Exposure to adverse life events during childhood has consistently been linked to increased risk of depression in adulthood. Reference Arnow1,Reference Chapman, Whitfield, Felitti, Dube, Edwards and Anda2 However, early life exposure to adversity alone is likely not sufficient to elicit depression later in life. In fact, evidence to date suggests that some individuals are more susceptible to negative outcomes following exposure to adversity and that this susceptibility in part may reside in an individual's genome.
Over the past decade, genetic variation in over 35 genes have been examined for their interaction with adverse life events. Reference Mandelli and Serretti3 Among these genes, the serotonin transporter (SLC6A4) and its corresponding length polymorphisms located in the 5′-flanking promoter region (5-HTT gene-linked polymorphic region, 5HTTLPR) Reference Heils, Teufel, Petri, Stöber, Riederer and Bengel4 is arguably the most studied in relation to depression and exposure to adverse life events, namely severe childhood abuse. The initial study conducted by Caspi et al Reference Caspi, Sugden, Moffitt, Taylor, Craig and Harrington5 reported that young adults with the s allele were more likely to develop depression after exposure to severe childhood abuse compared with those with the l allele and proposed that the s allele may be a moderator (effect modifier) of the relationship between stress and depression. However, meta-analyses have both supported Reference Karg, Burmeister, Shedden and Sen6 and rejected this notion Reference Wankerl, Wust and Otte7 and researchers have since criticised the reliance on cross-sectional designs, inadequate adjustment for potential confounders and clinical utility of the gene × environment studies conducted to date. Reference Uher8,Reference Keller9
In response to these criticisms, the present study examined the interaction between the 5HTTLPR polymorphism and history of severe childhood abuse on depressive symptom trajectories in a 5-year prospective cohort of primary care attendees. Linear mixed modelling was used to test whether 5HTTLPR genotype moderated the effect of severe childhood abuse on depressive symptom trajectories. We hypothesised that s/s carriers would be more likely to experience severe depressive symptom trajectories following exposure to severe childhood abuse compared with s/l or l/l carriers.
Method
Participants
Participants were recruited from the Diagnosis, Management and Outcomes of Depression in Primary Care (diamond) study, an ongoing prospective cohort that commenced in 2005 with an aim to document the experiences, health outcomes, treatment and service use of primary care patients identified as having clinically relevant depressed mood at screening from 30 rural and metropolitan general practices randomly recruited in Victoria, Australia. Reference Gunn, Gilchrist, Chondros, Ramp, Hegarty and Blashki10 Patients were eligible for the diamond cohort if they were: (a) aged 18–75 years, (b) able to read English, (c) not terminally ill, (d) did not reside in a nursing home and (e) scored 16 or higher on the Center for Epidemiologic Studies Depression Scale. Reference Radloff11 Participants were assessed annually using postal surveys as well as computer-assisted telephone interviews. In 2011 (cohort year 6), participants enrolled in the cohort were invited to provide a saliva sample for DNA extraction and genotyping.
All procedures were conducted in accordance with principles expressed in the Declaration of Helsinki and obtained approval from the University of Melbourne Human Research Ethics Committee (Ethics ID 1135247.1).
Measures
The main outcome of interest was depression symptom severity assessed at baseline and 1-, 2-, 3-, 4- and 5-year post-baseline using the Primary Care Evaluation of Mental Disorders Patient Health Questionnaire-9 (PHQ-9). The PHQ-9 is a self-administered questionnaire of depressive symptoms based directly on the nine signs and symptoms of major depressive disorder as described in the DSM-IV 12 and has been validated to screen and monitor depression severity in the primary care setting. Reference Spitzer, Kroenke and Williams13 The PHQ-9 asks respondents to rate their symptoms over the past 2 weeks and is scored on a scale of 0 (‘not at all’) to 3 (‘nearly every day’) for each item with a range of 0–27. Reference Kroenke, Spitzer and Williams14 Scores of 5, 10, 15 and 20 on the PHQ-9 represent cut-points for mild, moderate, moderately severe and severe depression respectively. Reference Kroenke, Spitzer and Williams14
