INTRODUCTION
Cryptosporidiosis is the most common reported cause of outbreaks of gastrointestinal disease linked to mains water supply and the fourth commonest reported cause of gastrointestinal infection in the United Kingdom [Reference Furtado1]. Certain characteristics of cryptosporidia such as low infective dose, single host life-cycle, resistance to chlorination and prolonged survival of the oocyst in the environment, make it an efficient cause of waterborne outbreaks. Disease can be severe in immunocompromised patients. The lack of effective specific treatments, and public anxiety leading to a loss of confidence in public water supplies add to its public health importance.
Following two major outbreaks of water-related cryptosporidiosis [Reference Morgan2, 3], the UK government strengthened regulations to minimize the risk of Cryptosporidium contaminating drinking water. Continuous monitoring was introduced for treatment plants assessed as posing a significant risk of cryptosporidiosis. Monitoring is against a standard of <1 oocyst/10 l of water. The Drinking Water Inspectorate (DWI) reports that none of the 146 307 samples taken between 2000 and 2003 exceeded the treatment standards, with most samples below 0·02 oocysts/10 l. The DWI was not aware of any outbreaks associated with water supplied from water treatment works identified as being at significant risk [4]. We describe an outbreak of gastroenteritis due to Cryptosporidium hominis in a population of 158 558 served by a mixture of water from a groundwater source and a surface water-treatment plant at significant risk, and where the continuous monitoring samples never exceeded treatment standards. Five water treatment plants serve the area covered by the Portsmouth office of the Hampshire & Isle of Wight Health Protection Unit (H & IOW HPU) with a population of 459 014. Four of them supply ground water and one a mixture of surface and ground water. This last supply has been assessed as posing a significant risk and is continuously monitored.
Thirty-five cases of laboratory-confirmed cryptosporidiosis were reported between 1 November and 31 December 2002 compared to the usual level of about 11 cases during these 2 months. An outbreak investigation and control team was promptly established.
METHODS
All faecal samples from the area were routinely tested for Cryptosporidium at the local laboratory using modified Ziehl–Nielsen staining. Positive samples were referred to the national reference laboratory for confirmation and speciation. Laboratory results, including full speciation and patient demographic details, were obtained and supplemented by direct contact with referring clinicians where incomplete. The case definition was any person who was resident in the catchment area of the Portsmouth office of the H & IOW HPU with diarrhoea with a date of onset between 1 November and 31 December 2002 and confirmed C. hominis infection. Exclusion criteria were travel outside the United Kingdom in the 2 weeks prior to onset of diarrhoea, secondary cases, defined as anyone having had contact with other individuals with diarrhoea in the 2 weeks prior to onset of illness, and cases suffering from chronic diarrhoea.
An epidemic curve was plotted and the distribution of cases in relation to water supply areas was mapped. The local water company's 24-h Cryptosporidium monitoring programme results were reviewed.
A case-control study was undertaken to assess hypotheses raised by the descriptive epidemiology. Because cases fell into three discrete groups (children, civilian adults and naval personnel) controls were frequency matched within these three groups. For naval personnel, we selected other personnel in the same living accommodation or work-place. For civilians we selected individuals in the same age group (within 5 years of the case) from the same general practice list. We aimed to recruit twice as many controls as cases in each group. A modification of the Bouchier questionnaire was used [5]. The main modifications were the removal of animal exposure questions and the addition of a range of questions concerning supply of food, use of local restaurants and takeaways and exposure to any new or unusual foods. Civilian cases and controls (or parents for children) were interviewed by telephone where possible. This was supplemented by face-to-face interviews for individuals who could not be contacted by telephone. Face-to-face interview of naval personnel still present locally was supplemented by a postal questionnaire.
Cases and controls were compared in terms of age, sex, method of interview, and distribution among the three study groups to assess comparability. Univariate analyses were performed by tabulation and χ2 testing for linear trend and rank sum testing to assess association with risk and possible confounding factors. Sex, age and all factors with associations stronger than P=0·2 were considered in the multivariate analyses using logistic regression. Stepwise exclusion removed one at a time on the basis of the largest P value >0·1. When the final model was obtained each of the initially included exposures, age and sex were re-entered singly to assess evidence for confounding or significant association with reported cryptosporidiosis in the reduced model. Multivariate analyses were performed using the full dataset and separately for the adult and child study groups.
RESULTS
Twenty-nine cases met the case definition. The other six incident cases were excluded because of recent contact with another individual suffering from diarrhoea (five cases) or a history of foreign travel (one case). Cases formed three distinct groups – naval personnel (11 cases, 38%), civilian adults (three cases, 10%) and children (aged <14 years, 15 cases, 52%). The mean age was 18 years (range 6 months to 58 years). The attack rate of 13·2/100 000 compares to ∼2/100 000 over this 2-month period in other years. The incidence peaked during the fourth week in November. Fourteen cases (48%) reported illness with onset during this week (Fig. 1).

