INTRODUCTION
Toxoplasmosis is a worldwide zoonosis due to the protozoan parasite Toxoplasma gondii. T. gondii can infect a wide range of animal species and has a complex life-cycle which involves felids as definitive hosts, many mammals and bird species as intermediate hosts and an environmental stage (oocysts). Approximately one in four people are infected with T. gondii [Reference Larkin1], and although most infections are asymptomatic, the social and economic impact of toxoplasmosis is estimated to be comparable to that of major foodborne diseases [Reference Kijlstra and Jongert2]. Severe clinical symptoms occur mainly after mother-to-foetus transmission and in immunocompromised patients when T. gondii infection, or reactivation of a latent infection, occurs [Reference Tenter, Heckeroth and Weiss3].
Humans may become infected by ingesting either oocysts of T. gondii, after contact with contaminated soil, water or raw vegetable, or infective tissue cysts containing bradyzoites, in raw or undercooked infected meat [Reference Hill4]. The relative importance of these two routes of contamination may vary in populations, depending on food habits and geographical location [Reference Kijlstra and Jongert2]. Therefore, knowledge of sources of infection for a given population is a prerequisite for toxoplasmosis risk prevention. Because the parasite may infect any mammalian or bird species, any meat is a potential source of human infection. In particular, the consumption of game has been found to be a risk factor of infection for pregnant women [Reference Cook5]. The wild boar (Sus scrofa) is a species that carries a particular risk, being one of the leading species consumed, and because the wild boar are frequently infected [Reference Diderrich6–Reference Antolová, Reiterová and Dubinský10]. Moreover, populations of wild boar have dramatically increased during the three last decades in Europe [Reference Schley and Roper11].
In this paper we study the presence of toxoplasmosis in wild boar in the French Mediterranean island of Corsica, where an increasing wild boar population is also observed [Reference Klein12]. The consumption of hunted wild boar meat is traditionally developed on the island [Reference Casabianca and Albin13]. Moreover, cooking practices are changing (F. Casabianca, personal communication), with an increase in barbecue cooking where the meat is not fully cooked. However, no data on toxoplasmosis in animals exist for this insular region. In a perspective of risk analysis, the first aim of the current study was to estimate the prevalence of toxoplasmosis in the wild boar population of Corsica.
We also aimed to analyse the local variations of prevalence, since a better knowledge of the conditions affecting the presence of the parasite in wildlife is necessary in order to better estimate the risk of toxoplasmosis in humans in relation to game meat consumption. Factors associated with variations of seroprevalence have not been studied in the wild boar. We hypothesized that wild boar acquire toxoplasmosis by rooting and feeding from soil contaminated with oocysts excreted by cats, as shown for other species with similar behaviour, e.g. poultry [Reference Ruiz and Frenkel14], or by incidentally ingesting infected rodents. We thus expected seroprevalence in wild boar to vary locally according to the concentration of oocysts in the soil used by wild boar, which itself should depend on cat density, the presence of areas where wild boar may be in contact with cats' habitat, and oocyst survival. We used farm density as an estimator of cat density, because farms constitute areas where cat groups may grow up due to the presence of prey and shelters [Reference Warner15]. To estimate the probability that a wild boar comes in contact with soil or a rodent contaminated by oocysts from cat faeces, we estimated the density of edges between cat habitat and wild boar habitat using information on land cover. Finally, because oocyst survival depends on local meteorological conditions (precipitation and temperature) [Reference Frenkel, Ruiz and Chinchilla16], we used meteorological data to characterize local physical conditions. We then used these three variables as potential explanatory factors of seropositivity in wild boar.
MATERIALS AND METHODS
Study area and sampling
The study involved the entire island of Corsica (8680 km2) (42° 9′ N, 9° 5′ E), i.e. a mountainous island mainly constituted of parallel valleys oriented north-east/south-west. The mean altitude of the island is 568 m, with a culminate point at 2710 m (Monte Cintu). It is constituted of 360 administrative counties (communes in French), with areas varying from 0·8 to 203 km2 and mean altitude ranging from 19 to 1554 m.
On the island, the wild boar's habitat is mainly constituted by sclerophyllous vegetation (maquis), non-deciduous (conifers) and mixed mountain forest. More than 20 000 wild boars are hunted each year on the island [Reference Saint-Andrieux and Barboiron17].
