Introduction
Horseweed [Conyza canadensis (L.) Cronquist] is a weed species native to North America, where it can emerge in the fall, overwinter, and complete its life cycle the following season or emerge in the spring and complete its life cycle in the same season (Weaver Reference Weaver2001). It has a ruderal nature and thrives across a wide geographic range, particularly in undisturbed environments such as no-till crop production fields. Conyza canadensis is a primarily self-pollinating species that can produce up to 200,000 seeds per plant. Each seed is 1-mm long with an attached pappus, facilitating seed dispersal via wind into the planetary boundary layer and potentially more than 550 km from the source plant (Bhowmik and Bekech Reference Bhowmik and Bekech1993; Shields et al. Reference Shields, Dauer, VanGessel and Neumann2006). However, wind-borne seed dispersal is complex and subject to variation due to air movement by gusts, updrafts, and boundary-layer interactions, and 99% of C. canadensis seeds remain within 100 m of the source (Dauer et al. Reference Dauer, Mortensen and Humston2006, Reference Dauer, Dauer, Mortensen and Vangessel2007). The first report of glyphosate-resistant C. canadensis was confirmed in Delaware in 2001 from seed collected in a field following three consecutive years of exclusive use of glyphosate (VanGessel Reference VanGessel2001). Despite the ability to disperse by wind, many glyphosate-resistant C. canadensis populations have evolved independently and share a common resistance mechanism (Dinelli et al. Reference Dinelli, Marotti, Bonetti, Minelli, Catizone and Barnes2006; Okada et al. Reference Okada, Hanson, Hembree, Peng, Shrestha, Stewart, Wright and Jasieniuk2013).
Evidence that correlates C. canadensis emergence to particular environmental cues is lacking; thus, predicting timely herbicide applications to manage C. canadensis can be difficult. Conyza canadensis seeds have been classified as nondormant (Buhler and Owen Reference Buhler and Owen1997) or having non–deep physiological dormancy (Baskin and Baskin Reference Baskin and Baskin2004; Karlsson and Milberg Reference Karlsson and Milberg2007) and can readily germinate once shed from the parent plant. Conyza canadensis emergence generally occurs after seed shed in early autumn or the following spring; however, emergence has been observed throughout the growing season (Buhler and Owen Reference Buhler and Owen1997; Tozzi and Van Acker Reference Tozzi and Van Acker2014). Base temperatures for germination vary by population (Tozzi et al. Reference Tozzi, Beckie, Weiss, Gonzalex-Andujar, Storkey, Cici and Van Acker2014), and germination occurs across a range of temperatures if adequate soil moisture is available (Main et al. Reference Main, Steckel, Hayes and Mueller2006). Although C. canadensis can complete its life cycle as a winter annual, a vernalization period has not been confirmed as a requirement for flowering. However, Flowering Locus C (FLC), a MADS-box gene responsible for vernalization in winter wheat (Triticum aestivum L.) and Arabidopsis thaliana, is present in C. canadensis (He et al. Reference He, Doyle and Amasino2004; Rudnoy et al. Reference Rudnoy, Bratek, Paldi, Racz and Lasztity2002). In these other species, flowering is repressed by high levels of FLC expression until a cold period attenuates expression (He et al. Reference He, Doyle and Amasino2004). The presence of this gene, if expressed, indicates that a vernalization period such as winter should be required for flower production in C. canadensis. However, C. canadensis that germinates in the spring is still able to flower and produce seed, though it never experiences a cold period in the seedling stage (Regehr and Bazzaz Reference Regehr and Bazzaz1979).
Fall-emerging C. canadensis overwinters as a rosette while spring-emerging C. canadensis seldom (or only briefly) goes through the rosette stage (Figure 1) before growing upright (Loux et al. Reference Loux, Stachler, Johnson, Nice, Davis and Nordby2006; Regehr and Bazzaz Reference Regehr and Bazzaz1979). The ability to skip the rosette stage and grow upright to set seed in the same season has been observed in many field populations (Buhler and Owen Reference Buhler and Owen1997; Regehr and Bazzaz Reference Regehr and Bazzaz1979; Tozzi and Van Acker Reference Tozzi and Van Acker2014). In 2018 and 2019, Michigan C. canadensis emergence primarily occurred in the spring, and all seedlings skipped the rosette stage, immediately growing upright (JAS, personal observation). Additionally, simultaneous emergence of rosette- and upright-type C. canadensis in the same field during mid- to late summer has been observed in Michigan (Figure 1). Whether upright C. canadensis are a separate biotype or are produced from the same parent as rosette C. canadensis may influence management practices and resistance dynamics. Therefore, our first objective was to determine whether two distinct growth types can result from one parent, thus shedding light on whether the growth type is a result of separate biotypes or environmental conditions before germination. Our observation of both growth types emerging simultaneously in the same field coupled with the presence of only rosette growth type emergence in previous greenhouse herbicide dose–response experiments lends support to the latter. Thus, we hypothesize that seeds from a single parent experience different environmental conditions before emergence and result in different growth types, rather than the presence of separate biotypes.

Figure 1. Upright- (left) and rosette- (right) type Conyza canadensis plants emerging simultaneously in a field in midsummer.
