INTRODUCTION
Haemophilus influenzae type b (Hib) conjugate vaccine was introduced into the United Kingdom's immunization schedule in October 1992. An initial 1-year ‘catch-up’ programme offered children up to 4 years a single dose of vaccine [Reference O'Brien1]. Infants were immunized at 2, 3 and 4 months of age without a ‘booster’ dose [2]. Resultant relatively low post-immunization antibody concentrations were further reduced by the use of a less immunogenic Hib conjugate vaccine combined with diphtheria, tetanus and acellular pertussis (DTaP-Hib) in 2000 and 2001 [Reference McVernon3]. Despite an initial marked reduction in cases, resurgence of infections was noted 8 years after vaccine introduction [Reference Trotter, Ramsay and Slack4], necessitating a national catch-up immunization campaign [Reference Heath and Ramsay5].
The United Kingdom's initial experience of high vaccine effectiveness despite low Hib antibody concentrations inspired confidence in the protective efficacy of immunological memory [Reference Heath2]. Memory immune responses are evidenced by the rapid production of high avidity antibodies on re-exposure to antigenic challenge. Direct protection following the UK infant primary course proved lower than anticipated, however, being only 61% over the first 2 years, and 27% thereafter [Reference Ramsay6], possibly masked by the catch-up campaign originally employed [Reference Ramsay6, Reference Trotter7]. The demonstration of increased risk of vaccine failure in recipients of DTaP-Hib vaccine confirmed the need for higher antibody concentrations to protect against Hib disease [Reference McVernon3].
Hib conjugate vaccines also provide indirect protection [Reference Heath2] by delaying carriage acquisition in infants [Reference Murphy8, Reference Barbour9], resulting in a decline in colonization prevalence with widespread use [Reference Takala10–Reference McVernon12]. However, episodes of carriage are temporally associated with development of natural immunity [Reference Michaels and Norden13, Reference Hall14], believed to be the reason for the observed decline in serious Hib infections with age [Reference Fothergill and Wright15–Reference Kayhty17]. It is therefore possible that lower Hib carriage prevalence may have negative consequences for maintenance of population immunity.
Mathematical models provide a useful framework within which to explore general principles regarding transmission of infectious agents and the likely impact of immunization [Reference Halloran18]. We developed a model to better understand interacting factors contributing to the rise in serious Hib infections observed in the United Kingdom. It was structured to allow examination of key assumptions about the degree and duration of natural and vaccine induced protection against carriage and invasive disease, and incorporated critical aspects of vaccine implementation. We further sought to assess the impact of a vaccine known to block Hib transmission on maintenance of immunity. Of particular interest was the way in which these effects were influenced by assumptions regarding social mixing.
METHODS
Model structure
We developed an age-structured deterministic susceptible–infected–resistant–susceptible model with modifications, expressed as a set of partial differential equations (PDEs) [Appendix 1 (available online), Fig. 1]. To avoid consideration of maternal immunity, subjects entered the model aged 6 months, progressing through until the age of 20·5 years. Initial conditions reflected the population distribution of individuals susceptible to, infected with, or resistant to Hib in the pre-vaccine era.

Fig. 1. Age-structured model. (a) Unvaccinated proportion. Mutually exclusive compartments represent the different epidemiological status of individuals in the population in relation to the infection. Arrows describe flows between these compartments. Individuals are born immunologically naive (N), until they acquire their first Hib infection (F). Following clearance of colonization, individuals seroconvert and move to the high antibody (AH) compartment. This state denotes a period of absolute resistance to reacquisition of infection. Over time, immunity wanes and individuals move into the low antibody (AL) category. Here the presence of low-level measurable immunity provides incomplete protection against infection. With the waning of antibody from low to undetectable levels, individuals are once again fully susceptible to acquisition (S). Infections acquired in the presence of low (IL) or no detectable (IN) circulating antibody are assumed to have a reduced likelihood of progressing to invasive disease compared with the first infection (F). With clearance of carriage from either the IL or IN state, immunity is stimulated to the protective (AH) level and the cycle of waning immunity, re-infection and stimulation of immunity begins again. (b) Vaccinated proportion. Immediately following primary vaccination, individuals enter the model with high (AHV), low (ALV) or undetectable (SV) antibody levels. This bypassing of the immunologically naive (N) state removes the risk of progression to invasive disease associated with the first ever infection (F) which is experienced by the unvaccinated group. The proportion in each immune category at the outset depends on the immunogenicity of vaccines in use at any given time. The degree of protection against acquisition in these three states is assumed to be identical to that experienced by unvaccinated individuals with high (AH), low (AL) or undetectable (S) immunity. As before, infections may arise in the face of low or unmeasurable antibody, in this instance denoted as ILV and INV respectively. The same risks of progression to invasive disease are assumed as from the IL and IN conditions. Following recovery from infection, immunity is boosted to a higher level than would be seen in an unvaccinated population (BV). From here, antibodies wane to the post-immunization level (AHV), with this additional step making the duration of protection following an episode of natural exposure longer in individuals who have been vaccinated. With waning of immunity, the cycle of reacquisition and boosting of antibody levels can recur.
