Introduction
The Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA–CNMNC) is an international non-profit organisation that evaluates new mineral proposals, defines mineral nomenclature guidelines, and establishes mineral classification schemes. The results of the votes on new mineral and nomenclature proposals are published bimonthly as Newsletters in the European Journal of Mineralogy and in Mineralogical Magazine, and an official IMA–CNMNC list of minerals is freely available on the CNMNC website (Pasero, Reference Pasero2023).
A careful examination of the CNMNC mineral name list shows that there are still some inconsistencies in the mineralogical nomenclature, mainly concerning the use of polymorph suffixes. The guidelines for the use of suffixes and prefixes in mineral names are reported in Hatert et al. (Reference Hatert, Mills, Pasero and Williams2013); however, these guidelines mainly concern chemical prefixes and suffixes. The present paper is a follow-up of our previous guidelines and presents recommendations for the nomenclature of polymorphs and polysomes approved by the CNMNC including suggesting some minor changes to a few mineral names, especially concerning the use of structural suffixes.
These recommendations have to be applied for future new mineral proposals, when the authors decide to use structural prefixes or suffixes, however modifications of historical and well-established names in the scientific literature have to pass through the CNMNC for approval. The goal of this paper is to provide consistent guidelines for future proposals and to fix a few minor inconsistencies in mineral names, but it is not to initiate a massive modification of mineral names.
Definitions
General guidelines for mineral nomenclature, currently in use by the CNMNC, were established by Nickel and Grice (Reference Nickel and Grice1998) and define a mineral species on the basis of its chemical composition and crystallographic properties: “If a mineral is found whose composition or crystallographic properties (or both) are substantially different from those of any existing mineral species, there is a possibility that it may be a new species.” Quasicrystals and amorphous substances can also be accepted as valid mineral species, if an enough appropriate characterisation of their chemical and physical properties demonstrates that they are distinct minerals (e.g. Bindi et al., Reference Bindi, Steinhardt, Yao and Lu2011, Reference Bindi, Yao, Lin, Hollister, Andronicos, Distler, Eddy, Kostin, Kryachko, MacPherson, Steinhardt, Yudovskaya and Steinhardt2015; Pratesi et al., Reference Pratesi, Cipriani, Giuli and Birch2003; Grey et al., Reference Grey, Elliott, Mumme, MacRae, Kampf and Mills2022).
Each mineral species is consequently characterised by an ideal chemical formula (often corresponding to an end-member formula), however mineral samples generally show significant chemical variations, compared to the ideal composition. The possible ranges of these variations, and the boundaries between mineral species involved in solid solutions, are defined by the Dominant-Constituent Rule and the Dominant-Valency Rule (Hatert and Burke, Reference Hatert and Burke2008; Bosi et al., Reference Bosi, Hatert, Hålenius, Pasero, Miyawaki and Mills2019).
Two substances with the same composition may also be defined as separate mineral species if their crystal structures are substantially different. This means that their bonding schemes must show significant differences. Many mineral species are known that show the same ideal formula but distinct crystal structures, such as pyrite/marcasite, calcite/aragonite, or andalusite/kyanite/sillimanite. These phases are known as polymorphs (Nickel and Grice, Reference Nickel and Grice1998; Fig. 1).
