Introduction
Depression is among the most common psychiatric disorders, affecting nearly 280 million people worldwide [1]. The total estimated number of depression cases has increased by approximately 15% over the past decade and is expected to increase continually [2, 3]. It has been shown that depression accounts for over 40% of the global mental health-related disease burden [Reference Whiteford, Degenhardt, Rehm, Baxter, Ferrari and Erskine4, Reference Kessler and Bromet5], and persons with depression have elevated mortality compared to those without depression [Reference Walker, McGee and Druss6].
Depression can occur at any age throughout one’s life; its prevalence is the highest in earlier life (i.e., 20–40 years), followed by mid-to-late life (i.e., 50–70 years) [Reference Malhi and Mann7]. Difference in the prevalence of depression at different ages may represent individuals’ vulnerability across the lifespan. It has also been shown that depression at different ages manifests differential etiology [Reference Papazacharias, Logroscino, Barulli and Nardini8, Reference Kennedy9]. As a result, the impact of depression on mortality may differ depending on the age at which depression occurs. However, studies looking into the association between depression and mortality by the age of depression occurrence remain limited.
Furthermore, several pathways through which depression increases the risk of mortality have been suggested, such as the dysregulation of stress systems and the production of cerebrovascular pathology [Reference Cuijpers, Vogelzangs, Twisk, Kleiboer, Li and Penninx10, Reference Taylor, Aizenstein and Alexopoulos11]. Depression has been associated with an increased risk of dementia in several studies, including our previous study [Reference Yang, Li, Pan, Yang, Song and Qi12–Reference Holmquist, Nordstrom and Nordstrom14], and dementia is one of the leading causes of death [15]. Thus, it is plausible that dementia serves as a mediator in the association between depression and mortality. Moreover, both genetic and early-life environmental factors have been related to depression and mortality [Reference Malhi and Mann7, Reference Heim and Binder16, Reference Hughes, Bellis, Hardcastle, Sethi, Butchart and Mikton17]. A twin study design is particularly useful to clarify whether and to what extent these unmeasured familial factors contribute to the association between depression and mortality, by matching twins who shared genetic background and were reared together [Reference McGue, Osler and Christensen18].
We hypothesized that depression is related to elevated mortality, and dementia may play a mediating role in this association. In this study, we aimed to verify this hypothesis by (a) examining the association between depression that occurs at different ages and the risk of mortality, (b) assessing the extent to which dementia mediates the association, and (c) exploring whether genetic and early-life familial factors could account for the depression-mortality association.
Methods
Study population
Participants of the current study were identified from the nationwide Swedish Twin Registry (STR) which was first established in the late 1950s and has been an important epidemiological resource for common complex diseases [Reference Lichtenstein, De Faire, Floderus, Svartengren, Svedberg and Pedersen19]. Between 1998 and 2002, a total of 44,919 twin individuals alive (aged 40 years or above) in this registry participated in a computer-assisted telephone interview called Screening Across the Lifespan Twin (SALT) study and were included in the current study. In the mediation analysis (details see statistical analysis section), there remained 35,032 individuals after excluding 941 who died before the age of 65 and 8,944 who were younger than 65 years at the end of follow-up (December 31, 2016). This was done because they were not at risk of developing dementia which was the mediator of focus in the analysis. We also excluded two individuals who had dementia before depression diagnosis (Figure 1).

Figure 1. Flowchart of the study population.
All participants provided informed consent, and this study was approved by the Regional Ethics Board at Karolinska Institutet, Stockholm, Sweden and the Institutional Review Board at the University of Southern California, USA. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Data collection
Information on age, sex, education level, marital status, smoking status, alcohol consumption, physical exercise, zygosity status, height, and weight was collected at baseline from the SALT interview. Education level was grouped into <8 years, 8–10 years, and ≥11 years according to the maximum years of formal schooling [Reference Qiu, Backman, Winblad, Aguero-Torres and Fratiglioni20]. Marital status was defined as married/cohabiting and single (including divorced and living alone). Smoking status was dichotomized as never smoking and former/current smoking. Alcohol consumption was categorized as no/mild drinking and heavy drinking. Frequency of physical exercise was dichotomized as inactive (including almost never, much less than average, and less than average) and active (including average, more than average, much more than average, and maximum) based on the annual exercise pattern. Zygosity status was classified as monozygotic, dizygotic, and undetermined zygosity. Body mass index (BMI, kg/m2) was calculated as weight (kg) divided by the square of height (m) and divided into underweight (<20), normal (20–24.9), overweight (25–29.9), and obese (≥30).