From immunization introduction, a fraction of each new birth cohort entered the vaccinated proportion. ‘Catch-up’ immunization was simulated by transfer of the relevant subjects in the target birth cohorts to the vaccinated high antibody-resistant compartment. Children infected at the time of immunization were transferred to the corresponding low or no antibody immunized infected compartment (i.e. IL→ILV, IN→INV) as conjugate vaccination does not clear established carriage [Reference Barbour9].
Vaccine-induced immunity was assumed similar to natural immunity with two important distinctions. Hib vaccine was highly immunogenic in infants aged <2 years, in whom natural exposure provoked a negligible immune response. Further, infants primed by immunization manifested lifelong ‘booster’ antibody responses on re-exposure to Hib.
All infections in the model were episodes of oropharyngeal carriage. Invasive disease cases, rare by comparison, were not explicitly described but were calculated as a proportion of colonization events.
Data sources
Hib carriage
The largest population-based study of Hib carriage prior to widespread vaccination involved 1110 children aged 0–16 years attending outpatient clinics in the United States (Fig. 2) [Reference Michaels19]. The only similar UK-based study included children up to 72 months, and employed less sensitive microbiological methods [Reference Howard, Dunkin and Millar20]. The larger dataset was therefore used.

Fig. 2. Best fit of the ODE model used for parameter estimation to: (a) Hib carriage prevalence in the United States by age, 1976 (error bars represent 95% confidence intervals of population estimates). (b) Hib antibody titres <1·0 μg/ml in English children by age, 1990–1991 (error bars represent 95% confidence intervals of population estimates). (c) Hib antibody titres <0·15 μg/ml in English children by age, 1990–1991 (error bars represent 95% confidence intervals of population estimates).
Model outputs describing the impact of immunization on colonization rates among pre-school-aged children were compared with a series of cross-sectional carriage studies conducted in British nurseries between 1992 and 2002 [Reference McVernon12].
Hib seroepidemiology
The seroepidemiological data used to parameterize and validate the model were taken from a study of changing population immunity to Hib, using excess diagnostic serum donated by English hospitals in 1990–1991, 1994, 1997 and 2000 [Reference Trotter7].
Hib disease
Hib cases were identified by the Health Protection Agency (HPA) through laboratory reports to the Haemophilus Reference Unit (HRU) and Communicable Disease Surveillance Centre [Reference Trotter, Ramsay and Slack4]. The strain had to be cultured from a normally sterile site, unless the diagnosis was epiglottitis. For organisms sent to the HRU serotype was confirmed by polymerase chain reaction.
Vaccine coverage
The proportion of children receiving three primary Hib doses by 12 months in the United Kingdom between 1992 and 2002 was taken from Cover of Vaccination Evaluated Rapidly data (http://www.hpa.org.uk/cdr/archive04/immunisation04.htm). Quarterly uptake estimates for the 1992–1993 catch-up campaign came from a North Thames study [Reference O'Brien1].
Model parameterization
The Table summarizes the parameters estimated, and gives the final values used in the model. Their derivation is described below.
Table. Summary of parameter values

Susceptible→Infected
Individuals with antibody titres below the assay detection limit (<0·15 μg/ml) were deemed fully susceptible to the force of infection (N, S, SV). Levels of antibody between 0·15 and 1·0 μg/ml (AL, ALV) provided incomplete protection against acquisition, represented by the parameter ε, which was subjected to sensitivity analysis. Titres ⩾1·0 μg/ml conferred complete resistance.