In addition, much of our understanding of C. canadensis management, particularly in glyphosate-resistant populations, stems from populations that emerge in the fall and form rosettes. Glyphosate resistance in many C. canadensis populations is due to reduced translocation of glyphosate to the target site (Dinelli et al. Reference Dinelli, Marotti, Bonetti, Minelli, Catizone and Barnes2006; Feng et al. Reference Feng, Tran, Chiu, Sammons, Heck and CaJacob2004, González-Torralva et al. Reference González-Torralva, Rojano-Delgado, Luque de Castro, Mülleder and De Prado2012; Koger and Reddy Reference Koger and Reddy2005; Moretti and Hanson Reference Moretti and Hanson2016; Nandula et al. Reference Nandula, Reddy, Duke and Poston2005). Reduced translocation is due to rapid sequestration into the vacuole; vacuolar sequestration can be suppressed under low temperatures (~12 C) and the lethality of glyphosate is reinstated in otherwise resistant populations (Ge et al. Reference Ge, d’Avignon, Ackerman and Sammons2010, Reference Ge, d’Avignon, Ackerman, Duncan, Spaur and Sammons2011). We have observed that spring-emerging, upright-type C. canadensis seedlings from a confirmed glyphosate-resistant population exhibited leaf chlorosis and plant death when exposed to glyphosate in the early spring in Michigan (JAS, personal observation). This level of control was unexpected and much greater than control of fall-emerged rosette C. canadensis plants treated in the same field under similar conditions the previous fall. Temperatures at the time of application may have been low enough to suppress vacuolar sequestration. Nevertheless, verification of sensitivity to glyphosate with a dose response experiment is crucial to determining optimal management of these two growth types. Therefore, our second objective was to determine whether growth type influences sensitivity to glyphosate in both glyphosate-resistant and glyphosate-susceptible populations. Based on anecdotal evidence from field observations, we hypothesize that rosette-type C. canadensis may be less sensitive to glyphosate than the upright type.
Materials and Methods
Seed Collection
Glyphosate-resistant C. canadensis seed samples were obtained in fall 2018 from two commercial fields in Isabella County near Mount Pleasant, MI (ISB-18) (43.61°N, 84.88°W) and (ISB-19) (43.63°N, 84.98°W) and two fields at the Michigan State University (MSU) Agronomy Farm in East Lansing, MI (MSU-18) (42.68°N, 84.49°W) and (MSU-19) (42.68°N, 84.48°W). Seed for a known susceptible C. canadensis population (S-117) was collected from a commercial field near Saint Johns, MI (43.09°N, 84.58°W). Seed heads were collected from naturally senescing C. canadensis plants that emerged in the spring as the upright type. Only seeds from upright-type plants were used, because we were interested in whether both growth types could form from one parent and parent type as well as whether formation of one growth type or the other was associated with certain conditions. Seed heads from each individual plant were kept separate and assigned a lot number. Each seed-head lot was threshed using a series of incrementally smaller sieves until seeds were adequately cleaned. Seeds were stored in labeled manila envelopes in the dark at 4 C until use.
Growth Type Experiment
Threshed Conyza canadensis seeds from one lot (i.e., one plant) of the ISB-18, ISB-19, MSU-18, and MSU-19 populations were weighed and divided into 5-mg allotments. Allotments of seed were planted in a 5 by 5 by 9 cm pot and watered. Pots were filled with potting media (Suremix Perlite, Michigan Grower Products, Galesburg, MI) to 2 cm from the top of the pot to limit light-quality interference between plants in adjacent pots. Seed lots were replicated eight times, placed in potting trays in growth chambers, and subjected to various biotic and abiotic stresses before germination (described later). Daytime light intensity was set to 240 µmol m−2 s−1 photosynthetic photo flux at plant height in a 15-h day/9-h dark photoperiod, unless otherwise stated. This light intensity falls within the range of light intensity measured in Michigan fields during the months C. canadensis typically emerges. Plants were watered as needed to maximize growth, except in the soil moisture experiment. After emergence, when plants had obtained two true leaves, pots were thinned to 1 C. canadensis plant pot−1, except in the competition experiment. Experiments were terminated when C. canadensis seedlings were large enough to determine the growth type. Conyza canadensis plants typically emerge as the rosette growth type under controlled greenhouse or growth chamber conditions (Davis et al. Reference Davis, Kruger, Stachler, Loux and Johnson2009; Dinelli et al. Reference Dinelli, Marotti, Bonetti, Minelli, Catizone and Barnes2006; Feng et al. Reference Feng, Tran, Chiu, Sammons, Heck and CaJacob2004; Koger and Reddy Reference Koger and Reddy2005; Koger et al. Reference Koger, Poston, Hayes and Montgomery2004; Main et al. Reference Main, Mueller, Hayes and Wilkerson2004; Page et al. Reference Page, Grainger, Laforest, Nurse, Rajcan, Bae and Tardif2018; Shrestha et al. Reference Shrestha, Hanson, Fidelibus and Alcorta2010; Tani et al. Reference Tani, Chachalis and Travlos2015; VanGessel Reference VanGessel2001; Zelaya et al. Reference Zelaya, Owen and VanGessel2004). Thus, the intent of testing stresses before emergence was to determine under controlled settings whether (and which) factors lead to the formation of one C. canadensis growth type more than the other.