Data on type 2 diabetes, hypertension, cardiovascular disease (including heart disease and stroke), and cancer at baseline were obtained through the linkage to the Swedish National Patient Registry (NPR). The NPR was launched in the 1960s and has been maintained by the National Board of Health and Welfare, where more than 99% of all diagnoses of somatic and psychiatric (from 1973) disorders in inpatient care were registered. The NPR also includes outpatient care (non-private specialist clinic) from 2001 [21]. The NPR has been validated, and its validity is high for different disease diagnoses [Reference Ludvigsson, Andersson, Ekbom, Feychting, Kim and Reuterwall22]. Currently, the coverage of the inpatient register and public outpatient care is mostly 100% [Reference Ludvigsson, Andersson, Ekbom, Feychting, Kim and Reuterwall22]. All diseases were diagnosed and coded based on the International Classification of Disease, the seventh to tenth revisions (ICD-7–ICD-10).
Assessment of depression and the age of diagnosis
Depression was ascertained based on the depression diagnosis records from the NPR where depression was diagnosed by doctors following the Swedish version of the International Statistical Classification of Diseases and Related Health Problems. The ICDs and corresponding codes for any diagnosis of depression in in- or outpatient registers included: ICD-7 (314.99), ICD-8 (296.00, 298.00, 300.40, 300.41, 790.20); ICD-9 (296C, 296D, 296W, 298A, 300E, 309A, 309B, 311X), ICD-10 (F32, F33, F34.1, F41.2).
The age of depression occurrence was estimated using the earliest recorded date of diagnosis. In the current study, depression diagnoses prior to baseline survey were included, and depression diagnosed at different ages in life was divided as early-life depression (occurring before 45 years of age), midlife depression (occurring between 45 and 64 years of age), and late-life depression (occurring at or after 65 years of age). Individuals without any depression diagnosis were considered as the reference group in all analyses.
Identification of deaths and dementia
During the follow-up, deaths from all causes recorded in ICD codes and the corresponding dates were confirmed by the Swedish Cause of Death Register in which the information on deaths before 1991 was almost complete. During 1991 and 1996, deaths reported to the tax authority with no completed death certificate were not included in the register (which were rather added retrospectively later on). Since 1997, all deaths have been included in the registry, regardless of the death certificate [Reference Brooke, Talback, Hornblad, Johansson, Ludvigsson and Druid23].
Information on dementia diagnoses was extracted via linkage with the NPR and the Cause of Death Register where the specificity for prevalent dementia is over 99% and the sensitivity is 62.7% [Reference Rizzuto, Feldman, Karlsson, Dahl Aslan, Gatz and Pedersen24]. The ICD codes included the following: ICD-8, 290; ICD-9, 290A, 290B, 290E, 290X, 290 W, 331A, 331C; ICD-10, F00–03, F05, G30, G310, G318, G318A. The age of dementia was estimated using the age of the first dementia diagnosis. Mortality with dementia was defined as death due to dementia and/or death accompanied by previous dementia.
Statistical analysis
All the analyses were performed using SAS 9.4 (SAS Institute) and STATA SE 15.0 (StataCorp, College Station, TX). Baseline characteristics of participants by depression status were compared with the Chi-square test for categorical variables or the independent sample t test for continuous variables. Generalized estimating equation (GEE) models (PROC GENMOD procedures in SAS) were used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for the association between depression and mortality in the unmatched analysis. GEE models are conceptually equivalent to logistic regression but able to control for correlations between the twins within a pair [Reference Lichtenstein, De Faire, Floderus, Svartengren, Svedberg and Pedersen19, Reference Xu, Atti, Gatz, Pedersen, Johansson and Fratiglioni25].
In order to further assess the mediating effect of dementia on the depression-mortality relationship, generalized structural equation modeling was performed. Dementia diagnosed after the depression diagnosis was considered as a mediator to address the issue of temporality between the exposure and the mediator. Bootstrapping method was applied to estimate the 95% CI of indirect (mediated) effect. The bias-corrected 95% CI of the indirect effect that does not include zero indicates a significant mediating effect.