Samples were collected throughout island with the help of volunteer hunters during two consecutive hunting seasons: August 2006 to January 2007 (year 1) and August 2007 to January 2008 (year 2). At carving, hunters removed the diaphragm muscle and placed it into plastic bags. The samples were kept frozen and transported to the National Reference Laboratory for Foodborne Parasites in Maisons-Alfort (Northern France). For each animal sampled, hunters recorded the county of hunting, gender and age. Age was judged on the basis of coat colour and estimated body weight.
Serological analysis
Muscle fluid was obtained from 25 g of diaphragm cut into small pieces and frozen overnight at −20°C in a plastic bag. After thawing at room temperature, the meat juice was collected with a pipette into a microtube as previously described [Reference Nöckler18]. The modified agglutination test (MAT) for the detection of T. gondii-specific immunoglobulin (IgG) antibodies was performed as previously described [Reference Dubey and Desmonts19].
Muscle fluid from year 1 samples were analysed using antigen from a commercial kit Toxoscreen® (bioMérieux, France). Fluids were diluted at titres of 1:4, 1:20 and 1:400. The manufacturer's instructions were followed except the threshold dilution, which was 1:4 and not 1:40 due to the lower concentrations of antibodies in muscle fluids compared to sera [Reference Nöckler18, Reference Le Potier20, Reference Nielsen21]. Year 2 samples from were analysed using formalin-fixed whole RH tachyzoites as antigen, and fluids were serially diluted twofold from titre 1:2 up to titre 1:128. Formalin-fixed whole RH tachyzoites were provided by the Biological Resource Centre for Toxoplasmosis, Laboratoire de Parasitologie, Reims, France. Muscle fluid samples reactive at a titre ⩾1:4 were considered indicative of T. gondii infection.
A cross-validation test of 120 randomly selected samples was performed in order to determine the agreement between the two MAT tests. The level of agreement was determined using the kappa statistic interpreted according to the usual scale: <0·4. poor agreement; 0·4–0·74, fair agreement; >0·74, good to excellent agreement [Reference Fleiss, Levin and Paik22].
Epidemiological analysis
Design of the statistical analysis
We estimated seroprevalence (percentage of seropositive individuals) in wild boar and then searched for factors influencing the probability of carrying antibodies. The statistical unit was the wild boar.
Since the antigens used in MATs for the two consecutive hunting seasons were different, we analysed the results of years 1 and 2 separately.
We also separated data according to the age class: we defined two age classes, corresponding to juveniles (<1 year) and adults (⩾1 year), and tested if seroprevalence depended on age using a Pearson χ2 test. Data were analysed separately for juveniles and adults because information on each age class brings distinct information. In wild boar, births mostly occur in March–May and July–August [23], while hunting occurs from August to January. Juveniles thus provide information on the risk of T. gondii infections during the year of capture, while infections in adults cannot be attributed to any particular period. We thus built four models: juveniles/year 1 (n=64), juveniles/year 2 (n=171), adults/year 1 (n=425) and adults/year 2 (n=739).
For each of the four models, we analysed the relationship between potential explanatory variables and antibody carriage, considered as a binary outcome. Since boars were located at the county level, values of environmental variables were estimated at the same level. Thus, all individuals hunted in a given county had the same value for environmental variables (see below for description of the environmental variables considered).
We first tested if seroprevalence varied between counties, using a log-likelihood ratio (G test) test of independence [Reference McDonald24]. As we grouped together individuals from the same county, we also checked that data were not over-dispersed by estimating the dispersion (or scale) parameter of the null model, computed as the residual deviance divided by the degrees of freedom. Then each of the four models was built using a binomial-logistic regression, including all variables and first-order interaction terms between altitude and the two other environmental factors (see below). To select significant variables, we simplified the model using a backward approach based on Akaike's Information Criterion (AIC) [Reference Burnham, Anderson, McCullough and Barrett25]. When models had similar AIC values (ΔAIC<2), we retained the most parsimonious model, i.e. the one with fewest parameters. The overall fit of the final logistic equation was assessed using a Pearson goodness-of-fit test [Reference Dohoo, Martin and Stryhn26]. Odds ratios (ORs) and 95% confidence intervals (CIs) were computed to quantify the association between each variable and serological T. gondii status. For each final model we calculated the part of inter-county variability explained by the final model using R 2 deviance [Reference Cameron and Windmeijer27], computed as the ratio of the null model deviance minus the final model deviance on the null model deviance minus the deviance of the model with county as an explanatory factor.
Statistical tests were considered significant if the P value was <0·05. All statistical analyses were performed using R software [28].