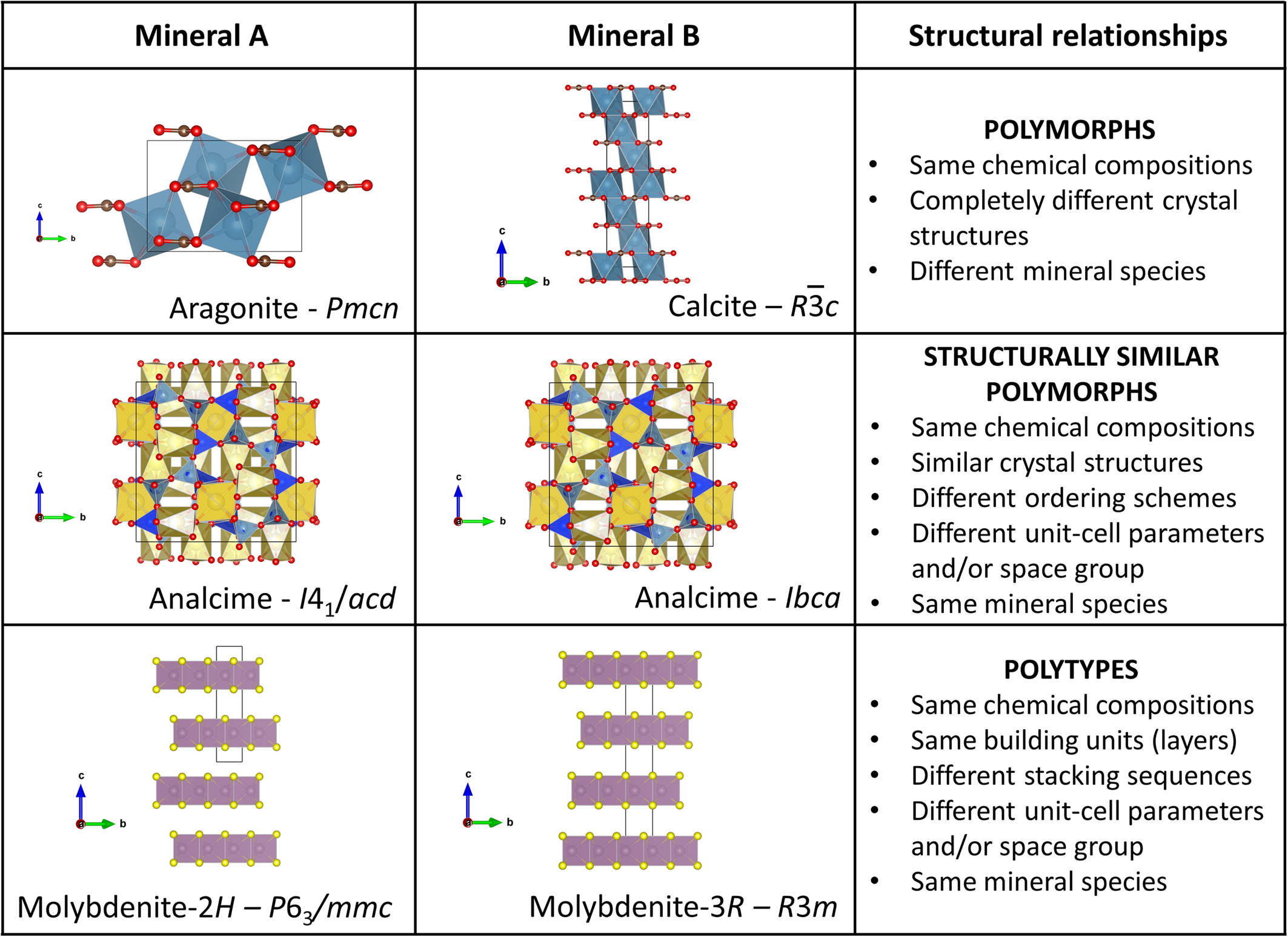
Fig. 1. Examples of crystal structures illustrating the difference between polymorphs, structurally similar polymorphs, and polytypes. The structure models were drawn with the program Vesta (Momma and Izumi, Reference Momma and Izumi2011) starting from the structural data of Graf (Reference Graf1961), de Villiers (Reference de Villers1971), Schönfeld et al. (Reference Schönfeld, Huang and Moss1983) and Mazzi and Galli (Reference Mazzi and Galli1978).
Most polymorphs show very different crystal structures, thus leading to distinct unit-cell parameters and space groups. However, space group and/or unit-cell parameters variations may also be induced by some order/disorder variations in crystal structures or structural distortion; in these cases, the two polymorphs show essentially the same bonding scheme. Such substances are named topologically similar polymorphs (we suggest here using the term ‘structurally similar polymorphs’), and are not considered as distinct mineral species (Nickel and Grice, Reference Nickel and Grice1998). A well-known example is given by analcime, which forms several structurally similar polymorphs characterised by different symmetries, induced by variations in the Al–Si ordering scheme (e.g. Mazzi and Galli, Reference Mazzi and Galli1978; Pechar, Reference Pechar1988).