Justification for these assumptions came from animal challenge experiments [Reference van Alphen21, Reference Kauppi-Korkeila22] and human studies describing delayed acquisition of Hib in the months following immunization [Reference Murphy8, Reference Barbour9]. While the antibody threshold necessary for protection against colonization remains undefined, the value of 5·0 μg/ml suggested by one study [Reference Fernandez23] was too high to explain herd immunity in the United Kingdom [Reference Heath2, Reference Goldblatt24]. Hib vaccine trials report immunogenicity in relation to purported correlates of long (1·0 μg/ml) and short-term (0·15 μg/ml) protection against invasive disease [Reference Kayhty17]. Use of these values to define cut-offs between high (AH, AHV), low (AL, ALV) and undetectable (S, SV) antibody compartments was therefore convenient, and consistent with the UK population experience.
Four age groups were defined: 0·5–1·99 years (home or nursery), 2–4·99 years (nursery or play group), 5–10·99 years (primary school) and 11–20·5 years (secondary or tertiary educational institution). The force of infection (λ) for each of the groups was calculated in Excel using a log-likelihood method to fit observed pre-vaccination carriage and seroepidemiology profiles to outputs of an ordinary differential equation (ODE) model (Appendix 2, online) representing the equilibrium, pre-vaccination age distribution of the PDE model.
The importance of between-group mixing was encapsulated by further defining λ as follows, where C(a, a′) determines the influence of infectious people of age group a′ in contributing to new infections in age group a, and β(a) denotes the age-dependent susceptibility to acquisition of individuals in age group a.
and
This effective contact parameter Cβ is a two-dimensional step function that can be represented by a matrix of constants. Values of β(a) were calculated according to the method of Anderson & May [Reference Anderson and May25] using the equilibrium distribution of the number of infections by age. Results comprised the elements of a ‘Who Acquires Infection From Whom’ (WAIFW) matrix under different assumptions of the relative values of C(a, a′) (Appendix 3, online). The WAIFW matrix was then used to calculate the force of infection at all subsequent time steps based on the number of carriers by age at each preceding time step:
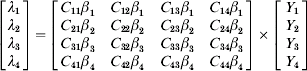
Infected→Resistant
Average carriage duration of 2·4 months was noted in toddlers in a nursery in the United States observed over one year [Reference Murphy26]. This figure was consistent with data from studies of babies in private homes [Reference Barbour9] and institutions [Reference Turk27] and was assumed independent of age and vaccination status.
Resistant→Susceptible
A temporal relationship between Hib carriage and rising antibody titres has been demonstrated [Reference Michaels and Norden13]. Age-dependent maturation of immune responsiveness to the Hib capsular polysaccharide poly-ribosyl-ribitol-phosphate (PRP) mirrors that observed following exposure to the whole organism [Reference Makela28] justifying its use as a proxy marker [Reference Robbins29], with an age-independent rate of antibody decay [Reference Kayhty30]. From these data, sensible estimates of the age-dependent duration of protection (1/ωH(a)) were made. Given the uniform waning rate, and the fixed thresholds defining boundaries between compartments, time spent in the low antibody (AL) category (1/ωL) was independent of age. These initial estimates of ωH(a) and ωL were optimized to fit population data using the same ODE model used to calculate the force of infection (Appendix 2, online).
Parameters describing resistance due to UK primary course conjugate vaccination (ωHV(a), ωLV) were estimated from clinical trials data [Reference Heath2, Reference Goldblatt24]. A series of trials conducted in US toddlers reported age-dependent antibody responses and persistence following a single dose of Hib-CRM197 conjugate (HbOC) [Reference Madore31, Reference Rothstein32], the vaccine predominantly used in the catch-up campaign. The estimated duration of low-level antibody persistence after immunization (1/ωLV) was later subjected to sensitivity analysis.
The concept of the boost response was key to considering long-term implications of immunization. Rising antibody titres following Hib colonization are an order of magnitude greater in children immunized with conjugate vaccines in infancy [Reference Barbour33]. The response to PRP boosting following receipt of an infant primary course was used as a proxy of natural exposure [Reference Robbins29]. Based on the similarity of recorded boost responses in children 1, 4 and 10 years following primary immunization [Reference Goldblatt34–Reference Makela36], a uniform peak post-boost titre of 100 μg/ml was assumed.