Variables describing landscape at the county level
We obtained estimates at the county level for the three factors that may be involved in the risk of toxoplasmosis in wild boar: farm density, edge density between cat and wild boar habitats, and meteorological conditions.
Farm density
We used farm density as an estimate of cat density because farms are a privileged area for the constitution of large groups of cats. Moreover, farms may serve as reservoirs of toxoplasmosis infection because of the frequent presence of rodents and cats [Reference Kijlstra29], and the high resistance of oocysts in moist and shaded environments of farm sites [Reference Weigel30, Reference Lehmann31]. We obtained the number of farms per county from the French Ministry of Agriculture (online AGRESTE data, www.agreste.agriculture.gouv.fr). We considered all farms regardless of the precise production involved, and we calculated farm density as the ratio of the number of farms in the overall area of the county. We expected seroprevalence to increase with farm density.
Edge index between wild boar and cat habitats
In order to calculate an index corresponding to the density of ecotone – or edge – between wild boar and cat habitats, we used the Corine Land Cover (CLC) map (IFEN® data) with a resolution of 0·25 km2. The CLC classifies land cover and use into 44 classes. We grouped these 44 CLC classes into five categories to characterize the openness of the landscape and urbanization: forests; mid-open areas (maquis, moors and heathland, transitional woodland, fruit plantations and olive groves); open areas (arable and cultivated land, vineyards, pastures, natural grasslands); urban areas (artificial surfaces); and others (beach, bare rocks, burnt areas, salines, water bodies). As populations of the European wildcat, Felis silvestris, are considered as residual in Corsica [Reference Fabrizy32], we only considered domestic cats to which we attributed patches of urban and open areas, both being their areas of residence or hunting [Reference Germain, Benhamou and Poulle33]. We attributed patches of forests and mid-open areas to wild boar habitat [Reference Meriggi and Sacchi34, Reference Boitani35]. Within each county, the edge index was calculated as a ratio of the sum of lengths of interface between wild boar areas and cat areas on the overall area of the county. We expected seroprevalence to increase with edge index, i.e. with increasing density of ecotone between wild boar and cat habitats. Analyses of the landscape composition were performed using ArcView 3.2a (ESRI Inc., USA). Calculation of the edge index was performed using the Shapefile package of R software.
Meteorological conditions
Meteorological conditions may affect oocyst survival, especially through desiccation when conditions are dry and hot [Reference Frenkel, Ruiz and Chinchilla16, Reference Barbier, Ancelle and Martin-Bouyer36]. We obtained meteorological data on rain (mean precipitation in summer) and temperature (mean temperature in summer) in 2006 and 2007 from MeteoFrance®. However, these data were collected from 20 field stations scattered over the island, and not at the county level. We thus used the relationship between altitude and meteorological conditions: in Corsica, due to the strong altitudinal gradient, both rain and temperature are correlated with altitude. We first verified these relationships for summers 2006 and 2007. We then used altitude of the centroid of the county as an indicator of precipitation and temperature conditions. We expected seroprevalence to increase with altitude.
In order not to impose a linear relationship between the seropositive response (logit scale) and each independent continuous environmental covariate (edge index, farm density, altitude), we tested them as discrete factors, after transformation into three modalities of equal sample size. In each model we included first-order interaction terms between altitude and the two other factors; because altitude describes meteorological conditions whereas farm density and edge index deal with the local landscape we hypothesized that both aspects could interact.
RESULTS
Sampling plan
We collected 1399 muscle samples, 489 during year 1 and 910 during year 2 (Fig. 1). Samples came from 129/360 counties in Corsica, with 1–251 samples per county (24 counties with one sample and 74 with a maximum of five samples). Fifty-three counties were sampled during the 2 years.
