Normal duration and timing of sleep is vital for mental and physical health and yet the majority of patients on a psychiatric ward experience sleep disturbance.Reference Muller, Olschinski, Kundermann and Cabanel1 Sleep disturbance is an independent risk factor for suicide.Reference Goldstein, Bridge and Brent2,Reference Pigeon, Pinquart and Conner3 Poor sleep is often attributed to the psychiatric disorder requiring in-patient treatment, but is also attributable to the ward environment. Noise, light and regular, typically hourly, overnight observations disturb patients independent of the psychiatric diagnosis.Reference Horne, Hay, Watson and Anderson4 Primary sleep disorders, including obstructive sleep apnoea and restless legs syndrome, are increased in psychiatry patients and also disturb sleep.Reference Stubbs, Vancampfort, Veronese, Solmi, Gaughran and Manu5 For a subgroup of patients, insomnia-specific cognitive–behavioural therapy can be effective within an acute psychiatry ward.Reference Sheaves, Isham, Bradley, Espie, Barrera and Waite6
Nursing observations are designed to enhance care and reduce risk, but may paradoxically increase sleep disturbance. To date, little research has focused on the effect of and need for regular overnight observations within psychiatry. Understandable concern about patient safety is one reason for frequent physical checks, despite no direct evidence that fixed timing checks reduces risk.Reference Veale7,Reference Flynn, Nyathi, Tham, Williams, Windfuhr and Kapur8
After review of current observation policy across a large mental health trust, a pilot scheme to enhance sleep was introduced (the SleepWell programme). This was a package including reduction of overnight noise and light, formal staff education about sleep and sleep disorders, a protected sleep period for those deemed safe and screening for sleep apnoea and restless legs syndrome for all in-patients. There was a service evaluation of adverse events, including harmful behaviours before and during the change in night-time observations, alongside detailed patient, staff and carer feedback over the assessment period. Cognitive–behavioural therapy for insomnia (CBTi) was made available to two of the seven wards that undertook the pilot. Issuing data of hypnotic medication was assessed before and during the pilot period.
Method
This was a service evaluation of a pilot scheme to enhance sleep and adjust overnight nursing policy. As such, formal ethical approval was not required, but the design, safety and existing trust operating procedures were reviewed before commencement, by the medical staff committee, the trust board and an existing trust safety programme ‘positive and safe’, which was aimed at reducing restrictive interventions while managing challenging or violent behaviour. Seven adult wards across one large mental health trust (Cumbria, Northumberland, Tyne and Wear NHS Foundation Trust) were used for the pilot. To ensure a range of patients they included a 16-bed male and two 16-bed female adult acute in-patient units based on two separate sites, a 26-bed long-stay rehabilitation unit with shared house, a 16-bed mixed neurorehabilitation ward, a 12-bed in-patient dementia service and an 18-bed psychiatric rehabilitation and recovery unit.
Staff training
Two designated staff representatives from each unit were identified as sleep leads to facilitate development and delivery of the necessary practice changes. Before implementation there was education about sleep and sleep disorders from K.N.A., and creation of an educational package labelled as SleepWell. The service evaluation was designed as a 6-month intervention, with 3 months to identify and educate sleep leads and then 3 months of the SleepWell programme in place with protected sleep during this time. Project supervision and clinical governance came within ‘positive and safe’, with monthly reports from all sleep leads.