Temperature and Photoperiod
Temperature and photoperiod effects on C. canadensis growth type were determined in growth chambers that were set to typical field conditions in May and July in Michigan. May and July were chosen because C. canadensis emerging in May are typically the upright growth type, while emergence of both rosette and upright growth types can occur on the same day in July (Figure 1). May and July daily average fluctuating temperatures and photoperiods in East Lansing, MI, are 16/4 C with a 10-h photoperiod and 27/16 C with a 15-h photoperiod, respectively (http://www.agweather.geo.msu.edu/mawn, Michigan State University, East Lansing, MI). Pots of C. canadensis seeds were placed in growth chambers set to the four combinations of May and July daily fluctuating temperatures and photoperiods (16/4 C, 10 h; 16/4 C, 15 h; 27/16 C, 10 h; 27/16 C, 15 h).
Competition
Because C. canadensis populations can be extremely dense, intraspecific competition was considered as a possible trigger for upright emergence. The effects of intraspecific competition on C. canadensis growth type was determined using the July daily average fluctuating temperatures and photoperiod. Pots of C. canadensis seed were placed in growth chambers set to 27/16 C with a 15-h photoperiod and subject to either intraspecific competition (5 plants pot−1) or no competition (1 plant pot−1).
Shading
The effects of shading on C. canadensis growth type were also determined using the July daily average fluctuating temperatures and photoperiod. Pots of C. canadensis seed were placed in growth chambers set to 27/16 C with a 15-h photoperiod and subjected to shading treatments of 0%, 30%, 60%, and 80%. These shading treatments were randomly chosen to simulate a range of potential shading effects from inter- and intraspecific competition. To obtain shading treatments, woven shade cloth colored forest green (Agriculture Solutions, Strong, ME) was placed over individual pots 5 cm above the soil surface to create shading treatments. Levels of shade were determined by using a MultispeQ (PhotoSynQ, East Lansing, MI) to measure photosynthetic photon flux at plant height.
Soil Moisture
Soil moisture effects on C. canadensis growth type were determined by placing pots of C. canadensis seed in growth chambers set to 27/16 C with a 15-h photoperiod. Soil moisture treatments consisted of 50%, 75%, and 100% field capacity. Field capacity of potting media was determined by saturating a pot with a known weight of oven-dried potting media for 24 h. After 24 h, potting media was considered to be at 100% field capacity, and pots were weighed to determine the amount of water being held. The amount of water needed in each pot for 50% and 75% field capacity treatments was determined by taking 50% and 75% of the water weight needed in 100% field capacity. Pots were weighed daily, and the appropriate amount of water was added to maintain soil moisture throughout the experiment. The biomass produced by plants from the time of germination until plants were large enough to determine the growth type was negligible; thus, weight adjustments for watering needs were not made.
Vernalization
The effects of vernalization before germination on C. canadensis growth type were determined for each population. Conyza canadensis seeds were surface planted in pots filled with potting media and watered as described earlier. Before germination, pots were placed in the MSU Wheat Breeding and Genetics Program’s vernalization room set to 4 C with an 8-h photoperiod. Conyza canadensis seeds were subject to vernalization period treatments of 2, 4, or 6 wk based on the vernalization requirement of A. thaliana (Nordborg and Bergelson Reference Nordborg and Bergelson1999). Following a vernalization period, pots were moved into growth chambers set to 27/16 C daily average temperatures with a 15-h photoperiod.
Dose–Response Experiments
Dose–response experiments were conducted to determine whether glyphosate sensitivity was affected by growth types from the same parent plant. Seeds from a single parent of two known glyphosate-resistant populations (ISB-18 and MSU-18) and a glyphosate-susceptible population (S-117) were used. To obtain the upright growth type, 0.5 g of seed from each population was surface planted in 28 by 55 by 6 cm trays filled with potting media (Suremix Perlite, Michigan Grower Products), watered, and placed in the MSU Wheat Breeding and Genetics Program’s vernalization room set to 4 C with an 8-h photoperiod for 4 wk. At the end of the 4-wk period, rosette-type trays were established by planting seeds in trays as described earlier with no vernalization period. Rosette and upright growth type trays were placed in the greenhouse at the same to time to ensure emergence of both types occurred simultaneously. Greenhouse conditions were set to 25 ± 5 C, and sunlight was supplemented to provide a total midday light intensity of 1,000 µmol m−2 s−1 photosynthetic photon flux at plant height in a 16-h day. Following emergence, seedlings were transplanted to 10 by 10 by 9 cm pots filled with potting media, 1 C. canadensis plant pot−1. Plants were watered and fertilized as needed to promote optimum plant growth. Glyphosate (Roundup PowerMax®, Bayer Crop Science, St Louis, MO) was applied to C. canadensis plants approximately 5 wk after emergence with a single-nozzle (8001E, TeeJet® Technologies, Wheaton, IL) track sprayer calibrated to deliver 187 L ha−1 at 193 kPa of pressure. Rosette plants were approximately 2-cm tall and 11-cm wide, while upright plants were approximately 15-cm tall and 19-cm wide at the time of application (Table 1). The glyphosate doses used for the susceptible population were 0, 0.04, 0.08, 0.16, 0.32, 0.64, 1.27, 2.54, 5.08, and 10.16 kg ae ha−1. The glyphosate doses used for the resistant populations were 0, 0.16, 0.32, 0.64, 1.27, 2.54, 5.08, 10.16, 20.32, and 40.64 kg ha−1. All treatments contained spray-grade ammonium sulfate (Actamaster, Loveland Products, Loveland, CO) at 2% w/w and nonionic surfactant (Activator 90, Loveland Products) at 0.5% v/v. Nontreated controls for each C. canadensis growth type from each population (S-117, ISB-18, and MSU-18) were also included in the experiment. Pots were arranged in the greenhouse in a randomized complete block design. Treatments consisted of growth type and glyphosate rate combinations for each population. Plants were blocked by plant size at the time of glyphosate exposure. Each treatment was replicated five times, and the experiment was repeated twice. Conyza canadensis control was evaluated visually at 7 and 14 d after treatment (DAT) on a scale of 0 to 100, with 0 representing no control and 100 representing complete plant death. Aboveground biomass was harvested at 14 DAT, dried at 60 C for 7 d, and weighed.