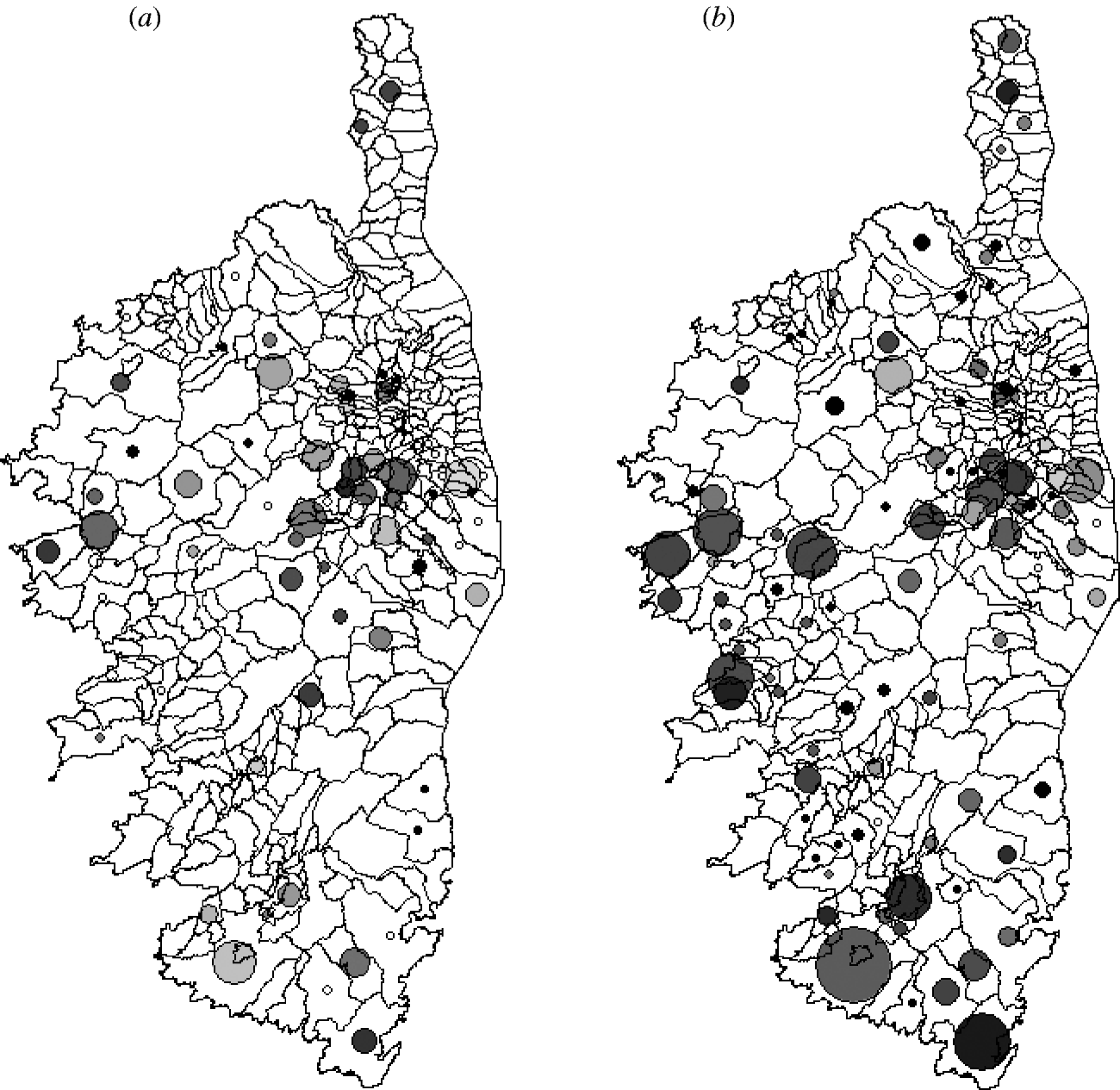
Fig. 1. Spatial distribution of toxoplasmosis in wild boar collected in Corsica from 2006 to 2008: (a) year 1 (hunting season 2006–2007), (b) year 2 (2007–2008). Circles are positioned at the centroid of the corresponding sampled county. The diameter of the circles is proportional to sample size in the county (from 1 to 98 samples) and the level of shading to seroprevalence (0% white; 100% black).
Serological results
The agreement between the two MATs was good to excellent (kappa=0·87) (Table 1). The overall seroprevalence during year 1 was 0·55 (95% CI 0·50–0·59) and was significantly higher than during year 2 (0·33, 95% CI 0·29–0·35) (Pearson χ2=64·415, P<0·001) (Table 2).