Polytypes show crystal structures built by the stacking of layers of nearly identical compositions, but due to differences in the stacking sequences of the layers, variations of unit-cell parameters and/or space groups are induced. Such polytypes are not considered as separate mineral species as all layers constituting their structures have essentially the same basic topologies. Many examples of polytypes exist in the mineral kingdom and are distinguished by the addition of a polytype suffix that is not part of the mineral species name [see Guinier et al. (Reference Guinier, Bokij, Boll-Dornberger, Cowley, Ďurovič, Jagodzinski, Krishna, De Wolff, Zvyagin, Cox and Goodman1984) for the nomenclature of polytype suffixes], e.g.: molybdenite-3R and molybdenite-2H (Traill, Reference Traill1963; Wickman and Smith, Reference Wickman and Smith1970; Frondel and Wickman, Reference Frondel and Wickman1970; Fig. 1); muscovite-1M, muscovite-2M and muscovite-3T (Velde, Reference Velde1965; Güven and Burnham, Reference Güven and Burnham1967; Takeda et al., Reference Takeda, Haga and Sadanaga1971); and sapphirine-1A, sapphirine-2M, sapphirine-3A, sapphirine-4M and sapphirine-5A (Grew et al., Reference Grew, Hålenius, Pasero and Barbier2008). Those polytype suffixes can also be used to distinguish structurally similar polymorphs.
Polytypoids are also characterised by layered structures in which different stacking sequences occur; however, they do not follow the strict definition of the polytype as their chemical compositions show significant variations. Such a situation occurs in pyrrhotite, for example, which exists in many structural forms having distinct stacking sequences of nickeline-type layers; the presence of vacancies in the layers induces variations of the Fe/S ratio (Pósfai and Dódony, Reference Pósfai and Dódony1990a and Reference Pósfai and Dódony1990b; Fig. 2). Polytypoids are not considered as separate mineral species (Nickel and Grice, Reference Nickel and Grice1998).

Fig. 2. Examples of crystal structures illustrating the difference between polytypoids and polysomes. The structure models were drawn with the program Vesta (Momma and Izumi, Reference Momma and Izumi2011) starting from the structural data of de Villiers et al. (Reference de Villiers, Liles and Becker2009), Powell et al. (Reference Powell, Vaqueiro, Knight, Chapon and Sanchez2004), Arakcheeva et al. (Reference Arakcheeva, Pushcharovskii, Rastsvetaeva, Kashaev and Nadezhina1995) and Armbruster and Feenstra (Reference Armbruster and Feenstra2004).
Polysomes are characterised by different arrangements of a few basic structural units (blocks or layers), leading to minerals with structural analogies, but with significant chemical variations. Such polysomatic and homologous series are well-known in sulfosalts (e.g. Makovicky, Reference Makovicky and Merlino1997; Moëlo et al., Reference Moëlo, Makovicky, Mozgova, Jambor, Cook, Pring, Paar, Nickel, Graeser, Karup-Møller, Balic-Žunic, Mumme, Vurro, Topa, Bindi, Bente and Shimizu2008), as well as in högbomite-supergroup minerals (Armbruster, Reference Armbruster2002; Fig. 2). They may be considered as separate valid mineral species (Nickel and Grice, Reference Nickel and Grice1998).
Suffixes in mineral nomenclature
There are many mineral names that have included a suffix. Various systems are described below, note however some of these suffixes have now changed due to the CNMNC proposal described in the new guidelines below.
Levinson suffixes. The most common suffixes are the so-called Levinson suffixes, to be used in all cases in which rare earth elements, excluding scandium [= yttrium (Y) + lanthanides (La–Lu), hereafter abbreviated as REE], are an essential chemical component (Levinson, Reference Levinson1966). In such cases the most abundant REE (in atoms per formula unit) is mentioned in parentheses after the root-name [e.g. bastnäsite-(Nd); Miyawaki et al., Reference Miyawaki, Yokoyama and Husdal2013]. Species differing only in the dominant REE are considered as separate species such as those in the epidote supergroup [e.g. allanite-(Ce) and allanite-(Y) or ferriallanite-(La) and ferriallanite-(Ce); Armbruster et al., Reference Armbruster, Bonazzi, Akasaka, Bermanec, Chopin, Gieré, Heuss-Assbichler, Liebscher, Menchetti, Pan and Pasero2006; Mills et al., Reference Mills, Hatert, Nickel and Ferraris2009; Kolitsch et al., Reference Kolitsch, Mills, Miyawaki and Blass2012].