Table 1. Shoot biomass, height, width, and leaf count of Conyza canadensis plants at the time of glyphosate application. a

a Glyphosate applications were made approximately 5 wk after emergence.
b Upright-type plants were achieved by a 4-wk vernalization period of 4 C with an 8-h photoperiod before germination.
Statistical Analysis
The growth type experiments were terminated when C. canadensis seedlings were large enough to determine the growth type. Rosette- and upright-type plants were typically distinguishable at the 4-true-leaf stage, approximately 4 wk after emergence. Rosette plants formed a basal rosette that was dark green in color (Figure 2). Upright type plants skipped the rosette stage, grew upright, and were light green in color (Figure 3). The proportion of upright plants out of the total was determined for each population and for all populations combined. A one-tailed binomial test was performed with a 95% confidence interval for each condition using the binom.test function in R (R v. 3.6.2; R Core Team 2019). Because we hypothesized that both growth types could originate from a single parent, we predicted that half of the seeds would germinate as rosettes and half as upright. Thus, we used 50% as our hypothesized probability of success. If conditions tested resulted in 100% rosette-type plants, the experiment was not repeated.

Figure 2. Rosette-type Conyza canadensis seedling identified in the growth type experiment as forming a rosette, dark green in color.

Figure 3. Upright-type Conyza canadensis seedling identified in the growth type experiment as growing upright, light green in color.
Dose–response data were analyzed using the drc package in R (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015). Statistical analysis was based only on final biomass following glyphosate exposure. Dry weights from each experiment were converted to a percent of the nontreated control within each block for each population (S-117, ISB-18, and MSU-18) (Nakka et al. Reference Nakka, Thompson, Peterson and Jugulam2017; Page et al. Reference Page, Grainger, Laforest, Nurse, Rajcan, Bae and Tardif2018). The appropriate model for each growth type (comparing populations) and for each population (comparing growth types) was determined using the mselect function. Four-parameter log logistic models (Equation 1) were fit to the data as selected by the drc modelFit function using the lack-of-fit test. The effective dose to reduce biomass 50% compared with the nontreated control (ED50) was determined using the ED function for each growth type and population.
For this equation, y is the biomass response (percent of nontreated control); x is the dose; c and d are the lower and upper limits, respectively; b is the relative slope around e; and e is the ED50 (Streibig Reference Streibig1988). For each growth type and population, c and d were set to 0% and 100%, respectively. Relative population differences in ED50 values (based on a t-statistic with P ≤ 0.05) were compared using the EDcomp function and selectivity indices (R/S ratio; Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015), which are the ratios between two ED50 values from dose–response curves. In addition, relative differences between upright and rosette ED50 values were compared (Up/Ro ratio) for each population using the same method.
Results and Discussion
Growth Type Experiment
Regardless of temperature and photoperiod combination, all plants emerged as rosettes (data not shown). Conyza canadensis that emerge in May in Michigan typically skip the rosette stage and immediately grow upright. For this reason, we hypothesized that May daily average fluctuating temperatures (16/4 C) and photoperiod (10 h) would result in a high proportion of upright-type C. canadensis. Simultaneous emergence of rosette- and upright-type C. canadensis generally occurs in July (Figure 1); thus, we hypothesized mimicking July temperatures (27/16 C) and photoperiod (15 h) would result in a split population between growth types. In addition, all other stresses occurring before emergence (intraspecific competition, shading, and soil moisture) resulted in all C. canadensis emerging as the rosette type (data not shown). Extensive research has been conducted on C. canadensis germination factors such as temperature, intraspecific competition, light, and soil moisture (Buhler and Hoffman Reference Buhler and Hoffman1999; Buhler and Owen Reference Buhler and Owen1997; Main et al. Reference Main, Steckel, Hayes and Mueller2006; Nandula et al. Reference Nandula, Eubank, Poston, Koger and Reddy2006; Palmblad Reference Palmblad1968; Steinmaus et al. Reference Steinmaus, Prather and Holt2000; Tozzi et al. Reference Tozzi, Beckie, Weiss, Gonzalex-Andujar, Storkey, Cici and Van Acker2014); however, we found that none of these conditions influenced growth type. Additionally, several other researchers have reported C. canadensis emerged as the rosette type when grown in controlled greenhouse or growth chamber conditions when screening herbicide-resistant C. canadensis populations (Davis et al. Reference Davis, Kruger, Stachler, Loux and Johnson2009; Dinelli et al. Reference Dinelli, Marotti, Bonetti, Minelli, Catizone and Barnes2006; Feng et al. Reference Feng, Tran, Chiu, Sammons, Heck and CaJacob2004; Koger and Reddy Reference Koger and Reddy2005; Koger et al. Reference Koger, Poston, Hayes and Montgomery2004; Main et al. Reference Main, Mueller, Hayes and Wilkerson2004; Page et al. Reference Page, Grainger, Laforest, Nurse, Rajcan, Bae and Tardif2018; Shrestha et al. Reference Shrestha, Hanson, Fidelibus and Alcorta2010; Tani et al. Reference Tani, Chachalis and Travlos2015; VanGessel Reference VanGessel2001; Zelaya et al. Reference Zelaya, Owen and VanGessel2004). Our results coupled with results of previous research lead us to believe these conditions do not contribute to C. canadensis emerging as the upright type. However, our results indicate that C. canadensis seed shed from an upright plant can emerge as a rosette type.
