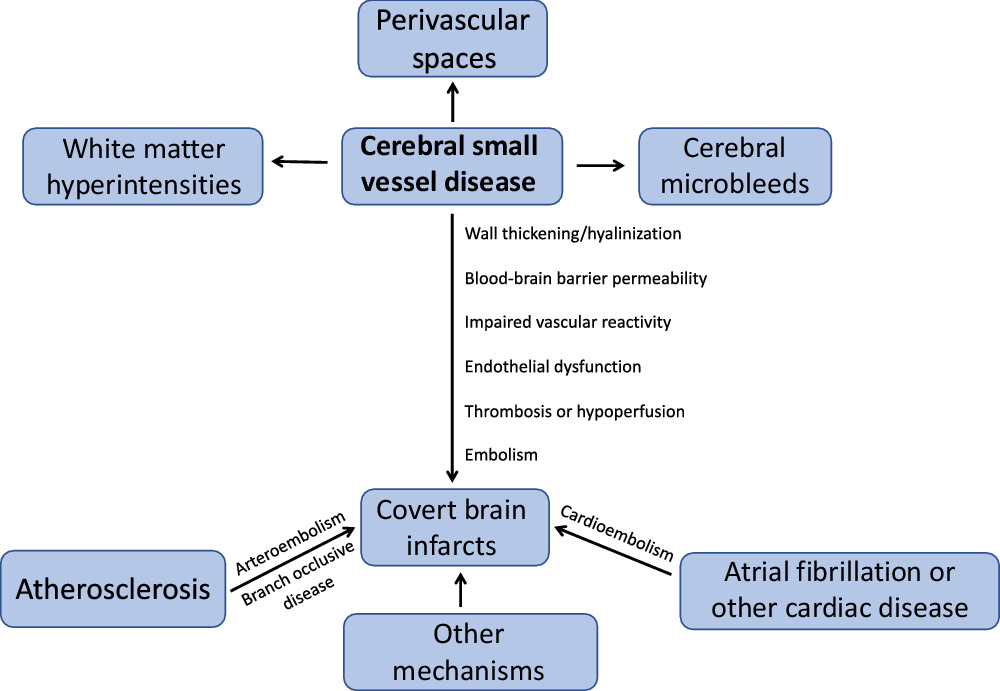
Figure 2: Pathophysiology of covert brain infarcts and cerebral small vessel disease.
CBI and Cognitive and Behavioral Impairment
CBIs are associated with cognitive impairment and dementia.Reference Vermeer, Longstreth and Koudstaal6 Cross-sectional studies generally find that the presence of CBI is associated with lower performance on cognitive screening tests such as Mini-Mental State Examination or Montreal Cognitive Assessment, and on neuropsychological tests.Reference Vermeer, Prins, den Heijer, Hofman, Koudstaal and Breteler18,Reference Warren, Weiner, Rossetti, McColl, Peshock and King19 Acute CBIs, which can rarely be detected incidentally on neuroimaging, are more common in cognitively impaired persons.Reference Saini, Suministrado and Hilal20 Microinfarcts have been associated with worse cognitive performance.Reference van Veluw, Shih and Smith21
Non-demented persons with CBI are at higher risk for future cognitive decline and dementia. Two recent systematic reviews provide pooled analyses of future dementia risk in patients with CBI. One review pooled findings from four general population studies and found a hazard ratio (HR) of 1.47 [95% confidence interval (CI) 0.97–2.22] for the association between CBI and dementia risk, while another review that included both general population and at-risk populations found an HR of 1.37 [95% CI 0.99–1.89].Reference Bos, Wolters and Darweesh22,Reference Debette, Schilling, Duperron, Larsson and Markus23
Risk Factors for CBI
Risk factors for symptomatic stroke have been heavily studied and include factors such as previous history of hypertension or blood pressure ≥140/90 mmHg, physical activity, apolipoprotein (Apo)B/ApoA1 ratio, diet, waist-to-hip ratio, psychosocial factors, current smoking, cardiac causes, alcohol consumption, and diabetes mellitus.Reference O’Donnell, Chin and Rangarajan24 While these risk factors have been heavily studied in the context of symptomatic stroke, CBI risk factors have been less studied. Risk factors for CBI have been investigated in several population-based studies and have been systematically reviewed, classifying risk factors as “strong,” “likely,” and “unclear” (Table 1).Reference Fanning, Wong and Fraser25 The “strong” risk factors were age, hypertension, metabolic syndrome, carotid artery disease, and chronic kidney disease.Reference Fanning, Wong and Fraser25 Additionally, the “likely” risk factors were coronary artery disease, heart failure, homocysteinemia, and obstructive sleep apnea.Reference Fanning, Wong and Fraser25 Treating these risk factors may reduce the incidence of CBI.