The Child Maltreatment History Self-Report (CMHSR) Reference MacMillan, Fleming, Trocme, Boyle, Wong and Racine15 was used to measure history of severe childhood abuse. The CMHSR includes questions relating to sexual and physical abuse by an adult before 16 years of age. The distinction of severe physical abuse was originally based on expert consensus that responses in this severe category were more likely to cause injury requiring medical treatment and has been shown to have replicable psychometric properties. Reference Straus, Straus and Gelles16 The distinction of severe sexual abuse was based on items from a national Canadian survey of unwanted touching in sexual areas and attempted or achieved unwanted intercourse. Reference Bagley17
At baseline, assessments were also made of age, gender highest level of completed education, smoking status, family history of depression, present DSM-IV diagnosis of depression, antidepressant use, anxiolytic use, herbal/alternative medication use, health status, Reference Ware, Kosinski and Keller18 quality of life, 19 alcohol abuse/dependence 20 and significant negative life events in the past year. Reference Norbeck21
Polymorphism selection, DNA extraction and genotyping
The 5HTTLPR and its A-to-G internal single nucleotide polymorphism (rs25531) were selected for genotyping. Individuals were categorised as s/s if they were s/s, s/lG or lG/lG . Those who had s/lA or lG/lA were categorised as s/l and individuals with l A l A were categorised as l/l. To detect the presence of population stratification, 60 unlinked ancestry informative markers (AIMs; Table DS1) representing the three HapMap phase III populations (Northern/Western European, Han Chinese and Yoruba in Nigeria) were also genotyped. Reference Enoch, Shen, Xu, Hodgkinson and Goldman22
DNA was recovered from stabilised saliva samples using the manual prep IT system according to manufacturer's instructions (Oragene DNA (OG-500); DNA Genotek, Ontario, Canada). DNA precipitates were allowed to resuspend for a minimum of 48 h before quantification by fluorimetry (QuantiFluor™ dsDNA System; Promega Corporation, Madison, Wisconsin, USA) in conjunction with a Gemini™ Spectramax XPS fluorescence microplate reader (Molecular Devices, LLC, Sunnyvale, California, USA). DNA stocks were adjusted to a working concentration of between 10 and 50 ng/µL for subsequent genotyping.
The SLC6A4 locus was amplified for both the 5HTTLPR and rs25531 polymorphisms. The promoter region was amplified using the primers SERT1 and SERT2. Reference Gelernter, Kranzler, Coccaro, Siever and New23 The 5HTTLPR polymorphism was analysed as described in Wendland et al Reference Wendland, Martin, Kruse, Lesch and Murphy24 with modifications. Reactions were performed in a total volume of 24 µL comprising ‘KapaTaq A’ polymerase (0.6 units), ‘High-Yield’ Reaction Buffer A (2.4 µL); both Kapa Biosystems, Boston, Massachusetts, USA), 0.48 µL dNTP mix (2.5 mM; Bioline Australia, Alexandria, New South Wales, Australia), 20–50 ng template DNA, and supplementary Mg2+ (1.0 mM final; MgSO4, 50 mM; Bioline Australia) and 7-deaza-dGTP (0.2 mM final; New England Biolabs, Ipswich, Massachusetts). After initial denaturation (95°C, 7 min), cycling parameters were 94°C (60 s), 61°C (60 s), 72°C (60 s) (20 cycles) followed by a further 20 cycles of 94°C (60 s), 63°C (60 s), 72°C (60 s), and a final extension period of 72°C for 30 min. All polymerase chain reactions were conducted using Veriti 96-well thermal cyclers (Life Technologies Australia Scoresby, Victoria, Australia). Genotypes were determined by capillary electrophoresis using an AB3730 Genetic Analyser fitted with a 36 cm array, with sizing determined against a Genescan LIZ500 molecular weight marker. Analysis was performed in Genemapper V3.7 software (Life Technologies Australia, Scoresby, Victoria, Australia).
Statistical analysis
Chi-squared analysis was used to detect departures from Hardy–Weinberg equilibrium of 5HTTLPR. To estimate the presence of population stratification, the 60 AIMs were used to assign each participant to the HapMap ancestral group for which they carried the greatest proportion of that population's AIMs.