Ward environment and protected sleep time
The ward environment was assessed for all pilot wards and all staff reported weekly during the 3-month pilot on night noise reduction measures. Eye masks and ear plugs were offered to all suitable patients. The trust estates department were involved for wards that required any adjustment to soft-closing bins and doors. Non-caffeinated drinks were offered to the patients in the evening. Set wake-up and bed times and reduction in large meals before bed was encouraged. An agreed addition to the assessment tool was developed to highlight those safe for protected sleep time after at least 72 h on the ward. This was set at 00.00–06.00 h. There was in addition screening for obstructive sleep apnoea, using the validated STOPbang screening questionnaire.Reference Chung, Subramanyam, Liao, Sasaki, Shapiro and Sun9 A score of >3 indicates an >50% chance of having obstructive sleep apnoea. Screening for restless legs syndrome was undertaken with a single validated screening question with additional prompt to differentiate from drug-induced akathisia.Reference Ferri, Lanuzza, Cosentino, Iero, Tripodi and Spada10 The SleepWell pathway is shown in Fig. 1. This included asking all patients ‘Is sleep a problem for you?’. On every pilot ward, information about the SleepWell project was displayed and all patients and carers were informed about the change in policy on admission (shown in Supplementary Appendix 1 available at https://doi.org/10.1192/bjb.2020.30). For those with persistent insomnia on two of the acute wards (with adequate and trained psychology provision), CBTi was offered on a weekly basis as a small group therapy and, following existing published protocols developed from the Oxford Ward Sleep Solution study, this is modified to allow for the in-patient setting and encompasses education about sleep, sleep hygiene, sleep scheduling and relaxation, but does not use sleep restriction.Reference Sheaves, Isham, Bradley, Espie, Barrera and Waite6

Fig. 1 SleepWell algorithm used on all wards. CBTi, cognitive–behavioural therapy; MDT, multidisciplinary team; SSRI, selective serotonin reuptake inhibitors.
Feedback and review of incidents and hypnotic prescribing
The quantity of hypnotics (zopiclone, temazepam, melatonin and promethazine) issued to each ward was examined across two time periods: January to March 2019 (the SleepWell pilot time period) and January to March 2018 (before the SleepWell intervention). The specific number of patients deemed safe for protected sleep time and the number who completed sleep disorder assessments were also recorded. Interviews with staff and patients across all wards provided feedback for qualitative analysis of the intervention. Incident rates are routinely recorded within the trust by incident report forms (IR1) via an electronic incident reporting system. The number and type were looked at during the time of the pilot and for a further 5 months after this period, and compared with a similar 8-month period before the SleepWell pilot. A comparison between incidents recorded over 24-hour periods and specifically during the protected sleep time was made. Ongoing review of the incident data continued after the service evaluation for an 8-month period in total, as all pilot wards elected to continue protected sleep time. No patient-identifiable data were used at any stage.
Results
Protected sleep time
After assessing those who needed more frequent observations for reasons of physical health or safety, an average of 50% of patients were able to have protected sleep time during their in-patient stay (range 44.3–60%); the data for the different wards is summarised in Table 1. The psychiatric rehabilitation and recovery ward was excluded from the data below because patients were not on hourly observations as standard, but instead had established protected sleep time of 00.00–07.00 h living within long-stay flats and houses.
Table 1 In-patients on the six wards during the 3 month SleepWell pilot where there was a change to night-time observations

Adverse events during protected sleep time
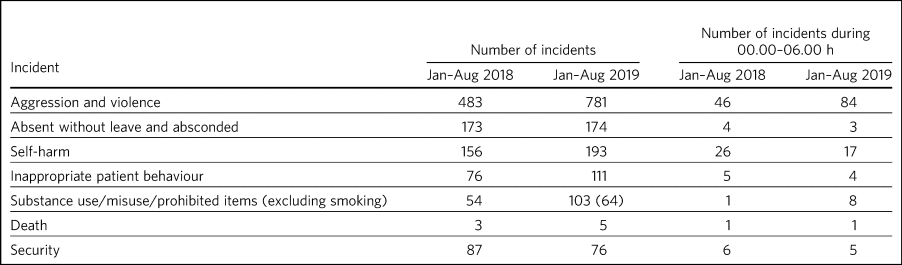
Incident data was compared between 1 January 2018 to 31 August 2018 and 1 January 2019 to 31 August 2019, which included the SleepWell protected sleep period. Far fewer incidents occurred at night in any of the different incident categories both in 2018 and 2019. No serious adverse event, suicide attempt or suicide occurred as a result of the overnight protected sleep period. The deaths that occurred were in-patients on the psychiatric rehabilitation and recovery and dementia wards and were attributed to an expected decline in physical health conditions. A single death occurred in an elderly patient overnight, but this was off-site in an acute medical ward and was attributable to perforated bowel. Across all seven pilot wards, the total number of patients absent without leave or absconding decreased during the SleepWell pilot. Serious incidents requiring security decreased and self-harm decreased during the night, although not during the day, as did inappropriate patient behaviour at night. Aggression and violence increased between 2018 and 2019, with most of the incidents reported on the male high-dependency unit (264 of 781 incidents). It should be noted that there was a trust-wide implementation of a no-smoking policy at the beginning of 2019, and an increase in aggression and agitation was noted (mostly related to wanting to smoke on the trust premises). The results are summarised in Table 2.
