Introduction
Antibiotics, since their discovery, have been used at therapeutic levels for the treatment of diseases, and at sub-therapeutic levels as growth promoters in animal feeds to improve production. Antibiotics had been considered as essential additives/supplements for better growth and maintaining gut ecosystem balance (Huyghebaert et al., Reference HUYGHEBAERT, DUCATELLE and VAN IMMERSEEL2011) for more than 50 years in poultry production. This supplementation was widely practiced for decades until questioned due to increasing frequency of resistance to antibiotics in chicken (Kabir, Reference KABIR2009) along with dwindling efficacy in humans (Dibner and Richards, Reference DIBNER and RICHARDS2005). In the past 25 years, 38 new pathogens have emerged, of which 75% have originated from animals, a number of them due to inappropriate use of antibiotics. Almost 800 pathogens have crossed the species barrier from animals out of 1,400 pathogens causing human diseases. Hence, the awareness among the general public has increased concerns towards the antimicrobial resistance in pathogens. In 2006 the European Union imposed a complete ban on the use of antibiotics in poultry feeds (Singer and Hofacre, Reference SINGER and HOFACRE2006; Vesna et al., Reference VESNA, LAZAREVIĆ, SINOVEC and TOKIĆ2007). As a consequence, the development of alternatives to antibiotics receives considerable attention. Ideally, alternatives to antibiotics should have the same advantageous properties. Isolated nutrients (amino acids, fatty acids, minerals, and vitamins), dietary supplements (probiotics, prebiotics, synbiotics, organic acids, antioxidants, and enzymes), herbal products (polyphenols, herbs, and spices) and genetically modified foods have been extensively studied in search of alternatives (Das et al., Reference DAS, BHAUMIK, RAYCHAUDHURI and CHAKRABORTY2012). Among these alternatives, organic acids are considered to be popular and suitable for in-feed use. These compounds are defined as short chain fatty acids that beneficially affect the host by selectively stimulating the favourable growth or activity of beneficial bacterial species and killing the harmful bacteria populations inhabiting the digestive tract of poultry. These are natural products of the microbial metabolism or fermentation of the carbohydrates in the intestine of animals. The most commonly known organic acids are acetic acid, propionic acid, and butyric acid, also known as volatile fatty acids (VFAs) or short chain fatty acids (SCFAs). Among these, butyric acid possesses the interesting characteristic features. It has a molecular weight of 88.12 g/mol, density 0.958 g/ml, and pKa 4.82. However, it is corrosive and volatile in nature, therefore the sodium salt of butyric acid is used which allows easy handling, stability and is less odorous.
Sodium butyrate is readily transformed into butyric acid within the digestive tract of the birds where it improves the intestinal health through various mechanisms. It is involved in the development of gut wall tissues and modulates the growth of symbiotic intestinal microflora (Van Immerseel et al., Reference VAN IMMERSEEL, FIEVEZ, DE BUCK, PASMANS, MARTEL, HAESEBROUCK and DUCATELLE2004; Reference VAN IMMERSEEL, BOYEN, GANTOIS, TIMBERMONT, BOHEZ, PASMANS, HAESEBROUCK and DUCATELLE2005; Friedman and Bar-Shira, Reference FRIEDMAN and BAR-SHIRA2005; Leeson et al., Reference LEESON, NAMKUNG, ANTONGIOVANNI and LEE2005). It improves body weight, feed conversion ratio (FCR), beneficial bacterial populations, and reduces the colonisation of harmful bacteria in the digestive tract of broilers (Chamba et al., Reference CHAMBA, PUYALTO, ORTIZ, TORREALBA, MALLO and RIBOTY2014; Zhang et al., Reference ZHANG, JIANG, ZHU, GAO, DAI, CHEN and ZHOU2011; Hu and Guo, Reference HU and GUO2007; Hernandez et al., Reference HERNANDEZ, AFANADOR, ARIZA-NIETO and AVELLANEDA2013). In addition, sodium butyrate supplementation has been linked to improving immunity in broilers (Zhou et al., Reference ZHOU, PACKIALAKSHMI, MAKKAR, DRIDI and RATH2014). In this review, published data relating to the used and benefit of sodium butyrate in poultry diets is discussed.