Modified Levinson suffix. This derives from the nomenclature adopted for REE-bearing minerals, and refers to specific cations, or anions, other than REE. Those suffixes are used in cases of two or more minerals having the same structural arrangement, and differing only in one chemical component at a given site [e.g. pumpellyite-(Al) and pumpellyite-(Mn2+); pertsevite-(F) and pertsevite-(OH); tetrahedrite-(Fe), tetrahedrite-(Hg), and tetrahedrite-(Zn); ardennite-(V) and ardennite-(As); Hatert et al., Reference Hatert, Pasero, Perchiazzi and Theye2007; Schreyer et al., Reference Schreyer, Armbruster, Bernhardt and Medenbach2003; Biagioni et al. Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020; Barresi et al., Reference Barresi, Orlandi and Pasero2007]. The use of modified Levinson suffixes aims at avoiding the proliferation of trivial names. Rarely, suffixes are multiples [e.g. fluorarrojadite-(BaFe), arrojadite-(BaNa) and dickinsonite-(KMnNa); jahnsite-(MnMnMg) and jahnsite-(MnMnFe); Chopin et al., Reference Chopin, Oberti and Cámara2006; Vignola et al. Reference Vignola, Hatert, Baijot, Dal Bo, Andò, Bersani, Pavese, Risplendente and Vanini2016, Reference Vignola, Hatert, Rotiroti, Nestola, Risplendente and Vanini2019a, Reference Vignola, Hatert, Baijot, Rotiroti, Risplendente and Varvello2019b].
Dominant extra-framework cations. These are occasionally used as suffixes, such as in minerals of the zeolite family. Such a notation is similar to the modified Levinson suffix, the only difference being that brackets are not used [e.g. dachiardite-Ca and lévyne-Na; labuntsovite-Fe, labuntsovite-Mg and labuntsovite-Mn; meurigite-K and meurigite-Na; obradovicite-KCu, obradovicite-NaCu and obradovicite-NaNa; Coombs et al., Reference Coombs, Alberti, Armbruster, Colella, Galli, Grice, Liebau, Mandarino, Minato, Nickel, Passaglia, Peacor, Quartieri, Rinaldi, Ross, Sheppard, Tillmanns and Vezzalini1997; Khomyakov et al., Reference Khomyakov, Nechelyustov, Ferraris, Gula and Ivaldi2001; Kampf et al., Reference Kampf, Adams, Kolitsch and Steele2009, Reference Kampf, Mills, Rumsey, Dini, Birch, Spratt, Pluth, Steele, Jenkins and Pinch2012].
Minerals of the högbomite supergroup. Suffixes such as –2N1S, –2N2S, –2N'2S, –2N3S, –2N4S, –2N6S, –6N'3S or –6N6S are used for the högbomite-supergroup members, which refer to polysomatic sequences of nolanite and spinel modules (Armbruster, Reference Armbruster2002).
Joséite. The suffixes -A and -B are used to denote two sulfide-tellurides with different Te:S ratios, Bi4TeS2 and Bi4Te2S (Cook et al., Reference Cook, Ciobanu, Wagner and Stanley2007, Reference Cook, Ciobanu, Slattery, Wade and Ehrig2021). These species are regarded as ‘questionable’ by the IMA.
Greek symbols α and β. These symbols, which denote polymorphs, are usually placed as prefixes in the chemical literature. In the CNMNC list of mineral names, these polymorph symbols are placed as suffixes, as recommended by Hatert et al. (Reference Hatert, Mills, Pasero and Williams2013): domeykite-β, fergusonite-(Ce)-β fergusonite-(Nd)-β, fergusonite-(Y)-β, lammerite-β, roselite-β, sulphur-β, uranophane-α and uranophane-β. The only exception is betalomonosovite, for which a prefix-type nomenclature has been preferred (Sokolova et al., Reference Sokolova, Abdu, Hawthorne, Genovese, Cámara and Khomyakov2015).
Roman numerals. The following cases exist with Roman numerals: andorite IV, andorite VI, baumhauerite II (questionable), mertieite-I, mertieite-II, metauranocircite-I, nováčekite-I, nováčekite-II, rathite-IV (questionable), taimyrite-I and uranocircite-II, ice-VII.
Gersdorffite. The suffixes -Pa3, -P213, and Pca21 are used to denote three distinct polymorphs with different space groups of gersdorffite, the compound NiAsS (Bayliss, Reference Bayliss1986).