Vaccine ‘take’
Changing vaccine immunogenicity was represented by the proportions of individuals achieving high (⩾1·0 μg/ml), low (0·15–1·0 μg/ml) and undetectable (<0·15 μg/ml) post-immunization antibody titres (p, q and z). Given 92% immunization coverage, values for p, q and z following Hib combined with diphtheria, tetanus and whole cell pertussis (DTwP-Hib) [Reference Heath2, Reference Goldblatt24] were 0·83, 0·07 and 0·02 respectively. Following administration of three doses of DTaP-Hib to one third of all infants in the 2000–2001 birth cohorts, corresponding values of these parameters were 0·63, 0·19 and 0·10 [Reference Bell37].
HbOC, used for catch-up immunization, was highly immunogenic in children aged 12 months and older, with 98–99% achieving post vaccination Hib antibody titres >1·0 μg/ml [Reference Madore31, Reference Rothstein32].
Progression to disease
The quasi-steady-state approximations used to calculate numbers of Hib cases were defined according to a series of equations (Appendix 4, online), which describe the age-dependent risk of invasive disease (δ(a)). The efficacy of low levels of detectable antibody (θ) and immunological memory (μ) at preventing progression to bacterial invasion were subjected to sensitivity analysis.
Implementation and simulation
The method of characteristics was used to find numerical solutions to the PDEs (Appendix 1, online). These were reduced to a series of ODEs along the characteristic lines t=a+constant, within 80 sequential 3-month age cohorts. The equations were solved by fourth-order Runge–Kutta integration using Model Maker Version 4 software (Cherwell Scientific, UK). The force of infection for each age category was integrated and updated at each time step.
RESULTS
Degree and duration of natural immunity and vaccine-induced protection against carriage (ε) and invasive disease (θ, μ)
Given 100% protection of the high antibody state against colonization, the best fit to observed data was achieved assuming 25% protection of low titre antibody against carriage acquisition. The corresponding level of invasive disease risk reduction attributable to low or unmeasurable immunity in those with prior Hib exposure was 35%.
The age-dependent distribution of invasive Hib infections could not be solely attributed either to the absence of circulating anti PRP antibodies, or a heightened risk of bacterial invasion linked to the ‘first ever’ Hib infection (F). Our model achieved an excellent fit to sequential cross-sectional English data describing Hib infections and immunity (Fig. 3). In order to then reproduce the observed disease epidemiology (Fig. 4), strong age dependence of the likelihood of progression to invasive disease (δ(a)) had to be assumed.

Fig. 3. PDE model fit to English data following vaccine introduction in October 1992 at 2, 3 and 4 months (error bars represent 95% confidence intervals of observed data). (a) Prevalence of Hib antibody titres ⩾1·0 μg/ml. (b) Prevalence of Hib antibody titres <0·15 μg/ml. (c) Hib carriage prevalence in children attending nurseries, England and Wales. □, Observed; –◆–, model.

Fig. 4. Observed (□) and model (–◆–) predicted invasive Hib incidence by age and year, 1991–2002.
Effects of changing vaccine use over time (p, q, z)
The catch-up immunization programme used in the United Kingdom over the first year of vaccine introduction caused rapid interruption of Hib transmission, resulting in marked transient effects. The time between peaks was relatively short, and the natural tendency of the system was a series of declining oscillations resolving to a new stable equilibrium within 25 years (Fig. 5).

Fig. 5. Effects of vaccine scheduling and immunogenicity on all infections in the population by age group and time (vaccine introduction at t=5 years). (a) Introduction of infant immunization alone. (b) Vaccine introduction accompanied by catch-up campaign. (c) Initial catch-up campaign, use of poorly immunogenic vaccine in years t=12 and t=13 (corresponding to 2000 and 2001).
An important consequence of the use of poorly immunogenic vaccines in 2000–2001 was an expansion of the susceptible infant pool, which amplified the second transient peak in infections (Fig. 5). More importantly, it ‘exposed’ the intrinsically higher risk of progression to invasive disease noted in younger children, leading to an associated increase in observed Hib disease (Fig. 4).
Impact of interrupting Hib transmission on maintenance of population immunity
A reduction in Hib circulation had a negative impact on maintenance of specific antibodies. The model reproduced the decline in the proportion of English children with high Hib antibody titres, and the rise in those with unmeasurable levels, noted in serosurveys from the late 1990s (Fig. 3).