Structure of butyric acid and sodium butyrate
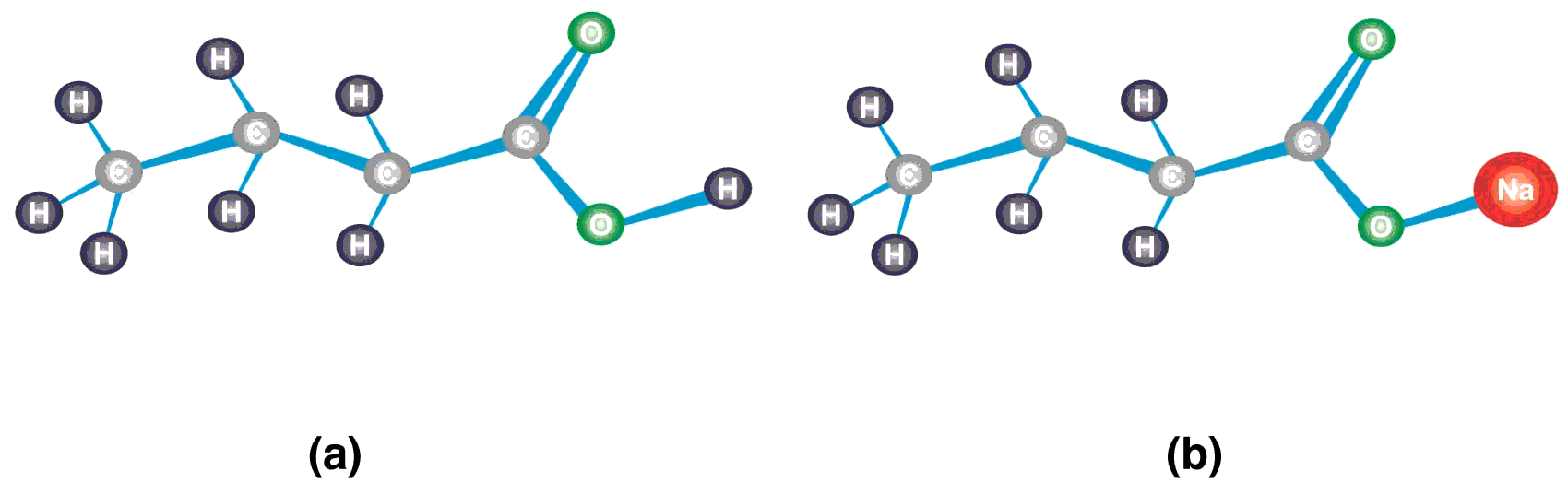
Butyric acid belongs to the class of carboxylic acids consisting of a four carbon atoms chain, thus named as butanoic acid. The terminal carbon is a carbonyl carbon of the carboxyl group (-COOH), the functional group of the carboxylic acids, also known as 1-butanoic acid. The hydrogen ion of hydroxyl group (-OH) is weakly bonded and replaceable. In solution, butyric acid loses its hydrogen ion to form butyrate ion (CH3CH2CH2COO-). Sodium butyrate is the sodium salt of butyric acid which contains sodium atom in place of hydrogen of -OH group. The structure of butyric acid and sodium butyrate has been shown in Figure 1.

Figure 1 Structure of butyric acid and sodium butyrate (a) The carbonyl carbon atom in the carboxyl group (-COOH) is attached to one oxygen atom with double covalent bond, a hydroxyl group (-OH), and the carbon chain. Since the structure contains four carbon atoms including the carbonyl carbon, it is named as butanoic acid or commonly called butyric acid. (b) The hydrogen atom of the -OH group of butyric acid is replaced with sodium (Na). When butyric acid loses hydrogen ion (H+) of the –OH group, it is called butyrate ion (CH3CH2CH2COO-). The attachment of sodium to butyrate ion makes its name sodium butyrate.
Dissociation and absorption of butyric acid
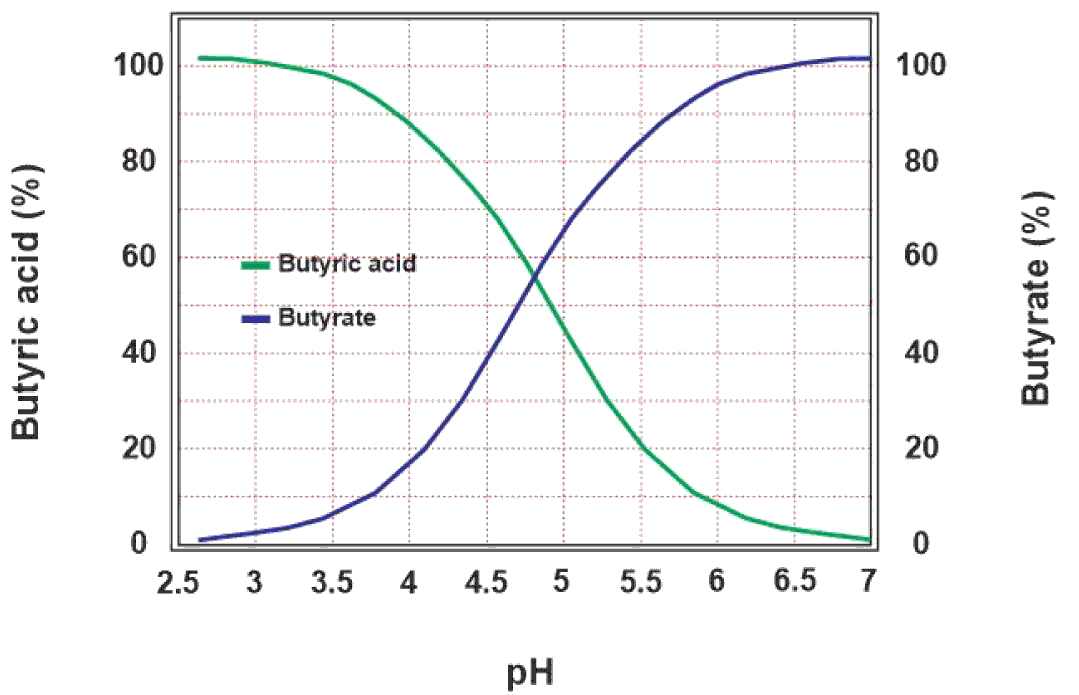
The efficacy of sodium butyrate depends upon the pKa value of butyric acid and pH of the corresponding part of the digestive system viz. crop, proventriculus, gizzard and small intestine. pKa is the pH value of an acid at which half the molecules of that acid are dissociated into positive and negative ions. At a pH of 4.82, butyric acid remains in equilibrium between butyric acid, and butyrate and hydrogen ions. If the pH of a medium is less than the pKa value of butyric acid, most of the molecules of butyric acid remain un-dissociated. Therefore, it is important for butyric acid to stay un-dissociated to be efficient in that medium. This equilibrium is shown in the equation below, and the theoretical dissociation of butyric acid as a function of pH is shown in Figure 2.