Halloysite-10Å and halloysite-7Å. These are both monoclinic, but due to different hydration degrees, the interlayer distances, d 001 = c.sinβ, are different (Bailey, Reference Bailey1980).
The use of chemical prefixes and suffixes in mineral nomenclature was recently revised by Hatert et al. (Reference Hatert, Mills, Pasero and Williams2013); however, no clear guidelines were established for the use of structural prefixes and suffixes, as demonstrated by the inconsistencies listed above. The aim of this paper is to provide CNMNC recommendations for structural prefixes and suffixes that have to be followed when naming minerals. In addition, as some mineral names appear to present unnecessary or ambiguous suffixes, they have been modified (Table 1).
Table 1. Summary of the mineral species renamed in the present paper.

*Due to their close chemical compositions, these minerals are considered as true polymorphs.
Guidelines for nomenclature of polymorphs
A variety of different symbol types have been used historically to designate polymorphs, for example Greek symbols, Roman numerals, or space-group notations. The use of a new coherent nomenclature scheme for these polymorphs is recommended for the following three different situations:
Polymorphs showing different crystal systems
When polymorphs show different crystal systems, we recommend adding a prefix to distinguish them. The root-name, which was the first to be historically defined, remains unchanged, and the prefix is added to the new polymorphs to designate their distinct crystal systems.
Accepted prefixes are: cubo- (cubic); hexa- (hexagonal); tetra- (tetragonal); trigo- (trigonal); ortho- (orthorhombic); clino- (monoclinic); and anortho- (triclinic). Some valid mineral names already use these prefixes to designate the crystal systems of polymorphs:
-
Cubic: cuboargyrite.
-
Hexagonal: hexacelsian, hexaferrum, hexahydroborite, hexamolybdenum.
-
Tetragonal: tetra-auricupride, tetraferroplatinum, tetrarooseveltite, tetrataenite, tetrawickmanite.
-
Orthorhombic: bario-orthojoaquinite, orthobrannerite, orthocuproplatinum, orthojoaquinite-(Ce), orthojoaquinite-(La), orthominasragrite, orthopinakiolite, orthoserpierite, orthowalpurgite.
-
Monoclinic: clinoatacamite, clinobehoite, clinobisvanite, clinocervantite, clinoenstatite, clino-ferri-holmquistite, clino-ferro-ferri-holmquistite, clinoferrosilite, clinohumite, clinojimthompsonite, clinokurchatovite, clinometaborite, clino-oscarkempffite, clinophosinaite, clinosafflorite, clino-suenoite, clinotobermorite, clinoungemachite, clinozoisite.
-
Triclinic: anorthominasragrite.
Other mineral species have used these prefixes to designate chemical, morphological or physical features of minerals e.g.: hexahydrite and hexahydroborite (six H2O molecules in the formula); tetradymite (fourling twins); tetraferriannite and tetraferriphlogopite (Fe3+ tetrahedrally coordinated); trigonite (triangular crystal shape); orthoclase (cleavage planes at 90°); clinochlore (inclined optic axis and green colour); clinoclase (oblique cleavage planes); clinohedrite (inclined faces of the crystals); and clinoptilolite s.l. (morphology of oblique feathers). We do not recommend changing the names of these valid historical species, however in the future, such prefixes should only be used to designate crystal systems.
It is worth mentioning that the prefix ‘iso-’ was also used to designate cubic polymorphs, in isocubanite (cubic polymorph of cubanite), isoferroplatinum (cubic polymorph of tetraferroplatinum), and isolueshite (cubic polymorph of lueshite). However, the prefix ‘cubo-’ is preferred.
This nomenclature scheme can be applied to simplify the nomenclature of several species names containing Greek symbols or Roman numerals:
• Domeykite is the cubic root-name, and domeykite-β is a trigonal polymorph. Domeykite-β consequently becomes trigodomeykite.
• Fergusonite-(Y) is the oldest tetragonal species, and fergusonite-(Y)-β is a monoclinic polymorph. Consequently, fergusonite-(Y)-β is re-named clinofergusonite-(Y). In the same way, fergusonite-(Ce) is unchanged, fergusonite-(Ce)-β becomes clinofergusonite-(Ce), and fergusonite-(Nd)-β becomes clinofergusonite-(Nd).
• Ice-VII is the cubic polymorph of hexagonal ice. Consequently, ice-VII is renamed cubo-ice (the name is hyphenated to improve readability).