Table 1. Agreement between modified agglutination test (MAT) using commercial antigen (Ag) Toxoscreen® (used for year 1) and MAT using antigen from the French Biological Resource Centre for Toxoplasmosis (BRC) (used for year 2)
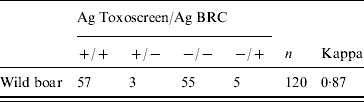
Table 2. Sample size and seroprevalence for Toxoplasma gondii in wild boars collected during year 1 (2006–2007) and year 2 (2007–2008) in Corsica (n=1399). Results are presented per age class and gender, and overall seroprevalence is given with 95% confidence interval (CI)

Epidemiological analysis and explanatory factors
Seroprevalence was lower in juveniles (0·45 and 0·25 for years 1 and 2, respectively) than in adults (0·56 and 0·35), but the difference was statistically significant for year 2 only (Pearson χ2=2·365 and 5·802, respectively, P=0·124 and P=0·016). For both years, there was a significant variability in the spatial distribution of seropositivity at the county level (G test statistic=163·498 and 197·416, respectively, both P<0·001).
As expected, meteorological conditions were related to altitude (Fig. 2). Altitude was correlated positively with mean rainfall in summer (r=0·881 and 0·453 for years 1 and 2, respectively, P<0·001 and P=0·045) and negatively with mean temperature over the period from June to August (r=−0·756 and −0·659, both P<0·001).
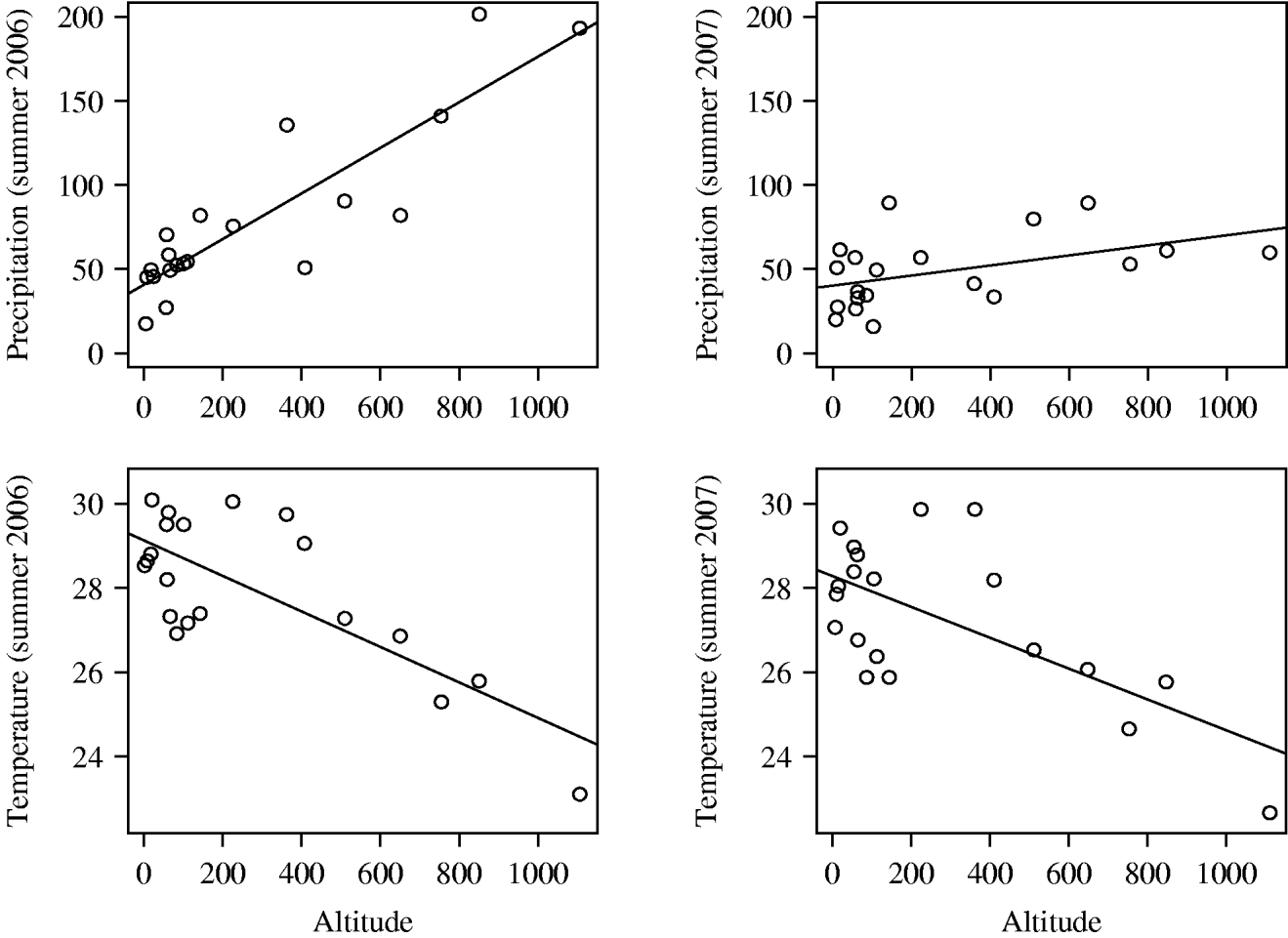
Fig. 2. Relationship between altitude and average temperature or precipitation for summers 2006 and 2007 in 20 meteorological stations from Corsica (MeteoFrance® data).