CH3CH2CH2COOH ↔ CH3CH2CH2COO- + H+
pH<4.82 pH=4.82 pH>4.82
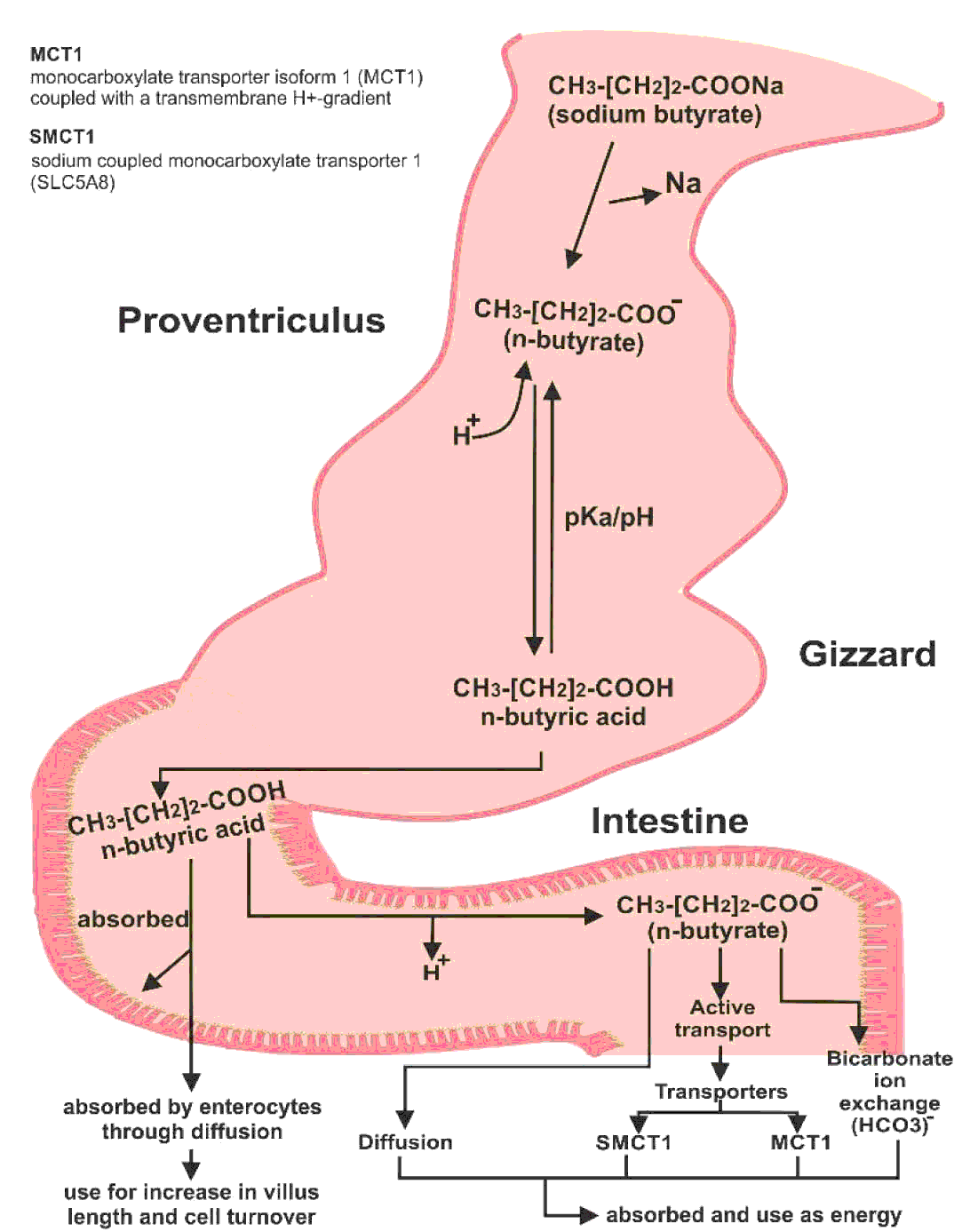
Sodium butyrate is converted into butyric acid after ingestion. The acidic pH of the crop, proventriculus, and gizzard allows butyric acid to stay in its un-dissociated form. As it enters the proximal small intestine, it is dissociated into butyrate and hydrogen ions (Figure 3). Butyric acid is readily absorbed by enterocytes via passive diffusion and used to increase villus length and cells turnover. However, most molecules of butyric acid are dissociated. Butyrate ions can be absorbed as a source of energy as well, which require different methods for their absorption. Butyrate ions can be transported either through diffusion, bicarbonate ion (HCO3-) exchange method or by active transport (McNeil et al., Reference McNEIL, CUMMINGS and JAMES1979; Velazquez et al., Reference VELAZQUEZ, LEDERER and ROMBEAU1997; Kawamata et al., Reference KAWAMATA, HAYASHI and SUZUKI2007). Two different transporters have been proposed for the absorption of dissociated form of short chain fatty acids: the monocarboxylate transporter isoform 1 (MCT1) coupled with a transmembrane H+-gradient, and SLC5A8 which is a Na+-coupled co-transport system also known as sodium coupled monocarboxylate transporter 1 (SMCT1) (Hamer et al., Reference HAMER, JONKERS, VENEMA, VANHOUTVIN, TROOST and BRUMMER2008). However, butyric acid is the preferred source of energy for the enterocytes (Mahdavi and Torki, Reference MAHDAVI and TORKI2009).
The bactericidal effects of butyric acid require it to be un-dissociated for entry into the bacterial cell. Therefore, it is necessary to use sodium butyrate in such a form that should protect it from dissociation, so enteric-coated forms of sodium butyrate have been developed in order to prevent its dissociation in proximal and distal portions of bird's intestine, and caking of the sodium butyrate. The different coatings include the palm stearin, vegetable fats, and salts of palm fatty acids. The palm stearin coating encapsulates only a low percentage of sodium butyrate, which requires a higher dosage in feed to reach a sufficient level of active ingredient in the animal (Puyalto and Mallo, Reference PUYALTO and MALLO2014). Vegetable fat and fatty acid salts coating processes protect a higher level of sodium butyrate that is dissociated slowly along the length of gastrointestinal tract and which is much more effective to reduce the level of infectious bacteria.

Figure 2 Effect of pH on dissociation of butyric acid. The acidic pH lower than the pKa (4.82) shifts the equilibrium towards the un-dissociated butyric acid on the left side of the graph whereas the increasing pH shifts the equilibrium towards the dissociated butyrate ions on the right side.