For the co-twin matched analysis, we applied conditional logistic regression models to estimate the OR and 95% CI of all-cause mortality in relation to depression, in which twin pairs discordant for vital status (one twin died but the other did not) were matched and compared within the pairs. As a result, potential confounding factors that twin pairs shared (such as maternal factors, early-life familial environment, and genes) could be controlled for [Reference Lichtenstein, De Faire, Floderus, Svartengren, Svedberg and Pedersen19]. Finally, logistic regression models were used to examine whether there was a difference in ORs between conditional logistic regression and GEE models, by estimating the difference between the proportion of depression in survivors from unmatched analysis and that from co-twin matched analysis. A significant difference in ORs between the two models suggests that early-life environment and genetic background may contribute to the depression-mortality association, whereas the absence of significant difference indicates otherwise.
Missing data on education level (n = 1,602), marital status (n = 1,021), smoking status (n = 1,546), alcohol consumption (n = 1,653), physical exercise (n = 6,851), and BMI (n = 2,388) were imputed using multiple imputation. To test the robustness of our results, we conducted several sensitivity analyses where (a) we distinguished non-suicide and suicide mortality, (b) we excluded 7,555 (16.82%) participants with missing data in covariates, (c) we further excluded 29 (0.8%) dementia cases diagnosed within 10 years following depression because the onset of dementia could be more than 10 years before the diagnosis and late-life depression in particular might be a prodromal phase of dementia [Reference Byers and Yaffe13], and (d) we only excluded participants who had dementia before depression in the mediation analysis to reduce the risk of survival bias resulting from deaths before the age of 65. A value of p < 0.05 (two-tailed) was considered statistically significant.
Results
Characteristics of the study population
Of the 44,919 participants, the mean age at baseline was 59.34 years (standard deviation [SD] = 11.10; range 41–103) and 53.52% were female. A total of 1,179 individuals had a history of depression at baseline, including 574 (48.68%, mean age at diagnosis 35.43 years [SD = 6.86]) with early-life depression, 487 (41.31%, mean age at diagnosis 52.40 years [SD = 5.19]) with midlife depression, and 118 (10.01%, mean age at diagnosis 73.72 years [SD = 6.07]) with late-life depression. Compared with depression-free participants, those with depression were more likely to be female, single, former/current smokers, heavy drinkers, physically inactive, underweight, and obese, and to have type 2 diabetes, hypertension, cardiovascular disease, and cancer. No statistically significant difference in age and education level was observed between the two groups (Table 1).