Table 1: Risk factorsReference O’Donnell, Chin and Rangarajan24,Reference Fanning, Wong and Fraser25
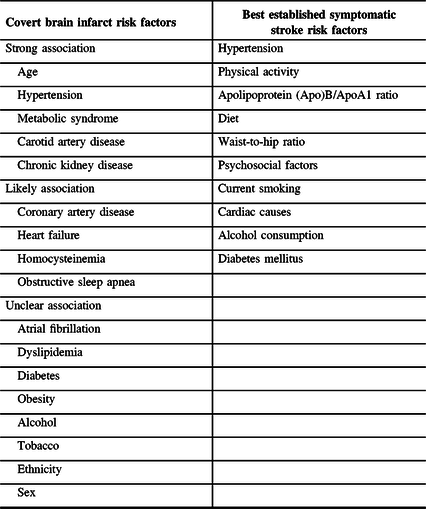
Covert brain infarct risk factors taken from the systematic review by Fanning et al.Reference O’Donnell, Chin and Rangarajan24 Symptomatic stroke risk factors taken from INTERSTROKE study by O’Donnell et al.Reference Fanning, Wong and Fraser25
These risk factors are also well-established risk factors for symptomatic stroke. However, it is unclear which factors are the most important for CBI, and how much of the overall risk they explain. In contrast, a large case–control study shows that ten risk factors account for 90% of the risk of symptomatic stroke, with hypertension accounting for the largest portion of risk.Reference O’Donnell, Chin and Rangarajan24 Despite the large degree of overlap between risk factors for both CBI and symptomatic stroke, these ten risk factors may not have the same level of importance for CBI, which is predominantly caused by CSVD, in contrast to symptomatic stroke which is predominantly caused by large artery disease or cardioembolism. Studies of the population attributable risk for CBI risk factors will be needed to identify the most important risk factors, which can then be used to guide public health prevention efforts.
A life-course approach to assessing dementia risk is increasingly being adopted, recognizing that risk factors for all-cause dementia may vary according to the life stage in which exposures were assessed.Reference Livingston, Sommerlad and Orgeta26 For example, some studies suggest that hypertension and obesity are associated with dementia risk when ascertained in midlife, but not later life.Reference Livingston, Sommerlad and Orgeta26 However, more long-term studies are required to substantiate the impact of midlife risk factors over later-life risk factors. Most studies of CBI have either been cross-sectional or have been done in the elderly, with few studies at other life stages. There is a need for longitudinal CBI epidemiology studies at different life stages, including in midlife when neuroimaging evidence of CSVD is beginning to accrue.Reference Smith, O’Donnell and Dagenais27
Another need is for data on CBI incidence and risk factors from a greater variety of populations, including in low- and middle-income countries.Reference Vermeer, Longstreth and Koudstaal6 Little is known about variation in CBI prevalence between countries and between races and ethnicities.