The results of the four models were different (juveniles/year 1, juveniles/year 2, adults/year 1 and adults/year 2). For both models on juveniles, no factor was significantly related to seroprevalence. In year 1 adults, edge index had no effect, but seroprevalence was related to farm density (Table 3): the probability of being seropositive for an adult increased with the farm density in the county of hunting. The odds ratio for being seropositive was 2·7 (95% CI 1·65–4·43) for areas with the highest farm density (⩾0·45 farms/km2) compared to the areas with low farm density (⩽0·22 farms/km2). The Pearson goodness-of-fit test indicated that the final model fit was adequate (P=0·449). The model including farm density as unique risk factor explained 9·4% of the spatial heterogeneity at the county level. In year 2 adults, we found an effect of farm density but in a farm density×altitude interaction a nonlinear effect for farm density was found (Table 3). The probability of being seropositive was highest when both farm density and altitude were high (farm density ⩾0·47 farms/km2 and altitude ⩾573 m), and lowest at high altitudes but low farm density. The odds ratio for counties with high farm density and high altitude was estimated as 25 compared to counties with low farm density and high altitude. Moreover, we detected an effect of edge index, which was also estimated to be nonlinear: wild boars were most often seropositive when edge index was low (OR 2·45 for low edge index). The selected model fitted observed values (Pearson goodness-of-fit test, P=0·361) and explained 19% of the spatial heterogeneity at the county level.
Table 3. Coefficients of the multivariable logistic regression models selected to explain Toxoplasma gondii seropositivity in adult wild boar from Corsica [for each modality, parameter estimate with its standard error (s.e.), P value of the Wald test and the adjusted odd ratio (OR) with 95% confidence interval (CI)]
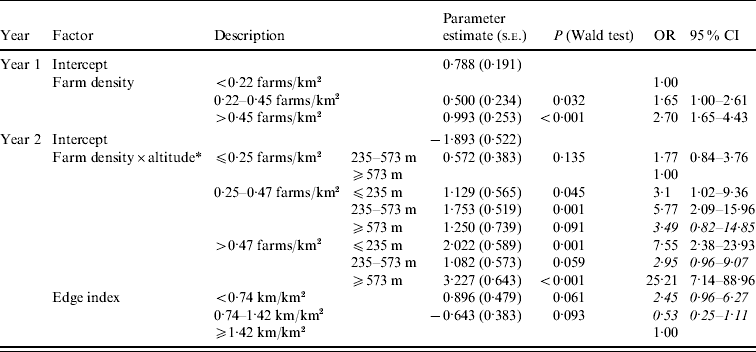
* No county had low farm density and low altitude.
Non-significant ORs are in italics.
DISCUSSION
To our knowledge the current study is the first survey to associate T. gondii seroprevalence in a game species with environmental risk factors. We observed a high overall seroprevalence, ranging from 33% to 55%, depending on the year of sampling, and a high level of spatial heterogeneity that could be partially explained by environmental features, especially farm density, altitude and edge index.
Seroprevalences are in agreement with data from previous studies using MAT to detect T. gondii infection in sera of wild boar (cut-off titre 1:25): in the USA, 18–37% [Reference Diderrich6, Reference Dubey7] of wild boar were found with antibodies to T. gondii, and 38·4% in Spain [Reference Gauss8]. Several other studies also showed that toxoplasmosis is common in wild boar in Europe, even if results are less comparable since distinct methods or thresholds are used: 19% in Austria (indirect immunofluorescence test) [Reference Edelhofer, Prosl and Kutzer37], 15% in the Czech Republic (Dye test, threshold 1:4) [Reference Hejlicek, Literak and Nezval38] or 26·2% (indirect fluorescence antibody test) [Reference Bártová, Sedlák and Literák9] in the Czech Republic and 8·1% in the Slovak Republic (commercial ELISA) [Reference Antolová, Reiterová and Dubinský10].