Table 1. Baseline characteristics of the study population by depression (n = 44,919).
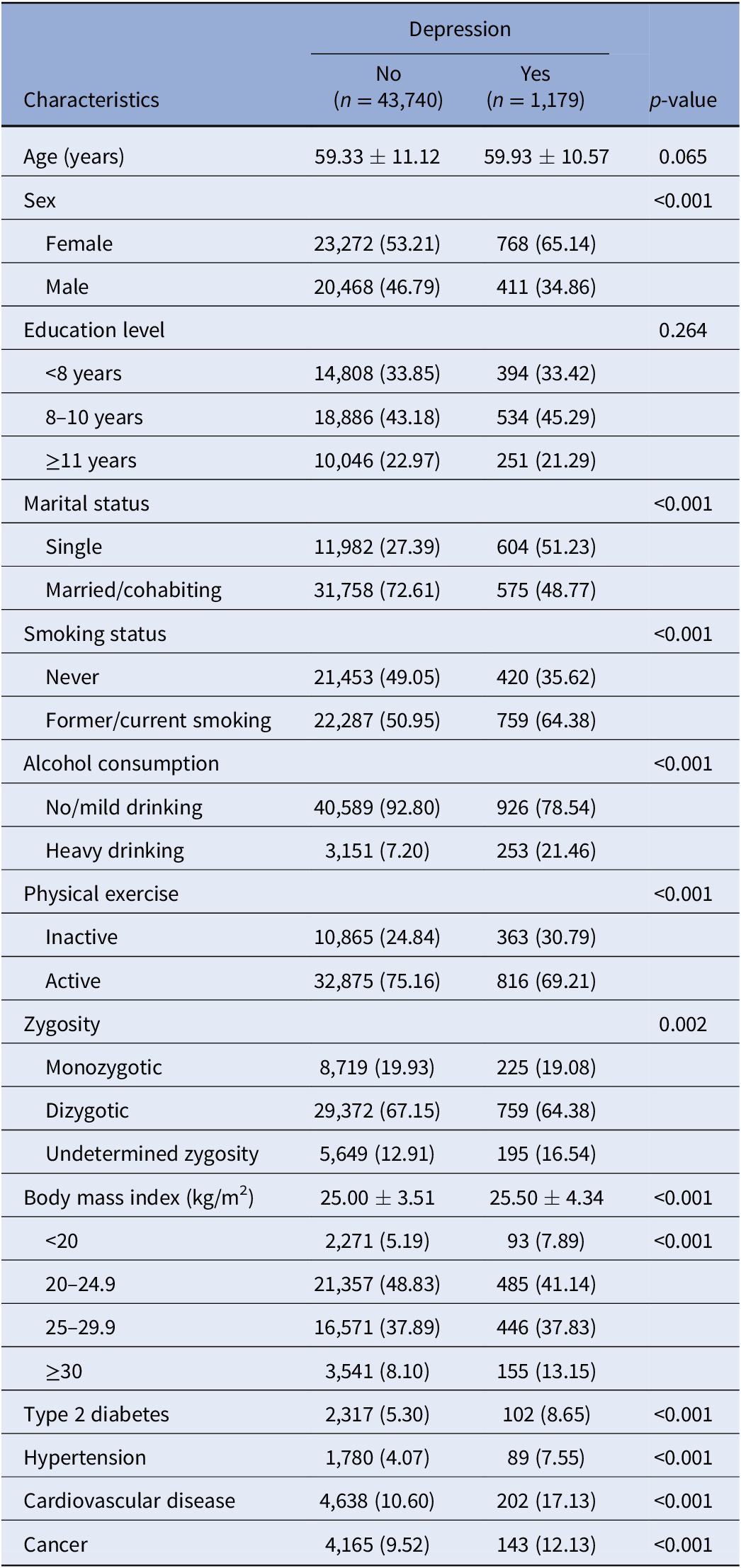
Note: Data were presented as means ± standard deviations or number (%).
Among the co-twin subsample, the baseline characteristics were similar to that in the entire population regarding sex, marital status, smoking status, alcohol consumption, and the history of hypertension or cardiovascular disease. However, education level, physical exercise, BMI, and the history of type 2 diabetes or cancer did not differ significantly between people with and without depression (Supplementary Table S1).
Association between depression at different ages and mortality in the unmatched analysis
During the follow-up (mean 14.61 years [SD = 4.20]), 12,761 died (28.41%, mean age at death 80.02 years [SD = 10.50]). In the entire study population, depression at any age was significantly associated with an increased risk of all-cause mortality in both basic- and multi-adjusted GEE models (OR 2.49, 95% CI 2.13–2.92; OR 1.71, 95% CI 1.46–2.00). Specifically, the multi-adjusted ORs (95% CIs) of all-cause mortality were 1.72 (1.36–2.17) for early-life depression, 1.51 (1.19–1.90) for midlife depression, and 4.10 (2.02–8.34) for late-life depression (Table 2). Furthermore, the risk of all-cause mortality was significantly higher in individuals with late-life depression than in those with early-life depression (OR 2.39, 95% CI 1.13–5.05) or midlife depression (OR 2.72, 95% CI 1.29–5.73), whereas mortality did not significantly differ between early-life depression and midlife depression. In addition, no significant additive (the relative excess risk due to interaction: 0.12, 95% CI −0.56–0.79; the attributable proportion: 0.05, 95% CI −0.23–0.32, and the synergy index: 1.09, 95% CI 0.66–1.81) or multiplicative (p = 0.53) interaction between depression and gender on all-cause mortality was detected (Supplementary Tables S2 and S3).
Table 2. Odds ratios (ORs, 95% confidence intervals) for the association of depression at different ages with all-cause mortality and mortality with dementia: results from generalized estimating equation models.

a Adjusted for age, sex, and education level.
b Further adjusted for smoking status, alcohol consumption, marital status, physical exercise, body mass index, type 2 diabetes, hypertension, cardiovascular disease, and cancer.