Linear mixed models were used to determine trajectory differences in PHQ-9 depressive symptom severity over the 5-year follow-up period by 5HTTLPR and history of severe child abuse. Before modelling, genotype variables and covariates were centred. Reference Kraemer and Blasey25 Potential covariates were assessed for their association with abuse history and genotype using chi-squared, Fisher's exact or analysis of variance tests, depending on the variable structure. Covariates with P-values ≤0.05 were retained for adjusted analyses (Table 1). The unadjusted model included fixed effects of time, genotype, child abuse, and a time × genotype, time × child abuse, genotype × child abuse, and time × genotype × child abuse interaction terms as well as random effects of individual, primary care site, intercept (baseline PHQ) and time. Adjusted models included relevant covariates as well as covariate × time, covariate × genotype, and covariate × child abuse interaction terms as recently recommended. Reference Keller26 Covariance models used for the random and repeated effects were unstructured and first-order autoregressive, respectively. To improve model fit, a stepwise elimination strategy was employed by comparing −2 log likelihood (−2LL) information criteria using unrestricted maximum likelihood estimations. The covariate term with the lowest F-test score was removed, one at a time, and discarded if the difference in the −2LL between the simpler model and more complex model was less than 3.86 (i.e. chi-squared value with 1 degree of freedom at P=0.05). The final reduced adjusted model was tested with restricted maximum likelihood estimation. Modelling was performed using the SPSS software (SPSS, version 22.0, IBM, Chicago, Illinois, USA). Reference West, Welch and Galecki27
Table 1 Participant characteristics measured at baseline by history of severe child abuse and genotype a

| Baseline variables | Full sample | No HoSCA | HoSCA | ss | sl | ll | ||
|---|---|---|---|---|---|---|---|---|
| (n=333) | (n=200) | (n=133) | P | (n=78) | (n=195) | (n=60) | P | |
| Severe childhood abuse, n (%) | ||||||||
| Either physical or sexual | 133 (39.9) | 133 (100) | 25 (32.1) | 87 (44.6) | 21 (35.0) | 0.110 | ||
| Both physical and sexual | 44 (13.2) | 44 (31.1) | 10 (12.8) | 28 (14.4) | 6 (10.0) | 0.679 | ||
| Only physical | 46 (13.8) | 46 (34.6) | 9 (11.5) | 32 (16.4) | 5 (8.3) | 0.228 | ||
| Only sexual | 43 (12.9) | 43 (32.3) | 6 (7.7) | 27 (13.8) | 10 (16.7) | 0.247 | ||
| Characteristic | ||||||||
| Female, b n (%) | 235 (70.6) | 149 (74.5) | 86 (64.7) | 0.054 | 55 (70.5) | 133 (68.2) | 47 (78.3) | 0.322 |
| Age, years: mean (s.d.) | 49.0 (11.9) | 48.3 (12.4) | 50.2 (11.1) | 0.101 | 49.1 (12.8) | 49.1 (11.5) | 48.5 (12.5) | 0.953 |
| Born in Australia, n (%) | 291 (87.4) | 177 (88.5) | 114 (85.7) | 0.453 | 72 (92.3) | 169 (86.7) | 50 (83.3) | 0.259 |
| English as a first language, n (%) | 327 (98.2) | 196 (98.0) | 131 (98.5) | 0.739 | 77 (98.7) | 192 (98.5) | 58 (96.7) | 0.609 |
| Northern European genetic ancestry, n (%) | 333 (100) | 200 (100.0) | 133 (100.0) | 78 (100.0) | 195 (100.0) | 60 (100.0) | ||
| Depression | ||||||||
| Diagnosed major depression, n (%) | 158 (47.4) | 84 (43.8) | 74 (56.1) | 0.290 | 35 (47.3) | 97 (50.5) | 26 (44.8) | 0.719 |
| Sum of negative life events over past 12 months, mean (s.d.) | 2.0 (1.6) | 1.9 (1.5) | 2.2 (1.7) | 0.152 | 2.1 (1.7) | 2.1 (1.5) | 1.9 (1.6) | 0.657 |
| Relative with a history of depression, n (%) | 207 (62.2) | 122 (72.6) | 85 (78.7) | 0.255 | 47 (74.6) | 128 (75.3) | 32 (74.4) | 0.990 |
| Medication use, n (%) | ||||||||
| Antidepressant | 198 (59.5) | 114 (57.0) | 84 (63.2) | 0.262 | 44 (56.4) | 122 (62.6) | 32 (53.3) | 0.365 |
| Anxiolytic | 78 (23.4) | 43 (21.5) | 35 (26.3) | 0.309 | 16 (20.5) | 49 (25.1) | 13 (21.7) | 0.674 |
| Sedative b | 57 (17.1) | 27 (13.5) | 30 (23.3) | 0.022 | 12 (15.8) | 39 (20.1) | 6 (10.2) | 0.194 |
| Antipsychotic | 34 (10.2) | 19 (9.5) | 15 (11.3) | 0.600 | 5 (6.4) | 22 (11.3) | 7 (11.7) | 0.447 |