Table 2 Adverse events before and during the SleepWell pilot
Hypnotic issuing before and during the SleepWell pilot
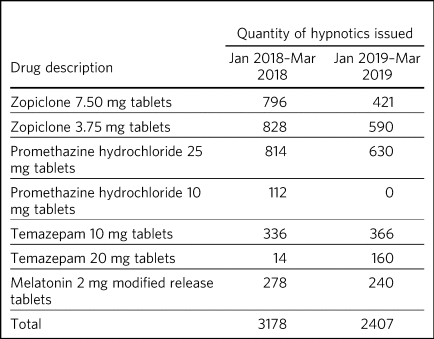
Review of hypnotics use (Table 3) showed a 25% decrease in the quantity of hypnotics ordered to the wards during the SleepWell pilot period. There were specific decreases in zopiclone 7.5 mg and 3.75 mg tablets, promethazine hydrochloride 10 mg and 25 mg tablets and melatonin 2 mg modified release tablets. There was an increase in temazepam 10 mg and 20 mg tablets, but the total number of all prescriptions issued decreased. Specific ward issuing data highlighted that six of the seven wards ordered less hypnotics from pharmacy.
Table 3 Hypnotic issuing during the SleepWell pilot compared with a similar time period before the pilot
Sleep environment
The input from estates varied upon the different wards, but included blackout blinds and dimmer lights to ensure light levels throughout the night were kept to a minimum. Soft-closing doors were fitted on two wards. Loud-closing bins were identified and replaced. Eye masks and ear plugs were available on request for all wards. The Rehabilitation and Recovery Unit was structured in bungalows, with fewer noises and disturbances, and so little adaptation was therefore necessary. The volume of night nurse calls was reduced to a minimum and staff were encouraged to report any issues relating to noise/light promptly during weekly meetings.
Posters were put on walls to remind staff and patients about the need try to keep the noise levels to a minimum at night-time. Carers and staff were invited to feedback on changes and given information about sleep and sleep hygiene (included in the Supplementary Appendices).
Staff and patient feedback
Before
One key theme running throughout the feedback collected before the pilot was about the negative effect the ward environment had on patient sleep. The main environmental factors noted were noise, temperature, lighting, bedding and other patients.
After
Post-pilot feedback from ward staff was universally positive and many commented that the ward environment was more peaceful and settled. A focus on bed-time routines was perceived by staff as helping better sleep and, overall, this was well-supported by staff, although there was initial anxiety before implementation about not checking on patients and assessing risk caused some disagreement about which patients were safe to go onto protected sleep. More standardised sleep assessments were not possible within the framework of a service evaluation primarily assessing safety and feasibility. Making this a documented, multidisciplinary team decision helped to reassure staff alongside involving the night coordinators.
Patients who did comment preferred being on protected sleep time: some did not notice a difference and had not been woken, but others expressed feeling safer without people looking into the room, and those who had been readmitted described it as better than previous admissions. All carers were positive about the intervention, with none asking for more frequent observations to be restarted. No patients or carers had concerns about the protected sleep time. All in-patients were asked about the SleepWell programme and typical comments from patients from all of the wards are summarised in Supplementary Appendix 2 but included ‘better than last admission’, ‘I feel safer now’ and ‘I don't worry about people looking into my room on a night’.