• Roselite was the earlier defined monoclinic species, and the later defined roselite-β is triclinic. Roselite-β is re-named anorthoroselite.
• Sulphur is the older species and is orthorhombic, whereas later defined sulphur-β is monoclinic. Sulphur-β becomes clinosulphur. Another monoclinic polymorph of sulphur is also known, which has been named rosickýite (nomenclature without suffix).
Polymorphs showing different crystal systems, but one polymorph shows a pseudosymmetry
When polymorphs with a different crystal system show a pseudosymmetry, we recommend adding the prefix ‘pseudo-’ to the secondly described polymorph. This situation occurs between pseudo-hexagonal mertieite-I, and hexagonal mertieite-II.
• Mertieite-II is renamed mertieite, and mertieite-I renamed pseudomertieite.
Other examples of minerals, in which the pseudo- prefix is used to designate a pseudosymmetry, are pseudoboleite, pseudosinhalite and pseudowollastonite.
Some mineral names have also used the prefix pseudo- to designate strong similarities with existing species (pseudobrookite, pseudocotunnite, pseudograndreefite, pseudojohannite, pseudolyonsite, pseudomalachite, pseudomarkeyite, pseudomeisserite-(NH4) and pseudorutile) or to designate polymorphic relationships (pseudolaueite).
Polymorphs showing the same crystal system, but different space groups
If two polymorphs show the same crystal system but different space groups, we recommend using the prefix ‘para-’ to distinguish them.
The prefix ‘para-’ is used widely in the mineralogical nomenclature to designate polymorphs (paraberzeliite, parabrandtite, parabutlerite, paracelsian, paracoquimbite, paracostibite, paradamite, parafransoletite, parageorgbokiite, paraguanajuatite, parahopeite, parakuzmenkoite-Fe, paralabuntsovite-Mg, paralaurionite, paralstonite, paranatisite, pararaisaite, pararammelsbergite, pararealgar, pararobertsite, pararsenolamprite, parascholzite, parascorodite, parasibirskite, parasymplesite, paratellurite, paratsepinite-Ba and paratsepinite-Na).
It has also been used for species with similar chemical compositions and/or structural features (para-alumohydrocalcite, paraershovite, parakeldyshite, paramelaconite, paramendozavilite, paramontroseite, paranatrolite, paraotwayite, parapierrotite, paraschachnerite, paraschoepite, parasterryite, paratacamite, paratacamite-(Mg), paratacamite-(Ni), paraumbite, paravauxite, paravinogradovite and parawulffite), and also for species occurring in close association (paradocrasite), or for resembling other species (paratimroseite).
The nomenclature of the following mineral species can hence be simplified:
• Uranophane-α and uranophane-β are monoclinic, however their space groups are different. As uranophane-α is the first defined species, it becomes uranophane, whereas uranophane-β becomes parauranophane.
• As the gersdorffite group contains two cubic polymorphs and an orthorhombic polymorph, it was very difficult to distinguish these species by prefixes. Consequently, the space group notations were placed as suffixes: gersdorffite-Pa3, gersdorffite-P213, and gersdorffite-Pca21 are the valid mineral names. As gersdorffite-P213 has historical priority, it has been renamed as gersdorffite. Gersdorffite-Pa3 becomes paragersdorffite, and the orthorhombic polymorph gersdorffite-Pca21 becomes orthogersdorffite.
If three or more polymorphs show the same crystal system but different space groups, the space group may be added as a suffix. Such examples are not yet known in mineralogy, and such a nomenclature should be avoided, if possible, as it is very cumbersome.
Polymorphs showing the same space group
If two polymorphs show the same space group, then the prefix ‘para-’ should ideally be used to distinguish them, though in some cases below further investigation is required.
• Baumhauerite was defined before baumhauerite II, which is a questionable species with similar chemistry. Both minerals are triclinic, P $\bar{1}$
 , therefore baumhauerite II should ideally be renamed parabaumhauerite, however we decided to keep the name of this questionable mineral unchanged as its restudy is required. ‘Baumhauerite-2a’, which appeared in the first versions of the CNMNC list of minerals, has recently been redefined and renamed argentobaumhauerite (Topa and Makovicky, Reference Topa and Makovicky2016).
