Article contents
Levantite, KCa3(Al2Si3)O11(PO4), a new latiumite-group mineral from the pyrometamorphic rocks of the Hatrurim Basin, Negev Desert, Israel
Published online by Cambridge University Press: 14 June 2019
Abstract
Levantite, with the end-member formula KCa3(Al2Si3)O11(PO4), is the phosphate analogue of latiumite, KCa3(Al3Si2)O11(SO4, CO3) found in gehlenite–wollastonite hornfels on Har Parsa, Negev Desert, Israel. Levantite forms later zones on long-prismatic crystals of latiumite. Rarer homogeneous colourless levantite crystals up to 0.2 mm long and with mean composition (K0.94Ba0.01Na0.01□0.04)Σ1.00(Ca2.96Mg0.03)Σ2.99{(Si2.69Al2.06Fe3+0.16P0.06)Σ4.97O11}[(PO4)0.65(SO4)0.35]Σ1.00 were noted. Minerals of the levantite–latiumite series are associated with gehlenite, wollastonite, clinopyroxene of the esseneite–diopside series, anorthite and Ti-bearing andradite. Levantite crystalises in space group P21 with unit-cell parameters a = 12.1006(9) Å, b = 5.1103(4) Å, c = 10.8252(9) Å, β = 107.237(8)°, V = 639.34(9) Å3 and Z = 2. The structure of levantite is analogous to latiumite. It is formed by tetrahedral hybrid zweier double layers [(Si,Al)10O22] connected by Ca atoms. Three Ca atoms linked to different double layers are bridged over by (PO4) and minor (SO4) groups. K atoms reside in the cavities between two superimposed zweier double layers. The measured micro-indentation hardness of levantite gave VHN50 = 580(19) (mean of 14), range 550–611 kg/mm2, which correlates with 5 on the Mohs scale. Cleavage is good on (100). Twinning on (100) is polysynthetic or simple. The calculated density is 2.957 g cm–3. Levantite is optically negative with α = 1.608(2), β = 1.618(2), γ = 1.622(2) (λ = 589 nm), 2Vmeas. = 70(5)° and 2Vcalc. = 64.3°. Dispersion of the optical axes r > v is weak; the optical orientation is: Z = b, X ^ c = 22–27°; and it is non-pleochroic. Minerals of the levantite–latiumite series from Israel show characteristic Raman spectra with the main bands at 994 cm–1 [ν1(SO4)2–] and 945 cm–1 [ν1(PO4)3–]. The band intensity ν1(PO4)3–/ν1(SO4) ratio is well correlated with P and S contents in the investigated minerals. The strongest lines in the powder diffraction pattern [dobs, Å (I, %) (hkl)] are: 3.0762(100)(310), 2.8551(96)(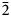 $\bar 2$13), 2.9704(92)(
$\bar 2$13), 2.9704(92)(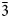 $\bar 3$12), 2.8573(83)(013), 2.5552(66)(020), 2.8228(48)(212), 2.8893(40)(400), and 3.0634(30)(103).
$\bar 3$12), 2.8573(83)(013), 2.5552(66)(020), 2.8228(48)(212), 2.8893(40)(400), and 3.0634(30)(103).
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2019
Footnotes
Associate Editor: Oleg I Siidra
References
- 7
- Cited by