Exposing C. canadensis seeds to a vernalization period of 4 or 6 wk resulted in all upright-type seedlings in all populations (Table 2). In addition, exposing ISB-19 and MSU-19 to a 2-wk vernalization period also resulted in all C. canadensis emerging as the upright type. The proportion of upright-type seedlings after a 2-wk vernalization period was 10/16 and 14/16 for ISB-18 and MSU-18, respectively. The proportion of upright-type seedlings in ISB-18 was not significantly greater than our expected 8/16 proportion (P = 0.2272). However, when we combined the results of the 2-wk vernalization period over all populations, the proportion was significantly greater than expected (P = <0.0001). Thus, C. canadensis seed exposed to a cold period of 2 wk or greater can trigger seedlings to emerge as the upright growth type. Furthermore, these populations and the susceptible population (S-117) were exposed to 4-wk vernalization periods for the following dose–response experiments, and all plants emerged as the upright growth type. A crucial next step would be to verify that seed shed from a rosette-type plant and exposed to a vernalization period also results in the upright-type plant.
Table 2. Effects of vernalization time on the proportion of Conyza canadensis emerging as upright type from seed collected from individual parent plants. a
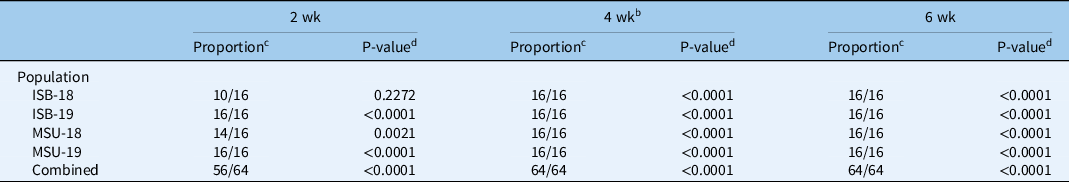
a Vernalization consisted of exposure to 4 C with an 8-h photoperiod following imbibition of water.
b Vernalization for 4 wk was repeated in time with the addition of the S-117 population. All C. canadensis plants from all populations formed the upright growth type.
c Proportion indicates the proportion of C. canadensis emerging as upright type out of the total emerged plants.
d P-value indicates the level of significance of the proportion of C. canadensis emerging as upright type being greater than the 1/2 proportion.
A vernalization requirement has not been confirmed for C. canadensis; however, Flowering Locus C (FLC), a MADS-box gene responsible for vernalization in winter wheat and A. thaliana, is present in C. canadensis (He et al. Reference He, Doyle and Amasino2004; Rudnoy et al. Reference Rudnoy, Bratek, Paldi, Racz and Lasztity2002). In these other species, flowering is repressed by high levels of FLC expression until a cold period attenuates expression (He et al. Reference He, Doyle and Amasino2004). Nondormant seeds, such as winter wheat, can imbibe low quantities of water and complete vernalization as seed before germination. In our study, the C. canadensis seeds that formed the upright type were sown, watered, and immediately exposed to a cold period before germination. Assuming C. canadensis seed is nondormant, seeds could partially imbibe water before winter without germinating and complete vernalization. This may suggest that C. canadensis does have a vernalization requirement and that a rosette is simply an overwintering structure to survive this cold period. Tozzi and Van Acker (Reference Tozzi and Van Acker2014) suggested that C. canadensis skipping the rosette stage and immediately growing upright at emergence resulted in less time and energy spent before flowering. However, overwintering rosettes can photosynthesize at low air temperatures and levels of light (Regehr and Bazzaz Reference Regehr and Bazzaz1976). Thus, skipping the rosette stage may only reduce time to flowering rather than energy expenditure. Our findings suggest that, if vernalization is required, C. canadensis can experience vernalization via imbibed seeds (spring-emerging cohorts) or in the form of a rosette structure (fall-emerging cohorts).
This theory fails to explain why rosettes are observed emerging alongside upright-type plants during midsummer. Interestingly, all seeds used in this experiment were stored in the dark at 4 C before planting, and only the seeds which were watered and reintroduced to a cold period formed the upright type, while the rest emerged as rosettes. Further research is needed to track the fate of late summer–emerging rosettes to determine whether or not they reproduce, which would verify whether vernalization is needed for flowering. Nonetheless, the majority of C. canadensis seed is shed over a 4-wk period, and the question of why some seeds germinate in the fall and others in the spring still remains. Perhaps whether seeds emerge immediately following seed shed, the following spring as upright type, or during midsummer is dependent on the ability to imbibe water. Seeds that emerge the following spring or summer may have non–deep physiological dormancy or a thicker seed coat, making them less water permeable than those that emerge in the fall. Conyza canadensis seed typically germinates on the soil surface where variable moisture conditions often exist and could create additional imbibition discrepancies. Further research is needed to explain this phenomenon. Additionally, future exploration of FLC expression in C. canadensis is needed to understand whether this species has a vernalization requirement, and how it can be met.