Screening for sleep disorders
The numbers documented as screened were small on the acute wards despite encouragement throughout the period of SleepWell. A total of 39 out of 125 and 37 out of 79 patients were assessed for obstructive sleep apnoea and restless legs syndrome on the acute adult wards, respectively, with nine positive screens for obstructive sleep apnoea (STOPbang score >3). Using the screening tools, no sleep disorders were identified on the rehabilitation wards or the dementia unit.
CBTi
CBTi was implemented on two adult acute wards (one male and one female), with 25 patients assessed as suitable based on length of stay, problematic insomnia and ability to attend therapy. Thirteen (52%) then accepted therapy and attended at least four sessions.
A total of 85 admissions came to the two wards during the 3-month assessment period; only 27 were suitable and approached, 18 accepted and 13 completed. The majority not suitable were either transferred or due for imminent discharge, but 15 had decline in mental state and were unable to engage in therapy. All treated had either paranoid schizophrenia, psychosis or depressive disorder, reflecting the typical case mix of the two wards. Mean insomnia severity index before treatment was 18 (range 6–28), and completion insomnia severity index was 14 (range 6–16).
Discussion
In this pilot study, a protected sleep time and improved education around sleep were safely incorporated into a personalised care plan for adult psychiatric in-patients. There were no serious adverse events or deaths related to the change in policy, ward demands for hypnotics were reduced and both staff and patient feedback was positive.
For psychiatry patients, sleep disturbance is an independent risk factor for suicide,Reference Goldstein, Bridge and Brent2,Reference Pigeon, Pinquart and Conner3 and has been shown to independently predict lower quality of life, higher symptom severity and less benefit from treatment, with Kallestad et al suggesting that sleep should be seen as a ‘stand-alone therapeutic entity, rather than an epi-phenomenon of existing diagnoses’.Reference Kallestad, Hansen, Langsrud, Ruud, Morken and Stiles11 However, there have been few systematic studies of the factors that adversely affect sleep on in-patient units. A large, questionnaire-based survey showed 66% of in-patients had poor sleep quality independent of gender or diagnosis.Reference Muller, Olschinski, Kundermann and Cabanel1 Previous work from our acute in-patient, psychiatry wards demonstrated high levels of objective sleep disturbance and suggested that a number of environmental factors within the ward, including noise levels at night and hourly observations, were disruptive and therefore paradoxically may be worsening mental health and delaying recovery.Reference Horne, Hay, Watson and Anderson4 Measures to stabilise sleep by using a high-intensity, modified version of CBTi has been shown to be possible and highly effective in acute psychiatry in-patients,Reference Sheaves, Isham, Bradley, Espie, Barrera and Waite6 although it was carried out maintaining overnight hourly observations.
The need to protect sleep as part of treatment has to be set against the importance of a safe level of observations for those at high risk of harm from mental or physical health problems. The National Institute for Health and Care Excellence guidelines define various levels of observation determined by a risk assessment, especially for severe self-harm, suicide, violence and absconding.12 Within physical health units, this initial assessment is rapidly followed by a personalised care plan, allowing for a step down to a protected sleep period where possible. This is shown to balance prevention of acute physical health deterioration with a minimum of intrusive night-time observations. The National Institute for Health and Care Excellence defined the purpose of observation as to ‘provide a period of safety… with observation levels set at the least restrictive level, for the least amount of time’.12
With specific regard to suicide risk, sleep deprivation owing to frequent checks may still be justified if it can be shown to reduce the frequency of suicide or severe self-harm. However, 91% of those who commit suicide do so while under intermittent observation,Reference Powell, Geddes, Deeks, Goldacre and Hawton13 and the most recent review from the National Confidential Enquiry into SuicideReference Flynn, Nyathi, Tham, Williams, Windfuhr and Kapur8 emphasised the avoidance of routine, non-personalised checklists. A recent review of the timing of suicide data highlighted a far lower risk of suicide occurring overnight during the night periods of 23.00–07.00 hReference Veale7 and challenged the perceived benefit of frequent observations. Despite these recommendations, frequent and typically hourly checks throughout the night remain widespread across acute mental health trusts throughout the UK.