Depression at any age was also related to mortality with dementia (OR 1.62, 95% CI 1.28–2.05). Midlife depression (OR 1.61, 95% CI 1.16–2.24) and late-life depression (OR 1.67, 95% CI 1.12–2.48), but not early-life depression (OR 1.56, 95% CI 0.86–2.85), were associated with an increased risk of mortality with dementia (Table 2).
The mediation of dementia in the association between depression and mortality
In the generalized structural equation modeling, the beta-coefficient (β) was 0.49 (95% CI 0.29–0.69) for the relationship between depression at any age and all-cause mortality after adjusting for dementia, together with other covariates. Depression was associated with an increased risk of subsequent dementia (β = 0.54, 95% CI 0.29–0.79), and dementia in turn was associated with increased mortality (β = 1.34, 95% CI 1.23–1.46), resulting in a significantly indirect effect of depression through dementia on all-cause mortality (β = 0.73, 95% CI 0.39–1.06). These reflected that dementia mediated 59.83% of the depression-mortality association (Figure 2 and Supplementary Table S4). Similar results were observed in the basic-adjusted model (Supplementary Table S4). Additionally, dementia mediated the association of early-life depression and midlife depression with all-cause mortality by over 60%, respectively, with a lower proportion of mediation regarding late-life depression (Supplementary Figure S1).

Figure 2. Mediating effects of dementia on the association of depression with all-cause mortality. Models were adjusted for age, sex, education level, smoking status, alcohol consumption, marital status, physical exercise, body mass index, type 2 diabetes, hypertension, cardiovascular disease, and cancer. Numbers next to arrows indicate beta-coefficients (95% confidence intervals). *p < 0.001.
Association between depression and all-cause mortality in the co-twin analysis
In the conditional logistic regression models, the relationship between depression and all-cause mortality was also significant (OR 1.70, 95% CI 1.22–2.38) and similar to that in the unmatched analysis (Table 3). Furthermore, no significant difference in ORs from unmatched analysis based on GEE models and co-twin matched analysis based on conditional logistic regression models was detected (p = 0.09), which suggested that genetic and early-life environmental factors might not play a role in the depression-mortality association.
Table 3. Odds ratios (ORs, 95% confidence intervals) for the association of depression with all-cause mortality in the co-twin matched analysis in survival/death-discordant twin pairs (n = 3,546 pairs): results from conditional logistic regression models.

a Adjusted for sex and education level.
b Further adjusted for smoking status, alcohol consumption, marital status, physical exercise, body mass index, type 2 diabetes, hypertension, cardiovascular disease, and cancer.
Sensitivity analysis
Results were generally similar to those from the initial analyses in the following analyses: (a) focusing on non-suicide mortality (Supplementary Table S5); (b) excluding participants with missing data in covariates (Supplementary Table S6); (c) excluding dementia diagnoses within 10 years after depression diagnoses (Supplementary Figure S2); and (d) only excluding participants who had dementia before depression in the mediation analysis (Supplementary Figure S3).
Discussion
In this large prospective cohort study of nationwide Swedish twins, we found that (a) depression at any age was associated with an elevated risk of all-cause mortality and mortality with dementia. Late-life depression in particular conferred a significantly greater risk of mortality compared to depression in early life and in midlife; (b) over half of the association between depression and mortality was mediated by dementia; and (c) genetic and early-life environmental factors could not account for the depression-mortality association.