Figure 3 Conversion of sodium butyrate to butyric acid in the bird's intestine and its absorption.
Productive performance
Beneficial health effects of butyrate or butyric acid are well documented, and it has been shown to have positive effects on broiler production parameters such as weight gain, feed intake, and FCR (Antongiovanni et al., Reference ANTONGIOVANNI, BUCCIONI, PETACCHI, LEESON, MINIERI, MARTINI and CECCHI2007; Leeson et al., Reference LEESON, NAMKUNG, ANTONGIOVANNI and LEE2005; Taherpour et al., Reference TAHERPOUR, MORAVEJ, SHIVAZAD, ADIBMORADI and YAKHCHALI2009). Chamba et al. (Reference CHAMBA, PUYALTO, ORTIZ, TORREALBA, MALLO and RIBOTY2014) reported that partially coated sodium butyrate in broiler diet significantly increased the feed intake and weight gain, and improved FCR as compared to positive and negative controls during grower and finisher phases but not in the starter phase. Mansoub (Reference MANSOUB2011) reported that dietary sodium butyrate increased the weight gain and FCR up to 28 days of age. (Antongiovanni et al., Reference ANTONGIOVANNI, BUCCIONI, PETACCHI, LEESON, MINIERI, MARTINI and CECCHI2007; Leeson et al., Reference LEESON, NAMKUNG, ANTONGIOVANNI and LEE2005; Taherpour et al., Reference TAHERPOUR, MORAVEJ, SHIVAZAD, ADIBMORADI and YAKHCHALI2009). Similarly, butyric acid enhanced the weight gain and FCR (Panda et al., Reference PANDA, RAO, RAJU and SUNDER2009). Likewise, partially protected and microencapsulated sodium butyrate positively affected the performance of broilers during the grower and finisher periods (Mallo et al., Reference MALLO, GRACIA, HONRUBIA and PUYALTO2010; Zou et al., Reference ZOU, YANG, YANG, JIANG, ZHANG and YU2010a). Hernandez et al. (Reference HERNANDEZ, AFANADOR, ARIZA-NIETO and AVELLANEDA2013) found a significant increase in weight gain and FCR of broilers in commercial flocks in response to both uncoated and coated sodium butyrate. The improvement in the performance of broilers is considered due to different functions accomplished by sodium butyrate. Butyric acid increases the villi length in small intestine (Chamba et al., Reference CHAMBA, PUYALTO, ORTIZ, TORREALBA, MALLO and RIBOTY2014; Adil et al., Reference ADIL, BANDAY, BHAT, SALAHUDDIN, RAQUIB and SHANAZ2011) and stimulates the pancreatic exocrine (Katoh and Tsuda, Reference KATOH and TSUDA1984; Reference KATOH and TSUDA1985) thus increasing the secretions of digestive enzymes such as amylase and lipase. Consequently, the feed digestion and nutrient absorption is improved.