Psychiatric nursing observations remain fundamental to the emotional and physical support of the patient, and current guidelines advise ‘minimising the extent to which patients feel they are under surveillance, while encouraging communication, listening, and conveying to the patient that they are valued and cared for’.Reference Powell, Geddes, Deeks, Goldacre and Hawton13,Reference Bowers, Gournay and Duffy14 This guidance is somewhat in contrast to typical night-time observations, which require the staff member to clearly see the patient is breathing. This can involve opening the window hatch in the door or entering the bedroom and shining a torch on the patient's face, switching on a light or physically waking the patient.15 Patient and staff feedback highlighted complaints about the intrusive nature of checks and dislike of the observation policy. During the pilot, support for nursing staff was vital so that staff felt protected and supported to change a policy that might expose them to criticism. In practice, only 50% of patients were deemed safe to be placed on protected sleep, with others requiring more regular observation and input for physical or mental health needs. This still allowed a greater level of necessary engagement for night staff for patients requiring more support or observation for their safety. However, the detailed work required to reassure staff before implementing the policy took an average of 3 months alongside the monthly meetings during the project. It is of note that all wards elected to continue the protected sleep period after the initial service evaluation.
A wide range of incident data is collected across the trust, and the main aim of the pilot was to use this data to show that serious adverse physical or mental health events were not increased in those on protected sleep time, and that there were no serious adverse events in those patients on protected sleep as an important safety measure. It would remain important to have ongoing monitoring of safety for those on protected sleep time and a flexible protocol that allows for any patient to have increased frequency of observation if there was clinical concern. Longer-term assessments would be required to assess for a consistent change in behaviour or any sustained improvements in night-time agitation.
High rates of obstructive sleep apnoea are found in those with severe mental illness, with a prevalence of 25% reported across all psychiatric disorders and the highest frequencies seen in major depressive disorder.Reference Stubbs, Vancampfort, Veronese, Solmi, Gaughran and Manu5,Reference Anderson, Waton, Armstrong, Watkinson and Mackin16 Risk factors for obstructive sleep apnoea include male gender, age >55 years, reports of sleepiness and obesity and the STOPbang questionnaire has recently been validated as an effective screening tool in the psychiatric population.Reference Knechtle, Economou, Nikolaidis, Velentza, Kallianos and Steiropoulos17 An in-patient admission is an opportunity to assess physical health, with increasing recognition of the poor cardiometabolic health of many patients with psychiatric disease.Reference Barber and Thornicroft18 Obstructive sleep apnoea screening should ideally be part of this screening or at least considered as a modifiable cause of poor sleep. Use of the STOPbang questionnaire in our pilot remained challenging, with small numbers of STOPbang scores recorded in records. This may reflect acutely unwell patients or the number of other assessments also required for this group; however, those who were screened were often at risk, which allowed further investigation and lifestyle advice.
Hypnotics carry a risk of diversion and respiratory depression in overdose. Those issued hypnotics while on a psychiatry ward in the UK will typically remain on them at discharge, with a substantial percentage still using them at 12 months.Reference Johnson, Nassr, Harpur, Kenicer, Thom and Akram19 A recent review of the side-effects and benefits of a range of hypnotics highlights the limited evidence base of antihistamines in particular, and the potential for dependency. There is also a falls risk in the elderly.Reference Wilson, Anderson, Baldwin, Dijk, Espie and Espie20 The total number of hypnotics issued to the wards decreased by 25% during the 3-month pilot period. This may reflect some hypnotic prescribing being partly attributable to a noisy environment and the observations themselves. However, the analysis did not include patient-level data, so future work would be needed to look at individual prescriptions over longer periods of time. The change in prescribing may also reflect increased knowledge of non-pharmacological strategies to manage poor sleep and the improved ward environment.