CBI as a Target for Preventing Cognitive Decline and Dementia
Given the high prevalence of CBI and their association with cognitive disorders, it should be considered a promising target for preventing dementia. Although this review focuses on CBI prevention, many patients with CBI also have CSVD that causes other brain lesions – including white matter hyperintensities (WMHs), cerebral microbleeds (CMBs), and enlarged perivascular spaces. These brain lesions may also be important causes of cognitive dysfunction and could warrant their own unique prevention strategies. However, in our view, CBI may be the most important focus for a few reasons. CBIs by definition are associated with tissue destruction (manifest as cavitation or encephalomalacia), while this is not clearly the case with WMH, enlarged perivascular spaces, or CMBs. Additionally, an American Heart Association statement has defined CBI as a form of “stroke,” a term that is widely understood by laypersons, patients, health professionals, health administrators, and policy makers. We consider it likely that persons will be more motivated to undertake new lifestyle, behavioral, and pharmacological interventions to prevent “covert stroke” than to prevent “white matter changes” or “larger spaces around arteries.” By focusing on “covert stroke,” the considerable public health effort to raise awareness for symptomatic stroke can be leveraged.
Preventing CBI-Related Cognitive Decline by Enhancing Cognitive Reserve
In addition to preventing CBI by addressing risk factors, enhancing resilience to CBI-related injury may be another useful approach to reducing the burden of CBI-related cognitive impairment. Interventions that enhance the brain’s ability to compensate for accruing age-related pathologies, such as CBI, could reduce the incidence of dementia even if the buildup of age-related brain pathology does not change. This concept follows from observations that cognitive performance can vary widely between persons with the same degree of pathology, such as Alzheimer’s disease.Reference Stern28,Reference Stern29 Enhancing cognitive reserve is an attractive approach to reduce the clinical burden of age-related brain pathologies, particularly those that are currently unmodifiable such as Alzheimer’s disease.
Cognitive reserve has been defined as the capacity of the brain to actively cope with brain pathologies by compensating for damage, thereby attempting to counteract the effects of these pathologies.Reference Stern28,Reference Stern29 Mechanisms for this compensation may include using brain networks more efficiently and enhancing the ability to use alternate brain networks to bypass damaged networks.Reference Stern28,Reference Stern29 Brain reserve has been defined as the structural brain features (i.e., brain size, synapse counts, or surrogates of brain size such as head circumference) that influence the clinical expression of a given level of brain pathology. Higher brain reserve is hypothesized to allow some individuals to better cope with pathologies.Reference Stern, Arenaza-Urquijo and Bartres-Faz30 With greater brain reserve capacity, more brain damage (i.e., more brain infarcts or greater brain atrophy) is required to reach the threshold for observable cognitive impairment.Reference Stern28,Reference Stern29,Reference Nitkunan, Lanfranconi, Charlton, Barrick and Markus31
Cognitive and brain reserve have been most commonly studied in the context of Alzheimer’s disease and Parkinson’s disease, and not CBI. Factors that may enhance cognitive reserve in this context include: education level, intelligence (measured by IQ), occupational attainment (professional, technical, and managerial positions), height, socioeconomic status (includes education, occupation, household income, and wealth), social participation, participation in cognitively stimulating activities, leisure time activities, and physical activity.Reference Stern28,Reference Stern29,Reference Armstrong, Naglie and Duff-Canning32–Reference Singh-Manoux, Marmot, Glymour, Sabia, Kivimaki and Dugravot37 However, it is unknown whether certain risk factors such as physical activity improve cognitive reserve through neurophysiological mechanisms or through improving vascular health.