Overall, depression has been associated with a 50% higher risk of all-cause mortality in a previous meta-analysis including more than 1.8 million participants from all populated continents [Reference Cuijpers, Vogelzangs, Twisk, Kleiboer, Li and Penninx10]. Similarly, in our study, we found a 70% higher risk of all-cause mortality in persons with a lifetime depression diagnosis. Few studies, however, have focused on the differential impact of depression or depressive symptoms across different periods in life on mortality [Reference Zivin, Yosef, Miller, Valenstein, Duffy and Kales26, Reference Bryant, Jannat-Khah, Cornelius, Khodneva, Richman and Fleck27]. One cohort study of veterans has shown that depression increases the risk of three-year mortality in persons with depression aged <40 and >70 years, but not in those aged 40–70 years [Reference Zivin, Yosef, Miller, Valenstein, Duffy and Kales26]. Another cohort study has revealed that the risks of mortality in relation to depressive symptoms do not differ between people aged 45–64 years and ≥65 years [Reference Bryant, Jannat-Khah, Cornelius, Khodneva, Richman and Fleck27]. Importantly, the current study contributes to the literature on depression and mortality by disentangling the impact of depression diagnosed at different ages in life on mortality. We found that early-life, midlife, and late-life depression were all associated with an increased risk of mortality. Notably, mortality related to late-life depression was also higher than early-life or midlife depression. Late-life depression may be part of the geriatric syndromes and a manifestation of poor physical fitness that contributes to the death, and thus monitoring and treatment of comorbid somatic conditions is emphasized. However, the coverage of inpatient psychiatric care in the NPR has only been complete since 1973, meaning that depression diagnoses before 1973 were not included in our study. This might lead to an underestimation of the number of early-life depression or even misclassification as later-life depression due to relapse or chronicity of earlier depression. Nevertheless, we also found that early-life and midlife depression were associated with a higher risk of suicide mortality than non-suicide mortality, which is in line with a previous study suggesting that the gap in life-expectancy between people with and without depression was largely due to suicide and other unnatural causes of death before age 60 years [Reference Korhonen, Moustgaard, Tarkiainen, Ostergren, Costa and Urhoj28].
In the current study, we found that depression was also associated with an increased risk of mortality with dementia. Given that depression is associated with an increased risk of dementia [Reference Byers and Yaffe13, Reference Bennett and Thomas29], it is plausible that dementia mediates the association between depression and mortality. Indeed, one population-based study in Korea has shown that dementia mediates the association between depressive and bipolar disorders and mortality [Reference An, Yang, Oh, Lim, Shin and Moon30]. The current study showed that more than half of the association between lifetime depression and mortality was mediated by dementia. Although late-life depression may be a prodromal symptom of dementia, the additional analysis excluding dementia occurring within 10 years after depression still yielded an over 40% of mediating effect by dementia in the association. Furthermore, a greater mediating effect of dementia was found with respect to early-life or midlife depression, compared to late-life depression. One possible explanation is that people with earlier-life depression may have recurrent or chronic conditions, resulting in more accumulated risk of dementia than late-life depression. As for those with depression in late life, they might have poorer health conditions and more risk factors for mortality, leading to a relatively lower mediating role of dementia. Our findings underscore the importance of early dementia prevention to further reduce mortality in persons with depression. Future studies are warranted to identify other potential mediators that contribute to the association between depression and mortality.
There are several potential mechanisms involved in the association between depression and mortality. Biologically, depression can activate several pathophysiological alterations, including the dysregulation of stress response, the production of peripheral and neural inflammation [Reference Raison, Capuron and Miller31, Reference Setiawan, Wilson, Mizrahi, Rusjan, Miler and Rajkowska32], and the increase in oxidative stress [Reference Black, Bot, Scheffer, Cuijpers and Penninx33]. These reactivities can subsequently increase the risk of somatic diseases and thereafter premature death. Behaviourally, persons with depression are more likely to have unhealthy behaviors, such as smoking, heavy alcohol consumption, and sedentary behavior, which can increase the risk of death afterwards [Reference Machado, Veronese, Sanches, Stubbs, Koyanagi and Thompson34, Reference Prince, Patel, Saxena, Maj, Maselko and Phillips35]. Furthermore, compared to persons with earlier-life depression, those with late-life depression have been shown to have more cardiovascular comorbidities, more severe vascular pathology in the brain, and structural brain abnormalities [Reference Papazacharias, Logroscino, Barulli and Nardini8, Reference Salloway, Malloy, Kohn, Gillard, Duffy and Rogg36, Reference Rapp, Dahlman, Sano, Grossman, Haroutunian and Gorman37], which have a detrimental impact on cardio-cerebral-vascular health, cognitive function, and survival. Moreover, older persons with depression are prone to have poorer adherence to prescribed medication, which may in turn worsen the health conditions and increase the risk of death [Reference Wei, Hou, Zhang, Xu, Xie and Chandrasekar38].
Both genetic background and early-life environmental factors, such as childhood adversity and family socioeconomic factors, are involved in the development of depression and premature death [Reference Hughes, Bellis, Hardcastle, Sethi, Butchart and Mikton17, Reference Jami, Hammerschlag, Bartels and Middeldorp39–Reference Kelly-Irving, Lepage, Dedieu, Bartley, Blane and Grosclaude41]. By utilizing the twin cohort and the co-twin matched analysis, we found that these factors shared by twins might not account for the association of depression with all-cause mortality. However, it is important to note that the null findings could be due to other reasons, for example, the smaller sample size in the co-twin matched analysis.