Fig. 1. Cases of cryptosporidiosis by onset date in South East Hampshire, November–December 2002 (n=29).
The water company's 24-h Cryptosporidium monitoring programme had recorded Cryptosporidium oocyst concentrations of <1/10 l of water, which is the regulatory standard, throughout October and November with completely negative sampling on 49 days, samples <4/1000 l on 10 days and samples showing 5/1000 l on 13 November and 8/1000 l on 28 November. There were no increases in water turbidity levels or changes in the pH, and no work was carried out on the water reservoir before or during the outbreak. No problems had been observed in the water treatment process.
Twenty-four cases (83%) lived in one water distribution area, which receives mixed surface and ground water. Three areas (population 300 456) which receive water supplies from other plants using only ground water recorded no cases. Five cases were recorded in an area that usually receives ground water but received temporary supplies from the plant associated with the other cases during the outbreak period (Fig. 2).

Fig. 2. Geographical distribution of cryptosporidiosis cases and areas supplied by each reservoir, South East Hampshire, November–December 2002 (n=29).
The strong geographic association of cases with supplies from one water treatment plant led to our primary hypothesis that low level contamination of drinking water might have caused the outbreak. A local food source was also a plausible explanation and formed the secondary hypothesis tested in our analytical study.
Parents of 13 of the 15 children participated and 26 controls were also recruited. All cases and 23 controls were interviewed by telephone and three controls face to face. Mean age of cases (3·5 years) and controls (3·9 years) was similar. All civilian adult cases were either not contactable or refused to participate. Six civilian adult controls were interviewed. Only five cases and four controls participated from among naval personnel. Most of the selected personnel had been deployed on duty in the Gulf of Arabia in the run up to the 2003 invasion of Iraq. The imbalance between cases and controls in each of the civilian and naval adult populations was also associated with different modes of questionnaire administration to adult cases and controls (50% of adult cases but no adult controls were interviewed face to face and 60% of adult controls, but no adult cases were interviewed by telephone). Multivariate analyses are presented separately for adults and children as well as overall since the validity of the child component of the study population is far stronger.
Exposures with associations statistically stronger than P<0·2 are shown in Table 1. The only exposure with a strong positive association with reported cryptosporidiosis is quantity of water consumed at home. Two cases and controls reported eating raw shellfish, which resulted in a weak positive association. Some other foods showed negative associations. Potential risk factors assessed for which statistical support was weaker (P⩾0·2) included age, sex, attendance at school or nursery, family size, travel in the United Kingdom, local shops, local restaurants, local takeaways, drinking milk, using water filters, consumption of ice, outdoor water contact, nine other specific food types and eating of any ‘new or unusual foods’. There was no association with consumption of bottled water or water consumed outside the home (P>0·5 in all cases).
Table 1. Exposures associated (P<0·2) with reported Cryptosporidium and degree of association for main water exposures (18 cases, 36 controls)

OR, Odds ratio; CI, confidence interval.
* Association with tomato eating is for frequency of tomato eating rather than whether or not tomatoes were eaten.
† χ2 test for trend.
‡ Fisher's exact test.
§ Rank sum test.
In the multivariate model (Table 2) only water consumed at home (expressed as number of 200 ml glasses per day and modelled as a continuous variable) and use of swimming pools were strongly associated with reported Cryptosporidium infection. There was no evidence for interaction between these two exposures. There was no evidence for substantial confounding by age, sex, or other measured exposures. Mean water consumption at home was 6·5 glasses per day among cases and 3·2 among controls. Thirty-nine percent of cases and 17% of controls reported use of swimming pools. Separate analysis by age group (child or adult) showed similar findings for water consumption with some suggestion of swimming pool risk being limited to children, although formal interaction testing indicates that the statistical evidence for a difference between adults and children is weak (P=0·18). One swimming pool was used by three cases and one control. Nine other pools were used by one study participant each (four cases and five controls).
Table 2. Association between reported cryptosporidiosis, water drinking and use of swimming pools from multivariate analysisFootnote *, Portsmouth, 2002
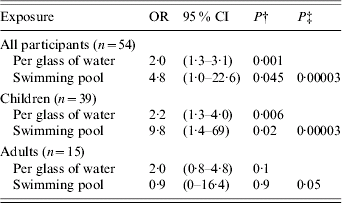
OR, Odds ratio; CI, confidence interval.
* Results are from a model including water consumption and swimming pool use.
† Probability of an association as extreme as that seen with this exposure if no true association exists (Wald test for each exposure in the model).
‡ Probability of this two-exposure model having as strong an association with illness by chance alone as was observed (likelihood ratio testing of this model against the null model).