Effect of social mixing assumptions on carriage and disease
Initial disease control was not achievable under homogeneous mixing assumptions. WAIFW matrices associated with best fit to population data assumed strongly assortative (within age group) mixing (Appendix 3, online). In contrast, for children aged <2 years, household mixing with an older sibling was the most potent source of infection. Infants thus colonized were further instrumental in re-infecting their older siblings.
DISCUSSION
How do natural and vaccine-induced immunity protect against Hib infections?
The model explored the contribution of high- and low-titre antibody and immunological memory to direct and indirect protection against Hib disease. Based on observation of delayed carriage acquisition over the first year of life following primary immunization [Reference Murphy8, Reference Barbour9], relatively low titres [Reference Leino38] of antibody (⩾1·0 μg/ml) were deemed sufficient to prevent colonization. With waning of indirect protection induced by catch-up immunization, true effectiveness of the infant primary vaccine schedule was unmasked, resulting in resurgence in infections. Direct efficacy estimates derived from the model compared favourably with those calculated from invasive disease incidence in the UK population using the screening method [Reference Ramsay6], which were considerably less than those assumed elsewhere [Reference Leino38]. Our results cautioned against over-reliance on immunological memory as a predictor of population protection [Reference Eskola39, Reference Poolman40].
The alternative conclusion was that the main effect of vaccination was to delay the age at first colonization with Hib until the intrinsic risk of invasive infection was reduced. Consistent with this viewpoint, among Alaskan populations with a high incidence of early onset Hib disease, a change of vaccine formulation in the mid 1990s resulting in lower post-primary antibody titres was associated with an increase in invasive disease [Reference Singleton41]. Rhesus monkey models of Hib infection confirm a heightened risk of meningitis secondary to bacteraemia in the first few months of life [Reference Smith42]. Such exquisite susceptibility of infants aged <2 years to bacterial invasion has previously been attributed to the inability of this age group to respond to polysaccharide antigens [Reference Fothergill and Wright15–Reference Kayhty17]. With others, we observed an age-related risk in excess of this marker of immunological immaturity [Reference Coen43]. Rather than focusing on maintenance of herd immunity [Reference Leino38], which is likely to fluctuate, ensuring direct protection by maintaining high antibody titres throughout the period of greatest disease risk may be the most effective immunization strategy.
How much of the observed epidemiology in the United Kingdom could be attributed to the way in which Hib vaccines were used?
The United Kingdom's use of a primary immunization series without a booster left infants with relatively low antibody titres through the vulnerable second year of life [Reference Heath2]. This strategy seemed effective while herd immunity was strong [Reference Rushdy44]. It was unfortunate that waning of this effect [Reference McVernon45] was superimposed on the use of less immunogenic vaccines for the primary course during 2000 and 2001 [Reference McVernon3], further exposing infants to risk of acquisition of Hib at a vulnerable age.
How important is Hib transmission for maintenance of vaccine-induced immunity?
The model's assumptions regarding the pre-vaccination force of infection and the impact of immunization on transmission resulted in an immunity profile in the population that was an excellent fit to observed UK data [Reference Trotter7]. A decline in antibody titres within 2 years of Hib vaccine introduction has subsequently been described in English adults, suggesting that recurrent Hib colonization is necessary for maintenance of immunity in all age groups [Reference McVernon45]. On these grounds, we feel that the relatively high [Reference Coen43, Reference Leino46, Reference Auranen47] forces of infection estimated in the model were justified.
Who acquires Hib infections from whom in the population?
Consistent with previous studies, within-age group mixing was necessary to explain observed patterns of Hib transmission [Reference Coen43, Reference Auranen47], and the impact of vaccination on disease control. This was consistent with the need for prolonged close contact to acquire Hib infections in families [Reference Michaels and Norden13], day-care centres [Reference Murphy26] and orphanages [Reference Turk27]. In addition, our final mixing matrix incorporated the importance of older siblings as introducers of infection to households, with subsequent risks for pre-school-aged children, consistent with ecological studies from the 1950s [Reference Ounsted48]. Without this added assumption, invasive disease in infants would be eradicated with ease.