A life-course approach to building resiliency against cognitive decline may be warranted. Similar factors may enhance resilience to CSVD pathology, but so far there are few studies. In the Leukoaraiosis and Disability (LADIS) study, higher education and higher occupational attainment were associated with weaker effects of WMH on cognition, and less decline in cognition.Reference Jokinen, Melkas and Madureira38 Education was a significant effect modifier of the effects of WMH on processing speed defined by the Stroop I (β = −0.09, p = 0.02) and Stroop II tests (β = −0.08, p = 0.04).Reference Jokinen, Melkas and Madureira38 Occupational attainment was a significant effect modifier for the effects of WMH on verbal memory measured by Immediate Word Recall (β = 0.11, p = 0.039).Reference Jokinen, Melkas and Madureira38 Similarly, education was a significant effect modifier of the effects of lacunes on Delayed Word Recall (β = −0.15, p = 0.008), while occupational attainment was a significant effect modifier for the effects of lacunes on Immediate and Delayed Word Recall (F = 6.1, p = 0.014; F = 6.17, p = 0.013, respectively).Reference Jokinen, Melkas and Madureira38 Cognitively stimulating leisure activities have also been identified as an effect modifier of the association between high WMH load and cognition, showing that high leisure activity is associated with significantly better processing speed (β = 0.15, 95% CI 0.01, 0.30), despite the presence of high WMH load.Reference Saczynski, Jonsdottir and Sigurdsson39
The few studies conducted on CBI-related cognitive decline suggest that those with high levels of education may show less cognitive impairment.Reference Vermeer, Longstreth and Koudstaal6,Reference Rapp, Espeland and Manson40 A study looked at the effect of education on cognition in the presence of ischemic lesions, finding that women with high ischemic lesion load and high education had significantly less cognitive decline measured on Modified Mini-Mental State: post high school −0.22 points/year, high school = −0.13 points/year, and less than high school −0.94 points/year.Reference Rapp, Espeland and Manson40 As mentioned above, in the LADIS study, higher education was associated with a weaker association between lacunes and lower performance on Delayed Word Recall.Reference Jokinen, Melkas and Madureira38
The relationship between reserve factors, CBI, and cognition is complex because many reserve factors, in addition to moderating the relationships between CBI and cognition, may also be risk factors for CBI or risk factors for cognitive decline independent of CBI. For example, there is evidence that late-life physical activity may increase cognitive resilienceReference Gow, Pattie and Deary41 and that childhood factors such as IQ are associated with later-life WMH burden.Reference Backhouse, McHutchison, Cvoro, Shenkin and Wardlaw42 Large datasets with advanced methods such as structural equation modeling may be needed to disentangle these complex relationships.
Clinical Trials of CBI Prevention
Because of the absence of adequately powered clinical trials, there is no consensus on how to prevent CBI.Reference Smith, Saposnik and Biessels2 Although there are no clinical trials with CBI as the primary outcome, there are a few substudies of clinical trials that include MRI in a subset of participants, with MRI-defined endpoints (Table 2). There are no definitive results from these studies because they have been underpowered to show differences. However, they do establish proof of concept that MRI endpoints are feasible in clinical trials.
Table 2: CBI clinical trialsReference Richard, Gouw, Scheltens and van Gool43–Reference Nasrallah and Pajewski46,Reference Cavalieri, Schmidt and Chen48–Reference Stephen, Liu and Ngandu50
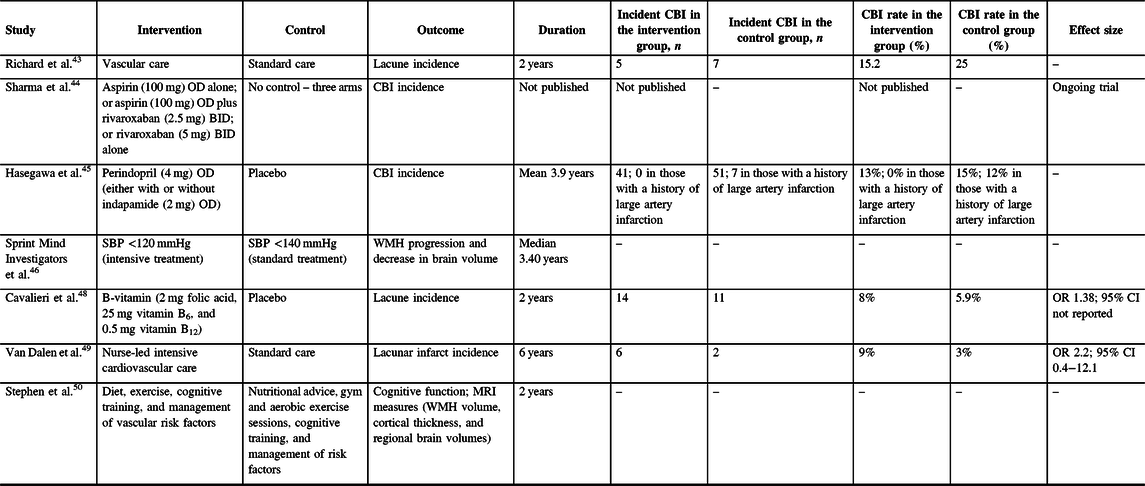
BID=twice per day; CI=confidence interval; OD=per day; OR=odds ratio; SBP=systolic blood pressure; WMH=white matter hyperintensity.