DISCUSSION
This outbreak of cryptosporidiosis showed a striking geographical pattern. This pattern was related to water supply. No other geographically patterned risk factors were obvious although a common leisure exposure to water or a localized source of food contamination could have produced a similar pattern. A case-control study showed a strong association with quantity of tap water consumed at home and an association with swimming pool use (Table 2). Cases reported drinking an average of 3·3 glasses of water more than controls. Although there may be differential recall among cases and controls or altered behaviours due to illness the difference reported was substantial. This is strongly supportive of a waterborne source for infection in this population. All cases drank tap water at home and some controls did not. The lack of statistical evidence for association between cryptosporidiosis and ‘fact of’ as opposed to the ‘quantity of’ water consumption is inevitable in an outbreak of this size in a population where drinking some tap water is very common. There may have been some transmission in swimming pools during the outbreak, particularly among children, but there is no evidence to implicate pools as a major or sustaining cause of the outbreak. There was no evidence for any other risk factors, geographically patterned or otherwise, despite detailed questions seeking local food and other exposures.
Our primary hypothesis from the descriptive epidemiology was that the risk would be associated with water consumption at home. There was no association with water consumption at work or elsewhere. Given that the area of suspected contamination was small, the work-place of many adults is likely to be outside this area, but children's schools and nurseries inside it. The affected children were young and histories were from their parents, which limited the quality of information on water consumption outside the home compared with information on water consumption at home. The low participation rate for adult cases and controls was a feature of difficulty in contacting individuals, especially among naval personnel, many of whom had moved on during this period. In combination with resultant differences in method of data acquisition between adult cases and controls these practical difficulties compromise the adult component of the case-control study, making the extent to which the adult controls represent the study base from which the adult cases arose and the comparability of the information obtained uncertain. However, the results in terms of the effect size of water consumption are remarkably similar among adults and children and even analysis restricted to the child cases and controls shows a strong association between water consumption and disease. The quality issues in the adult component of the study are not therefore likely to have led to the observed association.
The problems associated with the microbiological examination of water in cases of suspected waterborne gastrointestinal outbreaks are well documented [Reference Tillet, DE Louvois and Wall6]. There have been several reports of the continuing risk of gastrointestinal illness from water that apparently complies with current microbiological definitions of quality [Reference Barrell, Hunter and Nichols7, Reference Payment8]. This is because current quality definitions rely on total coliforms, faecal coliforms (E. coli) and total colony counts [Reference Gleeson and Gray9], and whilst most of these are killed by chorination, protozoan parasites such as Cryptosporidium oocysts are not [Reference Zmirou10]. Under these circumstances, there is more reliance on evidence from descriptive epidemiology, which apart from helping to inform the hypothesis may provide the only plausible link to the cause and route of infection. However, in this case, as well as no general evidence for microbiological contamination there had been detailed monitoring for Cryptosporidium with all results more than 10 times lower than national standards. Nonetheless the combination of descriptive and analytical epidemiological findings strongly implicates this drinking water in causing an outbreak of cryptosporidiosis. There are previous reports of waterborne outbreaks of cryptosporidiosis associated with water sources that are thought to be particularly wholesome and of good quality, such as treated boreholes [Reference Morgan2, Reference Willocks11] and in one of these, the oocyst concentrations were low [Reference Morgan2]. We believe the Portsmouth outbreak is associated with the lowest reported oocyst concentration in the literature. Such observations question the evidence base for the current normative standards of water quality for Cryptosporidium. Although the current UK legislation on Cryptosporidium recognizes the risk of waterborne outbreaks by the organism by explicitly setting standards using a single pathogen parameter (compared with the current bacteriological parameter for coliforms), the evidence for the standard of 1 oocyst/10 l of water appears weak [4, Reference Payment8, 12]. It may be that such an approach is effective in minimizing the risk of large, easily detectable, and high profile outbreaks while leaving a considerable burden of disease due to smaller outbreaks and sporadic cases. Relying on such monitoring in the absence of an evidence base of what the standards mean in terms of disease burden is therefore questionable. In the implicated water plant a decrease in the proportion of water from a surface source was adopted as a short-term measure.
Although systems such as membrane filtration remove Cryptosporidium more effectively than the sand filtration applied to this supply they are more expensive. It is difficult for water companies to justify this cost to shareholders or price regulators when their water is meeting current standards. Estimates of the potential disease burden remaining where water treatment meets current standards but without complete removal of oocysts are needed to inform a debate on the appropriateness of the current monitoring approach. It may be that additional infrastructure and process regulations are needed for identified at-risk sources as well as monitoring. This outbreak indicates that the disease burden in at-risk sources meeting the current standard may not be negligible and that work to estimate it should be treated as a priority. Otherwise the existence of such a standard may be a barrier to the implementation of available technologies to further protect the public from cryptosporidiosis.
DECLARATION OF INTEREST
None.