, therefore baumhauerite II should ideally be renamed parabaumhauerite, however we decided to keep the name of this questionable mineral unchanged as its restudy is required. ‘Baumhauerite-2a’, which appeared in the first versions of the CNMNC list of minerals, has recently been redefined and renamed argentobaumhauerite (Topa and Makovicky, Reference Topa and Makovicky2016).• Betalomonosovite and lomonosovite have close structural relations, however Sokolova et al. (Reference Sokolova, Abdu, Hawthorne, Genovese, Cámara and Khomyakov2015) showed that their structural formulae are slightly different. These minerals are both triclinic, space group P $\bar{1}$
 . In order to show the structural relations between these species, and to avoid the use of the prefix ‘beta-’ which is not used elsewhere in the mineralogical nomenclature, betalomonosovite is renamed as paralomonosovite.
. In order to show the structural relations between these species, and to avoid the use of the prefix ‘beta-’ which is not used elsewhere in the mineralogical nomenclature, betalomonosovite is renamed as paralomonosovite.• Lammerite is monoclinic, space group P21/c, and lammerite-β is a polymorph having the same space group. Consequently, lammerite-β becomes paralammerite.
Unnecessary polymorph suffixes
Several examples occur where polymorph suffixes are unnecessary. Two situations can be distinguished as follows:
Minerals with polymorph suffixes but with different chemical compositions
Some mineral names have a polymorph suffix, but cannot be considered as true polymorphs due to distinct chemical compositions. In those cases, we recommend using the prefix ‘meta-’, which indicates a close but significantly different chemical composition. This prefix can still be used for a mineral with different (generally lower) hydration degrees, as well as the prefix ‘hydro-’ which indicates a higher hydration degree, compared to the root species.
The prefix meta- is already widely used in mineral nomenclature, where it generally designates two species with different water contents: meta-aluminite, meta-alunogen, meta-autunite, metacalciouranoite, metahaiweeite, metaheinrichite, metahewettite, metahohmannite, metakahlerite, metamunirite, metanováčekite, metarauchite, metarossite, metasaléeite, metaschoepite, metasideronatrite, metastudtite, metaswitzerite, metatamboite, metatorbernite, metatyuyamunite, metauramphite, metauranocircite-I, metauranopilite, metauranospinite, metauroxite, metavandendriesscheite, metavanmeersscheite, metavanuralite, metavauxite, metavivianite, metazellerite and metazeunerite.
A few examples exist where the prefix designates minerals with chemical compositions significantly different from those of the root species: metadelrioite, metaköttigite and metaschoderite. The prefix meta- is also used for minerals with the same chemical composition as that of the root species, but with a different crystal structure: metacinnabar, metastibnite, metathénardite and metavariscite. Some of these names were given to denote the thermodynamic metastability of these species.
In addition, this prefix still exists in some species where the root-name has been discredited: meta-ankoleite, metaborite, metakirchheimerite, metalodèvite, metanatroautunite and metavoltine.
The following species are now covered by this guideline and are recommended for renaming though some require further study:
• Nováčekite-I contains 12 H2O molecules per formula unit (pfu), nováčekite-II contains 10 H2O molecules pfu, and metanováčekite contains 8 H2O molecules pfu. Consequently, nováčekite-I is renamed hydronováčekite, and nováčekite-II is renamed nováčekite. The name of metanováčekite remains unchanged, as this species is the weakly-hydrated end-member.
• Joséite-A corresponds to Bi4TeS2, and joséite-B to Bi4Te2S. As joséite-B was the first historically-defined species (Atencio, Reference Atencio2020), it should become joséite, whereas joséite-A should be renamed metajoséite. These minerals were investigated recently by Cook et al. (Reference Cook, Ciobanu, Slattery, Wade and Ehrig2021), who demonstrated that they are valid species, however their official CNMNC status is still ‘questionable’. For that reason, and while waiting for further structural investigations, we decided to keep these names unchanged.
• Rathite-IV is a questionable species, with a chemical composition significantly different from that of rathite. It should ideally become metarathite, but we decided to keep this name unchanged as a structural investigation of this questionable species is necessary.
• Halloysite-10Å and halloysite-7Å are both monoclinic, but due to different hydration degrees, the interlayer distances, d 001 = c.sinβ, are different. Bailey (Reference Bailey1980) recommended adding the approximate value of c.sinβ as a suffix, but this procedure is ill-advised as such suffixes are similar to polytype suffixes. Halloysite-10Å is the most hydrated species, Al2Si2O5(OH)4⋅2H2O, whereas halloysite-7Å is less hydrated, Al2Si2O5(OH)4. As halloysite-7Å corresponds to the first historically-defined species (Berthier, Reference Berthier1826), halloysite-7Å is renamed halloysite, and halloysite-10Å is renamed hydrohalloysite.