Contrary to these findings, Mahdavi and Torki (Reference MAHDAVI and TORKI2009) reported that different levels of dietary sodium butyrate did not improve the weight gain and FCR of broilers. Similarly, dietary sodium butyrate did not increase the weight gain and FCR of the broilers (Leeson et al., Reference LEESON, NAMKUNG, ANTONGIOVANNI and LEE2005). Zhang et al. (Reference ZHANG, JIANG, ZHU, GAO, DAI, CHEN and ZHOU2011) reported no significant difference in weight gain, feed intake and FCR of broilers fed different levels of sodium butyrate or without sodium butyrate. According to Zou et al. (Reference ZOU, YANG, YANG, JIANG, ZHANG and YU2010a; Reference ZOU, YANG, YANG, JIANG, ZHANG and YU2010b), feed intake was not increased in response to dietary coated sodium butyrate or antibiotics. The variable findings in productive performance are due to the fact that uncoated sodium butyrate, when converted to butyric acid, is dissociated in the small intestine because of its low pKa value in comparison with the pH of small intestine. Thus, a considerable quantity of butyric acid (undissociated) from uncoated sodium butyrate cannot be used by enterocytes due to low concentrations that may not result in higher villi length. Consequently, nutrient absorption will be lower which leads to poor FCR and reduced weight gain. As the functionality of intestines of day old chicks and the activity of the digestive enzymes is not sufficiently developed (Ravindran, Reference RAVINDRAN2003), and fat coating is not emulsified completely (Noy and Sklan, Reference NOY and SKLAN1994; Leeson and Summers, Reference LEESON and SUMMERS2001), fat-coated sodium butyrate is not released completely. As a result, the digestion of feed and absorption of nutrients are not completely accomplished. This causes lowered weight gain and poor FCR during the starter period of chicken growth in response to fat-coated sodium butyrate and the carry-over effects may affect overall performance of birds.
Gut microflora
Sodium butyrate is a selective bactericidal agent due to its activity of lowering the pH of crop and gizzard and in the upper part of the intestine, controlling harmful bacteria such as Salmonella spp., Escherichia coli and Campylobacter jejuni (Van Deun et al., Reference VAN DEUN, HAESEBROUCK, VAN IMMERSEEL, DUCATELLE and PASMANS2008). Chamba et al. (Reference CHAMBA, PUYALTO, ORTIZ, TORREALBA, MALLO and RIBOTY2014) did not report any effects of dietary sodium butyrate on E. coli populations in the jejunum in comparison with antibiotic supplemented diets. Similarly, the different concentrations of sodium butyrate in feed did not change E.coli populations in the jejunum (Hu and Guo, Reference HU and GUO2007). Likewise, a study showed that coated butyric acid proved to be the best bactericidal agent against Campylobacter jejuni in vitro in comparison with propionic acid, acetic acid and L-lactate (Van Deun et al., Reference VAN DEUN, HAESEBROUCK, VAN IMMERSEEL, DUCATELLE and PASMANS2008). The same effect was observed in the presence of intestinal mucous with a higher dose of sodium butyrate in vitro, however, sodium butyrate supplementation in feed was not effective against C. jejuni (Van Deun et al., Reference VAN DEUN, HAESEBROUCK, VAN IMMERSEEL, DUCATELLE and PASMANS2008). This implied that higher doses of sodium butyrate may be needed to be effective against C. jejuni. Another study revealed that sodium butyrate reduced the invasion of Salmonella enterica in intestinal epithelium of broilers (Van Immerseel et al., Reference VAN IMMERSEEL, FIEVEZ, DE BUCK, PASMANS, MARTEL, HAESEBROUCK and DUCATELLE2004) due to the downregulation of pathogenicity island 1 of S. enterica (SPI1; Gantois et al., Reference GANTOIS, DUCATELLE, PASMANS, HAESEBROUCK, HAUTEFORT, THOMPSON, HINTON and VAN IMMERSEEL2006).