Dose–Response Experiments
The dose–response experiments confirmed that the S-117 upright C. canadensis were more sensitive to glyphosate compared with upright plants from the ISB-18 and MSU-18 populations (Figure 4). The ED50 values for upright growth types were 6.35 (0.85), 23.32 (3.75), and 0.24 (0.05) for ISB-18, MSU-18, and S-117, respectively (Table 3). The respective R/S ratios were 26X and 97X for ISB-18 and MSU-18 upright-type plants, respectively. Similarly, rosette C. canadensis from the S-117 population were more sensitive to glyphosate compared with rosette plants from the ISB-18 and MSU-18 populations (Figure 5). The ED50 values for rosette growth types were 1.67 (0.27), 7.73 (0.92), and 0.02 (0.01) for ISB-18, MSU-18, and S-117, respectively (Table 3). The respective R/S ratios were 84X and 386X for ISB-18 and MSU-18 rosette-type plants, respectively.
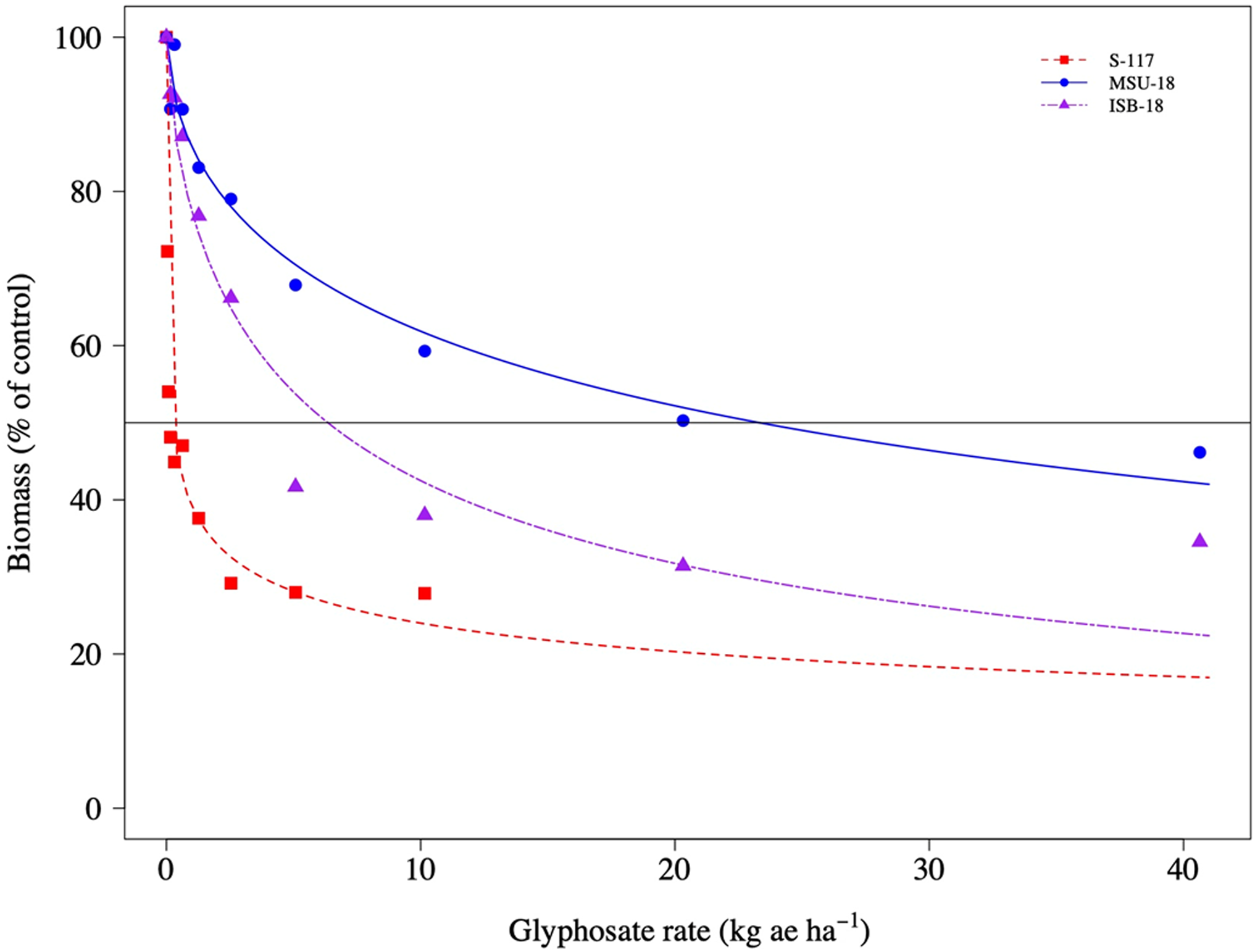
Figure 4. Biomass of upright Conyza canadensis plants of a susceptible population (S-117) and two resistant populations (ISB-18 and MSU-18) in response to applications of glyphosate.
Table 3. ED50 values (±SE), probability values (P), and ED50 ratios of Conyza canadensis populations (R/S) and growth types (Up/Ro) following glyphosate application.