The MAT with a cut-off titre 1:25 has been validated to detect IgG antibodies to T. gondii in sera of domestic pigs [Reference Dubey39]. In order to detect antibodies to T. gondii in muscle fluid in the current study, we chose the threshold 1:4 because previous studies indicated that antibodies are diluted at least tenfold in muscle fluid compared to serum [Reference Nöckler18, Reference Le Potier20, Reference Nielsen21]. The use of this threshold may have led to an over-estimation of the seroprevalence in the current study. Nevertheless the results of the analysis of explanatory factors were stable using a lower threshold (data not shown).
Seroprevalence was significantly different between the two study years. This interesting feature may be linked to climatic variations between the two consecutive years. Afonso et al. [Reference Afonso, Thulliez and Gilot-Fromont40] showed that seroprevalence in cats varied among years, cats being most often seropositive when temperature and precipitation prior to capture were high. High temperature and dryness decrease the survival of oocysts [Reference Frenkel, Ruiz and Chinchilla16, 41]. In Corsica, precipitation during summer 2007 was lower than during summer 2006 (975 mm vs. 1572 mm of water cumulated for the 20 main meteorological stations of Corsica; MeteoFrance data). The difference in prevalence may be related to drier climatic conditions during the second year of sampling. Moreover, domestic cats roam further away from farms when temperatures are high [Reference Germain, Benhamou and Poulle33]. The dissemination of T. gondii in the environment may be higher in these conditions. Variations of contamination of the environment and variations in survival conditions of parasite infective stages in external environment could explain the significant difference of seroprevalence we detected in wild boar in the sampling years. More data on the temporal variability of toxoplasmosis are required to further study the temporal variability of toxoplasmosis risk.
We investigated environmental risk factors at the county level, whereas, for a given individual host, risk factors are acting at the level of its home range, which is generally smaller than a county, but can be variable and encompass parts of several counties [Reference Keuling, Stier and Roth42]. However, this scale is appropriate to describe local landscape management, especially via agricultural practices. Under the same assumption, Afonso [Reference Afonso43] demonstrated that antibody prevalence in European wildcats was related to the number of farms per county. In agreement with the hypothesis that farm density is an indicator of the presence of favourable areas for cats and toxoplasmosis, we showed that antibody prevalence in adult wild boar is related to farm density in the county of capture. Moreover, we found a synergic interaction between high altitude and high farm density. In Corsica, the number of outdoor pig-breeding farms is highest at high altitudes (Fig. 3). Swine farms are reservoirs of T. gondii infection [Reference Weigel30, Reference Lehmann31] and, at high altitudes, high precipitation levels are observed, which favours the survival of oocysts in the environment [Reference Frenkel, Ruiz and Chinchilla16]. In Corsica, agricultural areas located at high altitude meet all conditions for the intense propagation of the parasite, and wild boar hunted in these areas are particularly at risk of transmitting infection to consumers.
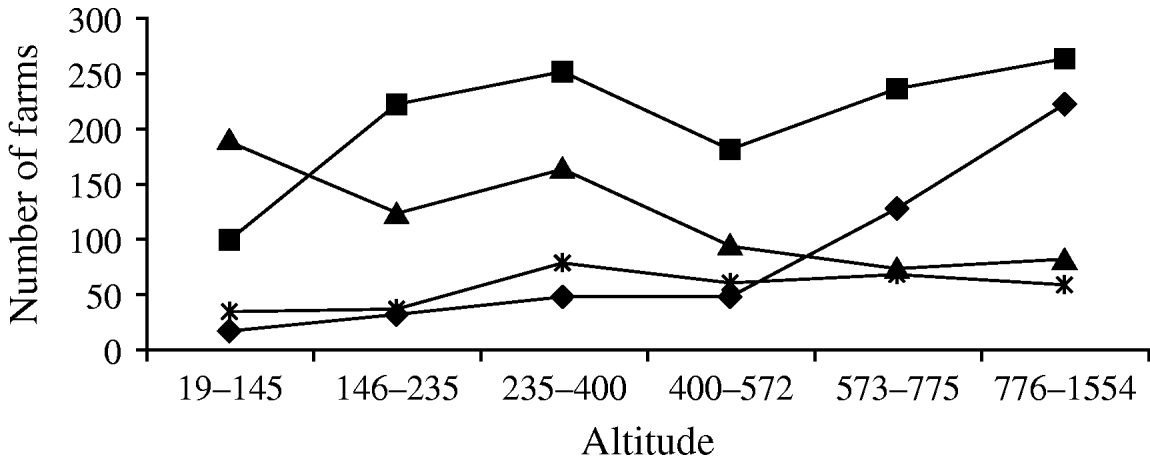
Fig. 3. Relationship between altitude and number of farms with pigs (–◆–), cattle (–▪–), sheep (–▴–) and goats (–*–) in Corsica (data from French Ministry of Agriculture and Fishing). We defined the same three altitude classes (19–235 m, 236–572 m and 573–1554 m) as used in the logistic regression analysis, then each class was separated in two parts with an identical number of counties.