Minerals with polymorph suffixes that are no longer necessary
For some minerals, a polymorph suffix exists, though the original un-suffixed root-name does not exist anymore. In those case, we recommend deleting the unnecessary suffix.
The species affected by this guideline are:
• metauranocircite-I that becomes metauranocircite;
• taimyrite-I that becomes taimyrite; and
• uranocircite-II that becomes uranocircite.
Polysomatic sequences and related phases
Polysomes are mineral species with distinct chemistries, which are produced by the stacking of different structure modules. Polysomatic symbols have to be placed as a suffix, which indicates the number and types of modules which alternate in the structure. In the högbomite supergroup, such suffixes are used in ferrohögbomite-2N2S, ferronigerite-2N1S, ferronigerite-6N6S, ferrotaaffeite-2N’2S, ferrotaaffeite-6N’3S, magnesiohögbomite-2N2S, magnesiohögbomite-2N3S, magnesiohögbomite-2N4S, magnesiohögbomite-6N6S, magnesionigerite-2N1S, magnesionigerite-6N6S, magnesiotaaffeite-2N’2S, magnesiotaaffeite-6N’3S, zincohögbomite-2N2S and zincohögbomite-2N6S (Armbruster, Reference Armbruster2002). The suffixes explicitly indicate the number of nolanite (N) and spinel (S) modules occurring in these mineral species.
In the sartorite group, the polysomes are produced by the stacking of the same basic unit with an a parameter of 4.2 Å (Topa et al., Reference Topa, Makovicky, Stoeger and Stanley2017). The polysomes show the same P21/c symmetry, and suffixes have been added to indicate the number of basic sartorite motifs involved in the stacking sequences. The crystal structures of heptasartorite, enneasartorite and hendekasartorite are characterised by the stacking of 7, 9 and 11 basic sartorite motifs, respectively.
In order to prevent any confusion, we recommend using different prefixes for crystal systems and for homologous series. For example, tetra- and hexa- prefixes are used to designate tetragonal and hexagonal crystal systems, however the prefixes quadra- and sena- can be used to distinguish minerals in homologous series.
This nomenclature scheme has been applied in the andorite series.
• Andorite VI and andorite IV are sulfosalts showing modular structures, characterised by the stacking of slabs along the c axis producing c unit-cell parameter values = n × 4.3 Å. These names were given by Donnay and Donnay (Reference Donnay and Donnay1954), to indicate the n value of four in andorite IV, and of six in andorite VI. Strictly speaking, these minerals cannot be classified as polytypes, but rather as chemical twins; their observed chemical compositions are slightly different (Nespolo et al., Reference Nespolo, Ozawa, Kawasaki and Sugiyama2012). In the literature the prefixed names quatrandorite and senandorite also appear (Moëlo et al., Reference Moëlo, Makovicky and Karup-Møller1984; Nespolo et al., Reference Nespolo, Ozawa, Kawasaki and Sugiyama2012), and recently, arsenquatrandorite was described as an As-rich analogue of andorite IV (Topa et al. Reference Topa, Makovicky, Putz, Zagler and Tajjedin2013). We consequently suggest uniformising the nomenclature of the andorite homologous series, by renaming andorite IV as quatrandorite, and andorite VI as senandorite.
Conclusions
These new guidelines for the nomenclature of polymorphs and polysomes must be followed for new mineral species proposals, when the authors decide to use structural prefixes or suffixes instead of a trivial name, as well as for nomenclature proposals. The guidelines will not be applied systematically to all existing mineral species, as the CNMNC recommends avoiding changing names, especially for grandfathered and well-established species (Hatert et al., Reference Hatert, Mills, Pasero and Williams2013). However, a few names have been modified for consistency; the species renamed in this paper are summarised in Table 1. Questionable species joséite-A, joséite-B, rathite-IV and baumhauerite II have not been renamed at this stage as future studies are necessary to clarify their status.
Acknowledgements
The authors thank the reviewers and Editors for their comments.
Competing interests
The authors declare none.