Direct bactericidal effect of sodium butyrate
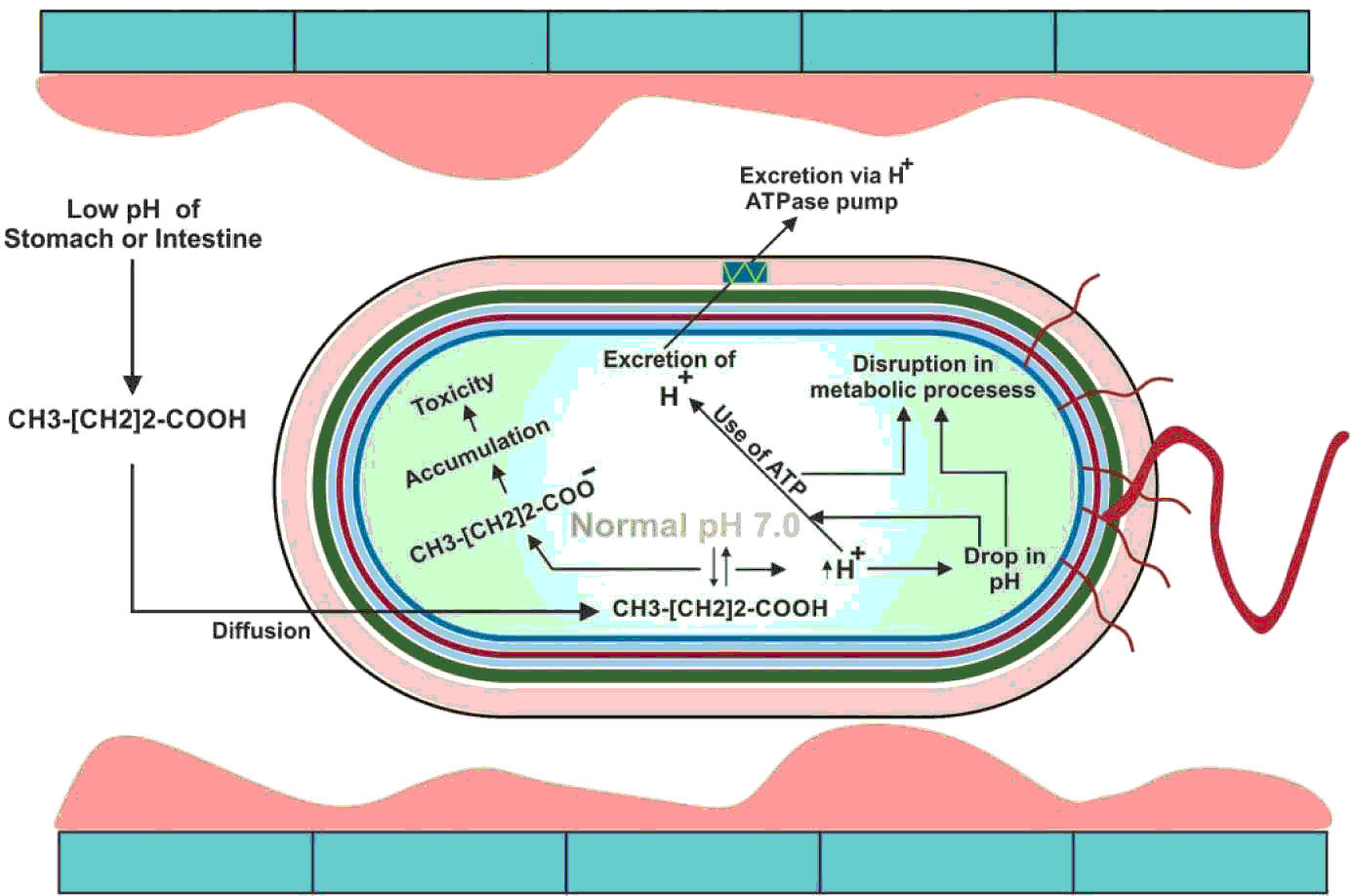
After sodium butyrate is converted to butyric acid, it has the ability to enter the bacterial cell wall mainly through diffusion (Clark and Cronan, Reference CLARK and CRONAN1996) which causes toxicity inside the bacterial cell (Warnecke and Gill, Reference WARNECKE and GILL2005). The reduction in the cytoplasmic pH of the bacterial cell which in turn affects the purine bases (Choi et al., Reference CHOI, BAUMLER and KASPAR2000) resulting in denaturing essential enzymes inside the cell (Roe et al., Reference ROE, O'BYRNE, MCLAGGAN and BOOTH2002), ultimately leading to the death of bacteria. Gantois et al. (Reference GANTOIS, DUCATELLE, PASMANS, HAESEBROUCK, HAUTEFORT, THOMPSON, HINTON and VAN IMMERSEEL2006) reported that when Salmonella spp. is grown in the presence of butyric acid, it downregulates the genes located in the SPI1 that results in reduced invasiveness of Salmonella spp. through intestinal epithelial cells. The bactericidal effect of butyric acid has been shown in Figure 4.