Contrary to our prediction, the probability of an adult wild boar being infected by T. gondii is highest at low edge index, i.e. in counties with a low level of landscape fragmentation. The edge index was expected to quantify the presence of places where infective contacts may occur between wild boar and areas frequented by cats. However, patches smaller than 0·25 km2 are not considered in the CLC data. We thus underestimated the level of fragmentation in counties with patches below this size. Moreover, urbanized areas can be smaller than 0·25 km2, as it is the case for small villages where the agricultural landscape is favourable to high cat density [Reference Pita44]. In our dataset, a main part of the counties with a low edge index corresponds to counties with small villages where toxoplasmosis might be favoured. Counties with a low edge index also tend to be situated at high altitude in Corsica (r=−0·28 between edge index and altitude), where swine farms are frequent and meteorological conditions favour oocyst survival (Fig. 3). Finally, the confusion between edge index, patch size and altitude may explain the counter-intuitive result found, and this confusion could not be disentangled at this level. This result underlines the need for investigation at a fine scale to better understand how landscape composition could model the wild boar–cat interface and influence the inter-specific transmission of T. gondii.
The datasets concerning juveniles did not enable us to detect any risk factor. One explanation may be the lack of statistical power due to too few animals being tested. Moreover, this population may be homogeneous regarding the risk of infection, and differences due to the environmental heterogeneity may appear only in adults. This would be in accord with the results found in adult populations where 9% and 19% of the variability in counties is explained by the variables tested. An important part of variability thus remains to be explained, possibly by other factors that we could not investigate here, e.g. direct estimation of cat density and infestation, local meteorological variables at the county level, or soil burden of oocysts.
The consumption of raw or undercooked meat is the main route of infection in humans in Europe, representing 30–63% of infections depending on the country considered [Reference Cook5]. Specifically, pork meat is considered as one of the major sources [Reference Dubey and Beattie45]. However, over the last two decades, infection in pigs decreased dramatically with changes in pig production and management [Reference Tenter, Heckeroth and Weiss3], which raises the question of the role of other species, including game, as a source of human infection. Traditionally, wild boar meat is consumed after lengthy cooking, which kills tissue cysts [Reference Dubey, Petersen and Ambroise-Thomas46]; however, consumption of raw or undercooked meat is increasing. In Corsica, raw and salted pork meat products are traditionally prepared, and may contain wild boar meat [Reference Casabianca and Albin13]. The curing of meat does not affect the parasite immediately and the survival time of tissue cysts varies with the concentration of the salt solution and the storage temperature [Reference Dubey47]. Finally, salting does not kill all tissue cysts in home-made pork sausages [Reference Tenter, Heckeroth and Weiss3]. Although the origin of most human infections cannot be documented precisely, acute toxoplasmosis has been reported in hunters following consumption of undercooked meat from wild pigs [Reference Choi48]. Thus, while prevalence of T. gondii in pork meat decreases, game meat should not be neglected as a significant and possibly increasing source of toxoplasmosis. However, the assessment of the risk related to wildlife is only possible through a better understanding of the complex life-cycle of T. gondii in the natural environment. This step can be achieved by combining information on toxoplasmosis, land use by intermediate and definitive hosts as well as physical characteristics of the environment, at different spatial and temporal scales.
ACKNOWLEDGMENTS
We thank all the volunteer hunters, especially Oscar Maestrini, for collecting field samples, the two Veterinarian Departmental Laboratories of Corsica for their technical help, and the Biological Resource Centre for Toxoplasmosis in Reims for formalin-fixed whole RH tachyzoites used in MAT. We thank Rémi Bouche and François Casabianca from INRA LRDE in Corte for their material and scientific support. This work was financially supported by Programme Bioscope (ANR 05 SEST 048-02) from the French National Research Agency and by the French Ministry of Agriculture and Fishing.
DECLARATION OF INTEREST
None.