Two studies have looked at the effect of antithrombotic medications for preventing CBI. In the Evaluation of Vascular Care in Alzheimer’s Disease study, patients with mixed Alzheimer’s disease dementia and small vessel disease randomly assigned to comprehensive vascular care had a similar number of new covert lacunes compared with standard care (5/36 vs. 7/29 treated for 2 years, p = 0.26).Reference Richard, Gouw, Scheltens and van Gool43 An MRI substudy of the Cardiovascular Outcomes for People Using Anticoagulation Strategies trial is planned and the trial protocol has been published.Reference Sharma, Hart and Smith44 The MRI substudy (n = 1760) will show whether CBI incidence differed in participants randomly assigned to take either aspirin (100 mg) per day (OD) alone, aspirin (100 mg) OD plus rivaroxaban (2.5 mg) twice per day (BID), or rivaroxaban (5 mg) BID was reduced to a similar degree.Reference Sharma, Hart and Smith44 Baseline MRI showed that 34.8% had CBI, a much higher prevalence than the 4.5% with a clinical history of prior symptomatic stroke, demonstrating that MRI may be able to increase statistical power by detecting more events in fewer participants.Reference Sharma, Hart and Smith44
Randomized trials of antihypertensives have so far provided mixed results on whether blood pressure lowering reduces the risk of cognitive decline or dementia. Two such trials have included MRI substudies. In the Perindopril Protection Against Recurrent Stroke Study (PROGRESS) study, patients with a history of stroke were randomly assigned to take either perindopril (4 mg) OD, either in combination with indapamide (2 mg) OD or alone, or placebo (treated for mean 3.9 years).Reference Hasegawa, Yamaguchi, Omae, Woodward and Chalmers45 There was no significant difference in the total number of new CBI between active and placebo arms.Reference Hasegawa, Yamaguchi, Omae, Woodward and Chalmers45 However, significantly more participants developed new CBI among those who had a history of large artery infarction and received placebo, compared to those who had a history of large artery infarction and received active treatment (7/55 vs. 0/40, p = 0.02).Reference Hasegawa, Yamaguchi, Omae, Woodward and Chalmers45 An MRI substudy of the Systolic Blood Pressure Intervention Trial showed that participants randomly assigned to a systolic blood pressure target of <120 mmHg compared with <140 mmHg (treated for median 3.40 years) had less WMH progression and decreased brain volume; however, the incidence of new CBI was not reported.Reference Nasrallah and Pajewski46 Ongoing studies are testing the effects of antihypertensives on cerebral blood flow.Reference Pauls, Moynihan and Barrick47
In the VITAmins TO Prevent Stroke trial, patients with a recent history of stroke were randomly assigned to take either B-vitamins (2-mg folic acid, 25-mg vitamin B6, and 0.5-mg vitamin B12) or placebo for 2 years.Reference Cavalieri, Schmidt and Chen48 Despite a significant reduction in median WMH volume change in a subset of participants with severe baseline CSVD who received B-vitamins compared to placebo (0.3 vs. 1.7 cm3, p = 0.039), there was no significant difference in the number of new lacunes on MRI between participants who received B-vitamins compared to placebo (8 vs. 5.9%, p = 0.43).Reference Cavalieri, Schmidt and Chen48