a ED50 is the required dose to reduce C. canadensis biomass 50%.
b R/S is the ED50 ratio of a resistant population and the susceptible population of the same growth type.
c P-value indicates the level of significance of ED50 differences between a resistant population and the susceptible population of the same growth type.
d Up/Ro is the ED50 ratio of upright- and rosette-type plants within a population.
e P-value indicates the level of significance of ED50 differences between upright and rosette plants within a population.

Figure 5. Biomass of rosette Conyza canadensis plants of a susceptible population (S-117) and two resistant populations (ISB-18 and MSU-18) in response to applications of glyphosate.
Sensitivity to glyphosate in the ISB-18 and MSU-18 populations was different among upright and rosette growth types, but not in S-117 (Table 3; Figures 6–8). The Up/Ro ratio was 4X and 3X for ISB-18 and MSU-18, respectively. The upright type had a significantly higher ED50 value than the rosette growth type in these two glyphosate-resistant populations. Koger et al. (Reference Koger, Poston, Hayes and Montgomery2004) studied growth stage, measured by the number of leaves per plant, of rosette-type C. canadensis plants and found no differences in glyphosate resistance between growth stages of three populations. However, Shrestha et al. (Reference Shrestha, Hembree and Va2007) reported tolerance to glyphosate increased when C. canadensis plants began to grow upright. In our study, rosette and upright growth types were placed in the greenhouse, emerged, and treated at the same time. Upright growth types grew faster and accumulated more biomass by the time of treatment compared with rosette plants of the same parent (Table 1). Thus, our results could be due to differences in total leaf surface, leaf morphology, or spray coverage. Dinelli et al. (Reference Dinelli, Marotti, Bonetti, Minelli, Catizone and Barnes2006) found no differences between ED50 values of glyphosate-susceptible and glyphosate-resistant C. canadensis exposed to glyphosate at the 2-leaf stage, but reported resistant biotypes were 4 to 4.7 times more resistant when exposed at the rosette stage. The differences in sensitivity to glyphosate between growth types was far greater than the differences in biomass in our study, and no differences in glyphosate sensitivity were observed between S-117 upright- and rosette-type plants. This indicates that the mechanism of resistance present in ISB-18 and MSU-18 in combination with growth type influences glyphosate sensitivity. However, arranging planting dates to obtain both growth types of similar size at the time of glyphosate exposure could help better explain the differences observed.

Figure 6. Biomass of upright and rosette Conyza canadensis plants of a susceptible population (S-117) in response to applications of glyphosate.
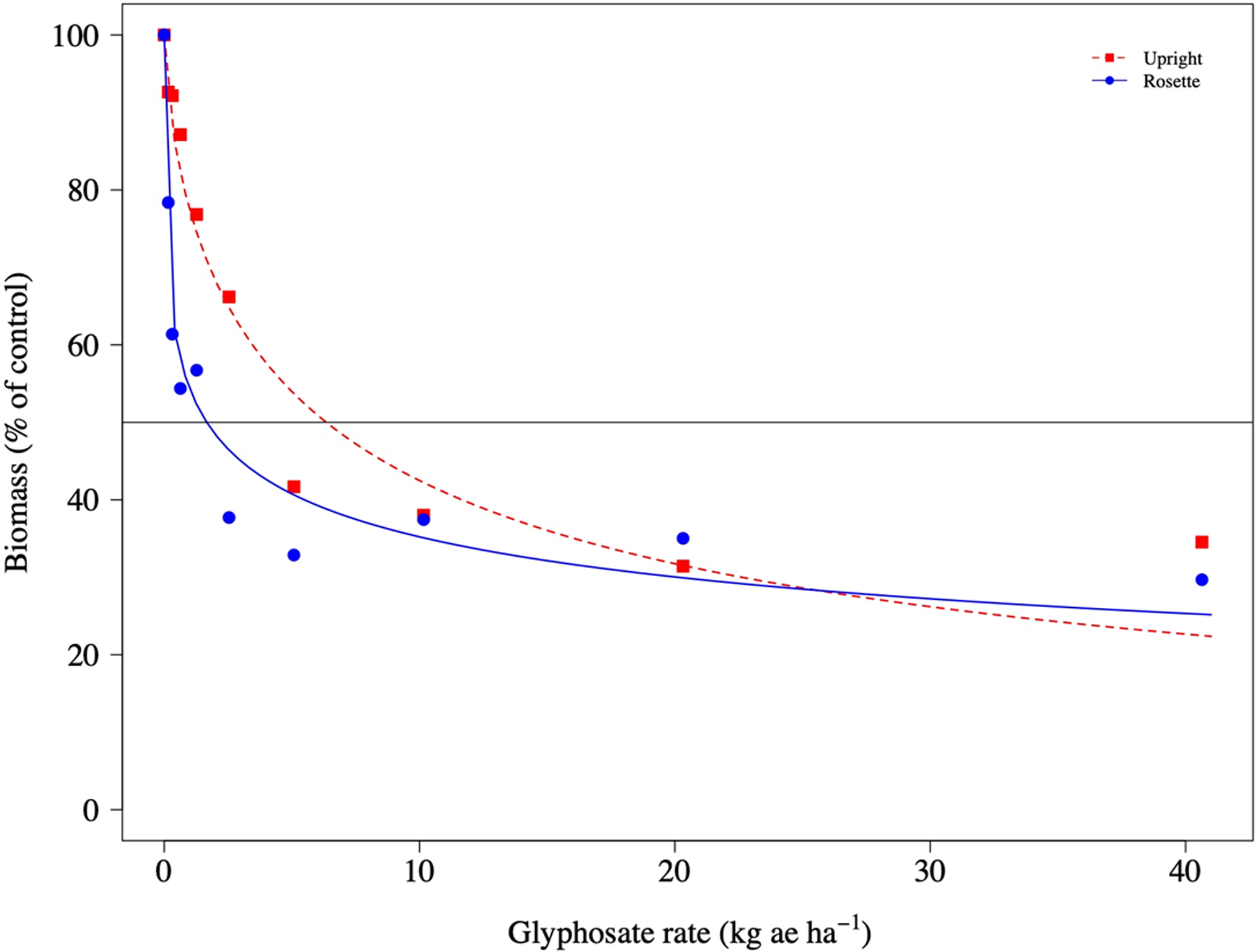
Figure 7. Biomass of upright and rosette Conyza canadensis plants of the ISB-18 population in response to applications of glyphosate.