The strengths of our study involve the access to a nationwide patient registry, which enabled the identification of depression diagnoses at different ages. Moreover, the large nationwide twin cohort provided us a unique opportunity to control for and explore the role of genetic and early environmental factors. We acknowledge several limitations in the present study. First, an important limitation is the reliance on depression diagnoses in the NPR. Although studies have shown high validation of different diagnoses in the NPR, there is no validation study of depression diagnosis in the NPR. Furthermore, the NPR mainly captures more severe depression cases including those who get hospital or specialized treatment and misses milder untreated cases or cases treated in primary care. It is important to note, however, that the Swedish healthcare system is tax-funded with limited financial barriers for care-seeking. Second, individuals with depression, compared to those without, could have been more regularly in contact with health care services, thus more likely to be diagnosed with dementia in an earlier stage. Therefore, the association between depression and dementia in our study could have been an overestimation. Nevertheless, the effect estimate of the association between depression and dementia in the current study appears to be similar to other population-based cohort studies [Reference Barnes, Yaffe, Byers, McCormick, Schaefer and Whitmer42, Reference Li, Wang, Shofer, Thompson, Peskind and McCormick43]. On the other hand, dementia diagnosis in the registers could have been an underestimation. As such, the mediating effect of dementia shown in the current study could have been the result of the two impacts that counteract each other. Third, individuals who were younger or died earlier than 40 years at baseline were not included in our study, which may have resulted in an underestimation of the association between early-life depression and mortality. Cautions are required when generalizing our findings to the younger population. Fourth, the exact dates of onset of chronic diseases, such as depression and dementia, are difficult to estimate. Late-life depression in particular has also been suggested to be a prodromal phase of dementia, which may have led to reverse causality in our mediation analysis. However, the exclusion of dementia cases identified within 10 years after depression diagnoses did not substantially alter our results. Fifth, due to the limited sample size, we were not able to perform co-twin analysis separately for monozygotic twins (who share 100% of their genetic background) and dizygotic twins (who share only 50% of their genetic background). Finally, it is important to emphasize that our findings based on the patient registry data could be suggestive to health professionals and need to be verified in large population-based cohort studies with medical examinations.
In conclusion, depression is associated with increased all-cause mortality. Almost 60% of the association between depression and all-cause mortality in late life is mediated by dementia. Genetic and early-life familial factors do not seem to play a significant role in the association between depression and mortality in late life. Our findings underline the importance of depression prevention or treatment in light of its substantial impact on dementia as well as mortality.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1192/j.eurpsy.2022.34.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank the Swedish Twin Registry for access to data and are grateful to all the twins who took part in the study, as well as the members of the survey teams. We are very grateful to Prof. Nancy L. Pedersen for her great contribution to the twin data. The Swedish Twin Registry is managed by Karolinska Institutet and receives funding through the Swedish Research Council under the grant No. 2017-00641.
Author Contributions
K-Y.P. and W.X. contributed to the conception and design of the study. W.Y. and Z.W. contributed to drafting of the manuscript. W.Y. did the analyses with support from X.L. W.X. obtained funding. W.Y. and W.X. were responsible for the integrity of the data and the accuracy of the data analysis. All authors contributed to acquisition or interpretation of data, critical revision of the manuscript for important intellectual content, and final approval of the version to be published. All authors had full access to the data in the study and had final responsibility for the decision to submit for publication. W.Y. and Z.W. contributed equally as first authors. K-Y.P. and W.X. contributed equally as last authors.
Financial Support
This study was supported by grants from the Swedish Research Council (No. 2017–00981 and No. 2021–01647), the Swedish Council for Health Working Life and Welfare (2021–01826), Alzheimerfonden (2021–2022), Karolinska Institutet Research Foundation (2020–01660), the Lindhés Advokatbyrå AB (2021–0134), and Stiftelsen För Gamla Tjänarinnor (2021–2022).
Conflicts of Interest
The authors declare none.









Comments
No Comments have been published for this article.