Two trials of comprehensive vascular risk reduction for dementia prevention have included MRI substudies. In the Prevention of Dementia by Intensive Vascular Care trial, a 6-year nurse-led intensive cardiovascular care regimen did not reduce the incidence of new lacunar infarcts on MRI (n = 126; 64 randomized to active treatment) compared with control intervention (odds ratio (OR) 2.2, 95% CI 0.4–12.1, p = 0.36; six intervention participants and two control participants had new lacunar infarcts).Reference van Dalen, van Charante and Caan49 The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability trial showed that nutrition, exercise, cognitive training, and management of vascular risk factors (2-year intervention) improved cognitive function; a small MRI substudy of 112 patients did not find differences in WMH volume between groups but the effect on infarcts was not reported.Reference Stephen, Liu and Ngandu50
Trials for treating vascular dementia have recently been systematically reviewed.Reference Smith, Cieslak and Barber51 Interventions included vasodilators, antithrombotic medications, and neuroprotectants.Reference Smith, Cieslak and Barber51 However, none of the reviewed trials reported whether these interventions prevented new brain infarcts.Reference Smith, Cieslak and Barber51
Conclusions and Future Directions
CBIs are highly prevalent with aging, and the appearance of new CBI is associated with cognitive decline and dementia. They share many risk factors with symptomatic stroke. However, the epidemiological story of CBI is not complete. More data are needed on CBI incidence, and from non-Western countries and non-White populations. Associations with conventional vascular risk factors are well established, but more data are needed on lifestyle and behavioral risk factors. A life-course approach is needed to examine how exposures to risk factors in different stages of life influence the risk of later-life CBI.
The associations of modifiable vascular risk factors with CBI suggest that they may be preventable. However, this can only be proven by clinical trials. Given the expense of MRI, we consider it unlikely that widespread MRI screening will be part of the solution to reducing CBI-related cognitive decline. Instead, research is needed on how to identify persons at risk for CBI using feasible screening methods (including medical history and blood pressure) followed by interventions proven by clinical trials to reduce CBI incidence and enhance cognitive reserve. This targeted screening strategy should be accompanied by a complementary public health strategy to promote better brain health, free of CBI, informed by epidemiological research identifying the most important risk factors for CBI throughout the life span.
Acknowledgments
We would like to thank Dr. Feryal Saad for providing us MRI images of CBI.
Conflict of Interest
Ms. RD has nothing to disclose. Dr. EES reports consulting fees from Portola Pharmaceuticals and Alnylam Pharmaceuticals, and royalties from UptoDate. Dr. MDH has no disclosures directly relevant to this manuscript. Outside the submitted work, Dr. MDH reports personal fees from Merck, non-financial support from Hoffmann-La Roche Canada Ltd, grants from Covidien (Medtronic), grants from Boehringer-Ingleheim, grants from Stryker Inc., grants from Medtronic LLC, and grants from NoNO Inc. In addition, Dr. MDH has a patent Systems and Methods for Assisting in Decision-Making and Triaging for Acute Stroke Patients pending to US Patent office Number: 62/086,077 and owns stock in Calgary Scientific Incorporated, a company that focuses on medical imaging software, is a director of the Canadian Federation of Neurological Sciences, a not-for-profit group, is a director of Circle NeuroVascular Inc., and has received grant support from Alberta Innovates Health Solutions, Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, and National Institutes of Neurological Disorders and Stroke.
Statement of Authorship
RD wrote the draft and revised the manuscript. MDH and EES edited and revised the manuscript.