Figure 8. Biomass of upright and rosette Conyza canadensis plants of the MSU-18 population in response to applications of glyphosate.
The R/S ratios in ISB-18 and MSU-18 are higher than those previously reported, 4- to 13-fold resistant, in populations with non–target site resistance (Main et al. Reference Main, Mueller, Hayes and Wilkerson2004; VanGessel Reference VanGessel2001). The ED50 values of the resistant populations in these studies ranged from 1.6 to 2.8 kg ha−1, whereas the range of ED50 values was 1.67 to 23.32 kg ha−1 in our study. Non–target site resistance generally results in lower R/S ratios than target-site resistance. Page et al. (Reference Page, Grainger, Laforest, Nurse, Rajcan, Bae and Tardif2018) determined the ED50 of a rosette-type accession from Michigan with known non–target site resistance to be 1.3 kg ha−1. This level of resistance is similar to ISB-18 rosettes but lower than MSU-18 rosettes in our study. A Pro-106-Ser substitution in EPSPS2 was recently characterized in an Ontario C. canadensis population, and the R/S ratios of this population as well as C. canadensis populations in Ohio and Indiana were reported to be as high as 20X (Beres et al. Reference Beres, Giese, Mackey, Owen, Page and Snow2019; Page et al. Reference Page, Grainger, Laforest, Nurse, Rajcan, Bae and Tardif2018). The high level of resistance observed in ISB-18 and MSU-18, particularly upright-type plants, would seemingly suggest the presence of target-site resistance. However, as both growth types in our study were derived from the same parent, the differences observed between rosette and upright growth types seems incompatible with target-site resistance. Nonetheless, the mechanisms of resistance present in ISB-18 and MSU-18 have not been characterized, and characterization may help explain observed differences in ED50 values between growth types.
In greenhouse and growth chamber studies, variations in temperature and photoperiod, competition, shading, and soil moisture only resulted in the rosette growth type of C. canadensis. However, a vernalization period before germination, yet after imbibition, resulted in the upright growth type for all populations. One limitation of our study was that seed was derived from a single parent, which resulted in testing only a few individuals from each population. A vernalization period before germination resulted in hundreds of upright-type plants in preparation for the dose–response experiments. However, additional experimentation with this process on other C. canadensis populations and the inclusion of more individuals is needed to verify our findings. In previous research, a Mississippi C. canadensis population was identified that grew upright 21 d after planting (Koger et al. Reference Koger, Poston, Hayes and Montgomery2004); however, our study is the first report of intentionally triggering C. canadensis to skip the rosette growth stage and immediately grow upright in a controlled setting.
Additionally, we confirmed that both rosette and upright growth types could originate from seed from the same parent plant. That being said, we focused on parent plants that emerged as the upright type, and additional experimentation with rosette-type plants is needed to confirm if this holds true, regardless of parent type. Upright plants in two glyphosate-resistant populations were less sensitive to glyphosate than rosette plants from the same parent. This was contrary to what we observed in the field. Future research to characterize the mechanisms of resistance in these populations could provide insight into the differences among growth types. The most common mechanism of resistance identified in previous research has been non–target site resistance mechanisms such as vacuolar sequestration and impaired translocation (Dinelli et al. Reference Dinelli, Marotti, Bonetti, Minelli, Catizone and Barnes2006; Feng et al. Reference Feng, Tran, Chiu, Sammons, Heck and CaJacob2004; Ge et al. Reference Ge, d’Avignon, Ackerman and Sammons2010; González-Torralva et al. Reference González-Torralva, Rojano-Delgado, Luque de Castro, Mülleder and De Prado2012; Koger and Reddy Reference Koger and Reddy2005; Moretti and Hanson Reference Moretti and Hanson2016). If non–target site resistance is present in these populations, how does growth type influence this mechanism? Also, does C. canadensis growth type affect resistance to other herbicide sites of action? Our results show that the recent shift of C. canadensis from winter-annual plants emerging as rosettes to a primarily summer-annual life cycle emerging as upright-type plants could result in new management challenges.
Acknowledgments
We thank Jinyi Chen, Gary Powell, and Brian Stiles for their technical assistance. This research received no specific grant from any funding agency or the commercial or not-for-profit sectors. No conflicts of interest have been declared.
