There are several limitations to this small study. Standardised sleep assessments were not undertaken, partly because of the variable ward populations and need to assess initial feasibility of protected sleep time. It was not possible to assess any effect on duration of in-patient stay or whether different mental health diagnoses were more or less able to have protected sleep time. Although a small number were able to have CBTi, many were excluded because of short-stay rehabilitation, highlighting the need to communicate to community teams for follow-up therapy. No cases of restless legs syndrome were detected, which likely reflects the lack of recognition of this syndrome and the need for more training. This pilot was designed to evaluate patient safety first and foremost, but a future, much larger trust-wide research study is underway to address patient-level data regarding diagnoses, patient-level prescribing data and patient stay for those on protected sleep time compared with those not on protected sleep time. Although some categories of incident increased, including aggression, this was felt to relate to factors outside of SleepWell, including the particular patient group on the high-dependency male rehabilitation ward and the implementation of the trust-wide smoking ban. It should be noted that even within this increase, far few incidents of any type occurred during the 00.00–06.00 h time window.
In summary, this is the first pilot trial within a UK adult psychiatry unit to formally evaluate the feasibility and safety of a protected sleep period. A trust wide review of the observation policy is now underway, but any personalised care plan for a patient should include an evaluation of the patient's sleep. Improving and stabilising sleep disturbance should be part of routine in-patient psychiatric care, with a personalised assessment of the risk versus the benefit of waking the patient at night.Reference Veale, Ali, Papgeorgiou and Gournay21
About the authors
Chloe Novak is a psychology undergraduate in the Department of Psychology at Newcastle University, UK. Emma Packer is an undergraduate in biomedical sciences in the Department of Psychology at Newcastle University, UK. Alastair Paterson is a pharmacist in the Department of Psychology at Cumbria, Northumberland, Tyne and Wear NHS Foundation Trust, UK. Ambrina Roshi is a speciality trainee in psychiatry in the Department of Psychology at Cumbria, Northumberland, Tyne and Wear NHS Foundation Trust, UK. Rosie Locke is a psychology research assistant in the Department of Psychology at Cumbria, Northumberland, Tyne and Wear NHS Foundation Trust, UK. Patrick Keown is a consultant psychiatrist and Associate Medical Director at In Patients South in the Department of Psychology at Cumbria, Northumberland, Tyne and Wear NHS Foundation Trust, UK. Stuart Watson is a consultant psychiatrist and academic clinical senior lecturer in the Department of Psychology at Cumbria, Northumberland, Tyne and Wear NHS Foundation Trust and in the Department of Psychology at Newcastle University, UK. Kirstie N. Anderson is a consultant neurologist and honorary clinical senior lecturer with the Regional Sleep Service at Newcastle upon Tyne Hospitals NHS Foundation Trust and in the Department of Psychology at Newcastle University, UK.
Supplementary material
Supplementary material is available online at http://doi.org/10.1192/bjb.2020.30.
Data availability
Data are available from the author.
Author contributions
C.N. led on project design and development of all SleepWell material and CBTi. E.P. analysed incident data and contributed to manuscript writing. A.P. contributed to analysis of all prescribing data, manuscript preparation and review. A.R. contributed to project design, dissemination of SleepWell and data analysis. R.L. contributed to qualitative feedback and data analysis. P.K. contributed to project design, implementation and assistance with manuscript preparation. S.W. contributed to project design and implementation, supervision of students and manuscript preparation. K.N.A. contributed to project conception, design, development of SleepWell material, supervision, data analysis and manuscript preparation.
Declaration of interest
None.
ICMJE forms are in the supplementary material, available online at https://doi.org/10.1192/bjb.2020.30.








eLetters
No eLetters have been published for this article.