Our model structure was novel in incorporating the notion of the boost response following conjugate immunization. The average duration of vaccine protection in the model was intimately related to the ongoing force of infection. This was in contrast to other models of conjugate vaccination that specify vaccine efficacy for a fixed period [Reference Trotter, Gay and Edmunds49]. In this way, we were able to gain unique insights into medium- and long-term effects of immunization.
Another assumption that differed from previously published Hib models was the deliberate exclusion of boosting exposures to cross-reactive organisms. It has been observed that carriage of a range of encapsulated organisms may result in production of antibodies cross-reactive to PRP [Reference Myerowitz, Norden and Demchak50, Reference Robbins51]. One model has attributed 90% of natural immunizing contacts in British infants to such antigens [Reference Leino46]. Further, it has been speculated that ongoing colonization with these organisms would be sufficient to naturally boost immunity to Hib following a reduction in Hib circulation [Reference Leino38, Reference Auranen47, Reference Coen, Heath and Garnett52]. While antibodies stimulated by cross-reactive bacteria bind to PRP, Hib vaccination or natural infection results in the production of PRP-specific antibodies that do not necessarily bind cross-reactive antigens [Reference Insel and Anderson53]. It is thus unclear whether cross-reactive bacterial exposures would boost vaccine-induced immunity. The rapid decline in Hib titres seen among English adults following vaccine introduction further suggests that exposures to organisms other than Hib play a negligible role in boosting immunity to PRP [Reference McVernon45].
The model had the limitation of being highly parameterized to allow exploration of epidemiological questions of interest. Parameters were, however, based on a large number of data points. Outputs describing the impact of immunization were validated against 10 years' invasive disease incidence data, three serosurvey collections and three cross-sectional carriage studies. Simulating initial excellent disease control followed by late recurrence of disease could only be achieved for a narrow range of assumptions, increasing confidence in the model's theoretical conclusions.
While rapid shifts in Hib disease in temporal concordance with observed UK data were well reproduced, we were unable to exactly replicate the incidence. This was expected, given the inherent limitations of models to fully capture the complexity of population effects. Predicted rates of invasive Hib infection in 1- to 2-year-olds were higher than observed levels in the late 1990s. Non-homogeneous reseeding of infections within small groups of immunized children may have resulted in a slower rise in disease incidence than was observed in the simulation. The importance of such ‘patchy’ transmission effects has been noted in the breakdown in correlation between measles outbreaks in large cities in England following vaccine introduction [Reference Bolker and Grenfell54]. The later rise in disease cases in this age group did not fully achieve the height of the observed incidence in 2002. This may in part have been due to the absence of adults in the model, because of insufficient pre-vaccination data upon which to parameterize their force of infection. Adult Hib disease rates in Britain returned to pre-vaccination levels in 2002, in close temporal association with the rise in paediatric incidence [Reference McVernon45]. Hib carriage prevalence is high among parents of Hib carriers [Reference Barbour33] and cases [Reference Michaels and Norden13, Reference Boisvert55], who may represent the primary household infection [Reference Glode56].
Our model has informed understanding of declining Hib vaccine effectiveness in Britain, cautioning against over-reliance on immunological memory as a strategy for control of invasive bacterial disease. Alternatively, emphasis has been placed on maintenance of high post-immunization antibody titres among at-risk age groups. Recent experience with the meningococcal serogroup C conjugate vaccine in the United Kingdom has similarly highlighted the relationship between waning antibody titres and declining vaccine efficacy [Reference Trotter57]. In subsequent work, we will use the model to address the likely impact of a range of interventions on disease rates. Meanwhile, the importance of ongoing surveillance of vaccine-preventable disease in order to identify unanticipated effects of immunization programmes is reinforced.
ACKNOWLEDGEMENTS
The studies that were the basis for model parameterization represent collaborative efforts over many years and J. M. was fortunate to be able to discuss their implications with investigators who have been involved in this work. J. M. gained useful insights into key modelling concepts through conversations with Dr John Edmunds of the HPA's Statistics, Modelling and Economic Department and was further grateful for the imparted experience of Dr Caroline Trotter and Andrew Sutton in the use of Model Maker software. No specific external funding support was provided for this work.
NOTE
Supplementary information accompanies this paper on the Journal's website (http://journals.cambridge.org).
DECLARATION OF INTEREST
None.