Figure 4 The bactericidal action of butyric acid. At low pH, the un-dissociated sodium butyrate enters into the bacterial cytoplasm. The pH of cytoplasm is neutral which dissociates the butyric acid into H+ and butyrate ions. Increase in H+ ions decreases the pH of the cytoplasm which disrupts the metabolic process. The bacterial cell, at the expense of ATP, excretes the H+ ions from its cytoplasm via H+ ATPase pump. On the other hand, butyrate ions are accumulated, which causes toxicity in the cytoplasm. The bacterial cell is eventually exhausted and killed.
Indirect bactericidal effect of sodium butyrate
Sodium butyrate lowers the pH of intestine that favours the growth of lactic acid producing bacteria such as Lactobacilli and Bifidobacteria spp. (Vogt et al., Reference VOGT, MATTHES and HARNISCH1982) as they require an acidic medium for their growth. It has been reported that lactic acid producing bacteria compete for space and nutrients with pathogenic bacteria within the intestine (Furuse and Okumura, Reference FURUSE and OKUMURA1994; Rolfe, Reference ROLFE2000). Lactobacilli spp. produce bacteriocins (Joerger, Reference JOERGER2003) whereas Bifidobacteria spp. secrete some organic acids (acetic acid and lactic acid) and bactericidal substances (Gibson and Wang, Reference GIBSON and WANG1994) which moderate the pathogenic bacterial count and maintain a healthy environment in the bird's intestine. Similarly, Audisio et al. (Reference AUDISIO, OLIVER and APELLA2000) reported that sodium butyrate favours the growth of Lactobacilli spp. that converts glucose to lactic acid within the intestine of birds, causing the inhibition of pathogenic bacteria such as Salmonella spp. and E.coli.
Gut morphology
Characteristic features of a bird's digestive tract for the optimal functions include large surface area covered with long healthy villi having shallow crypts (Ferket et al., Reference FERKET, PARKS and GRIMES2002). Deeper crypts are indicative of rapid tissue turnover in order to permit renewal of villi and normal sloughing or pathogenic invasion resulting in inflammation (Miles et al., Reference MILES, BUTCHER, HENRY and LITTELL2006). Long villi and shallow crypts provide a larger surface area for the absorption of nutrients and low renewal rate, allowing efficient enzyme production and maturation of the intestinal cells (Yang et al., Reference YANG, IJI and CHOCT2009). Any alteration in the diet and the intestinal microflora can alter the morphology of gastrointestinal tract of broilers. Response of the bird's intestine to dietary changes may result in either shortening or lengthening of each villus which further affects the digestion and absorption of nutrients (Yang et al., Reference YANG, IJI, KOCHER, MIKKELSEN and CHOCT2007). As sodium butyrate is converted to butyric acid after ingestion, it is preferably absorbed by enterocytes as a source of energy (Mahdavi and Torki, Reference MAHDAVI and TORKI2009). It accelerates the growth of enterocytes and villus elongation that results in increased villi height and deeper crypts. It has been reported that villus height increased in the jejunum and ileum in response to dietary sodium butyrate (fat coated) whereas crypt depth and villus height to crypt depth ratio were not affected (Chamba et al., Reference CHAMBA, PUYALTO, ORTIZ, TORREALBA, MALLO and RIBOTY2014). Significantly higher duodenal villi density was reported in response to 3% sodium butyrate in comparison with 2% sodium butyrate (Adil et al., Reference ADIL, BANDAY, BHAT, SALAHUDDIN, RAQUIB and SHANAZ2011). Adil et al. (Reference ADIL, BANDAY, BHAT, MIR and REHMAN2010) reported that dietary butyric acid (3% application rate) significantly increased the villus height in the duodenum and jejunum only. Similarly, many researchers have described the beneficial effect of dietary sodium butyrate on villi height and crypt depth in broilers at different phases of their growth (Antongiovanni et al., Reference ANTONGIOVANNI, BUCCIONI, PETACCHI, LEESON, MINIERI, MARTINI and CECCHI2007; Mallo et al., Reference MALLO, PUYALTO and RAMA RAO2012; Panda et al., Reference PANDA, RAO, RAJU and SUNDER2009; Sayrafi et al., Reference SAYRAFI, SOLTANALINEJAD, SHAHROOZ and RAHIMI2011; Smulikowska et al., Reference SMULIKOWSKA, CZERWINSKI, MIECZKOWSKA and JANKOWIAK2009). On the contrary, some researchers did not find any effect of dietary sodium butyrate on villi height and crypt depth (Leeson et al., Reference LEESON, NAMKUNG, ANTONGIOVANNI and LEE2005).
The variation in results may be due to the fact that uncoated sodium butyrate, with a pKa value being lower than the pH of intestine, is dissociated into ions which cannot be readily absorbed by the enterocytes. Therefore, activity relating to improved intestinal function is limited only to the upper part of the intestine. However, the fat-coated sodium butyrate may overcome this problem as it is available to the lower parts of the small intestine. In addition, the release of sodium butyrate from the fat covering needs it to be degraded by the activity of lipase enzyme. The inability of the younger chick's pancreas to produce sufficient quantity of lipase enzyme (Ravindran, Reference RAVINDRAN2003) may result in decreased release of sodium butyrate leading to the lowered villi height and crypt depth in starter phase.
Immunity
Not much data is available describing the effect of sodium butyrate on the immune functions of broilers. Host defence peptides (HDPs), also known as antimicrobial peptides, exist in almost all forms of life and are an integral part of innate immunity (Brogden et al., Reference BROGDEN, ACKERMANN, MCCRAY and TACK2003; Ganz, Reference GANZ2003). Chicken genome encodes for 14 β-defensins (AvBD1-14) and four cathelicidins (fowlicidins 1-3 and cathelicidin-B1) as a member of HDPs system (Goitsuka et al., Reference GOITSUKA, CHEN, BENYON, ASANO, KITAMURA and COOPER2007; Lynn et al., Reference LYNN, HIGGS, LLOYD, O'FARRELLY, HERVE-GREPINET, NYS and BRINKMAN2007; Sunkara et al., Reference SUNKARA, ACHANTA, SCHREIBER, BOMMINENI, DAI, JIANG, LAMONT, LILLEHOJ, BEKER, TEETER and ZHANG2011). HDPs exhibit their properties as broad spectrum antimicrobials against bacteria, enveloped viruses, fungi, and protozoa by direct binding and lysis of microbial membranes (Ganz, Reference GANZ2003), which prevents the development of resistance to HDPs in pathogens. Sunkara et al. (Reference SUNKARA, ACHANTA, SCHREIBER, BOMMINENI, DAI, JIANG, LAMONT, LILLEHOJ, BEKER, TEETER and ZHANG2011) reported that sodium butyrate induced HDP gene expression in chicken macrophage cells, monocytes, bone marrow cells, and jejunal and caecal explants. In addition, sodium butyrate enhanced the antibacterial activity of chicken monocytes and reduced the colonisation of Salmonella spp. in S. enteritidis challenged chickens. Similar findings were reported in response to oral supplementation of sodium butyrate or butyric acid that reduced the colonisation and shedding of S. enteritidis in broilers (Van Immerseel et al., Reference VAN IMMERSEEL, BOYEN, GANTOIS, TIMBERMONT, BOHEZ, PASMANS, HAESEBROUCK and DUCATELLE2005; Fernandez-Rubio et al., Reference FERNANDEZ-RUBIO, ORDONEZ, ABAD-GONZALEZ, GARCIA-GALLEGO, HONRUBIA, MALLO and BALANA-FOUCE2009). These findings may have arisen either due to the direct antibacterial activity of butyric acid (Van Immerseel et al., Reference VAN IMMERSEEL, DE BUCK, PASMANS, VELGE, BOTTREAU, FIEVEZ, HAESEBROUCK and DUCATELLE2003) or due to the decreased invasiveness of Salmonella spp. (possibly due to the downregulation of genes in SPI1) through intestinal epithelium (Van Immerseel et al., Reference VAN IMMERSEEL, DE BUCK, PASMANS, VELGE, BOTTREAU, FIEVEZ, HAESEBROUCK and DUCATELLE2003; Gantois et al., Reference GANTOIS, DUCATELLE, PASMANS, HAESEBROUCK, HAUTEFORT, THOMPSON, HINTON and VAN IMMERSEEL2006). Sodium butyrate can inhibit the nitric oxide production and expression of cytokines such as IL-1β, IL-6, IFN-γ, and IL-10 in chicken macrophage cells stimulated by the presence of S. typhimurium lipopolysaccharides (Zhou et al., Reference ZHOU, PACKIALAKSHMI, MAKKAR, DRIDI and RATH2014).
Conclusions
The effects of dietary sodium butyrate supplementation on productive performance, gut microflora, and gut morphology are well understood, although its effects on immunity of the chicken are not completely clear. However, varying results of uncoated and fat coated sodium butyrate supplementation in broilers have been reported. There is a room for further research on the use of sodium butyrate, particularly in the fatty acid-coated form and appropriate dose rates. Further studies are suggested to investigate the effects of sodium butyrate on immunity of the chicken.
Acknowledgements
The authors gratefully acknowledge the fellowship to Umair Ahsan from The Scientific and Technological Research Council of Turkey (TÜBİTAK) under the BİDEB-2215 programme.