| St John's wort | 31 (9.3) | 21 (10.6) | 10 (7.5) | 0.345 | 5 (6.4) | 18 (9.3) | 8 (13.6) | 0.363 |
| Health | ||||||||
| Self-rated health, good-to-excellent, b n (%) | 208 (62.5) | 137 (68.5) | 71 (53.4) | 0.005 | 47 (60.3) | 116 (59.5) | 45 (75.0) | 0.085 |
| Substance use, n (%) | ||||||||
| Alcohol abuse/dependence | 44 (13.2) | 26 (13.5) | 18 (13.6) | 0.980 | 12 (16.2) | 27 (14.1) | 5 (8.6) | 0.429 |
| Substance abuse/dependence b | 306 (91.9) | 6 (3.1) | 12 (9.1) | 0.021 | 7 (9.5) | 10 (5.2) | 1 (1.7) | 0.148 |
| Current smoker | 74 (22.2) | 38 (19.8) | 36 (28.8) | 0.064 | 20 (26.0) | 40 (21.7) | 14 (25.0) | 0.723 |
| Socioeconomic status, n (%) | ||||||||
| Highest level of education | 0.098 | 0.598 | ||||||
| Year 12 or less | 161 (48.3) | 92 (46.2) | 69 (52.3) | 42 (53.8) | 93 (47.9) | 26 (44.1) | ||
| Certificate or diploma | 84 (25.2) | 52 (26.1) | 32 (24.2) | 14 (17.9) | 48 (24.7) | 22 (37.3) | ||
| Bachelor's degree of higher | 86 (25.8) | 55 (27.6) | 31 (23.5) | 22 (28.2) | 53 (27.3) | 11 (18.6) | ||
| Managing on available income | 0.060 | 0.713 | ||||||
| Easily/not too bad | 160 (48.0) | 103 (52.0) | 57 (42.9) | 37 (48.1) | 90 (46.4) | 33 (55.0) | ||
| Difficult some of the time | 116 (34.8) | 67 (33.8) | 49 (36.8) | 31 (40.3) | 68 (35.1) | 17 (28.3) | ||
| Difficult all of the time/impossible | 55 (16.5) | 28 (14.1) | 27 (20.3) | 9 (11.7) | 36 (18.6) | 10 (16.7) | ||
| World Health Organization Quality of Life and Functioning, mean (s.d.) | ||||||||
| Environmental context | 64.1 (13.0) | 65.1 (13.2) | 62.7 (12.6) | 0.097 | 64.1 (13.9) | 64.0 (12.9) | 64.6 (13.0) | 0.940 |
| Social context b | 49.2 (23.8) | 52.1 (23.2) | 44.5 (23.9) | 0.008 | 52.1 (23.6) | 48.0 (22.9) | 49.4 (26.7) | 0.435 |
HoSCA, history of severe child abuse.
a Categorical and continuous variables were compared using chi-squared test and analysis of variance respectively.
b Inclusion of variable as a covariate to be adjusted for in the linear mixed model.
Results
A total of 498 individuals were enrolled in the diamond study at the time of DNA collection in cohort year 6 and 344 (69%) participants returned a DNA sample. Six individuals who were missing genotype data and five individuals who were missing childhood abuse data were excluded, resulting in a total of 333 individuals available for analysis (Table 1). No differences in demographic or clinical characteristics were detected by genotype. However, sedative use, self-rated health, non-alcohol-related substance abuse and the abbreviated World Health Organization Quality of Life and Functioning instrument of social relationships (WHOQOL-BREF social) differed by history of severe childhood abuse (Table 1) and as such were included in the adjusted models. 5HTTLPR genotype was in Hardy–Weinberg equilibrium (χ 2=3.51, P≥0.06) and all participants were of Northern/Western European ancestry based on 60 unlinked AIMs.
The effect of severe child abuse on depressive symptoms trajectories was moderated by 5HTTLPR genotype (F 2, 318.2=3.1, p covariate adjusted=0.047). Post hoc analysis (Fig. 1) showed that s/s genotype carriers with a history of child abuse had significantly greater baseline depressive symptom severity compared with those without a history (mean difference=3.4, 95% CI 1.2–5.6, p covariate adjusted=0.003) and this effect persisted throughout the 5-year period of observation. However, individuals with the l/s or l/l genotype had similar depressive symptom trajectories regardless of severe child abuse history (s/l: mean difference=0.8, 95% CI −1.1–1.3, p covariate adjusted=0.892; l/l: mean difference=0.3, 95% CI −1.9–2.5, p covariate adjusted=0.773). Furthermore, among individuals without a history of severe child abuse, s/s genotype carriers appeared to have more favourable depressive symptom trajectories compared with s/l and l/l genotype carriers, albeit not statistically significant (F=2.72, p covariate adjusted=0.068, Fig. DS1). Parameter estimates for all terms included in the unadjusted and adjusted models are provided in Tables DS2 and DS3 respectively.
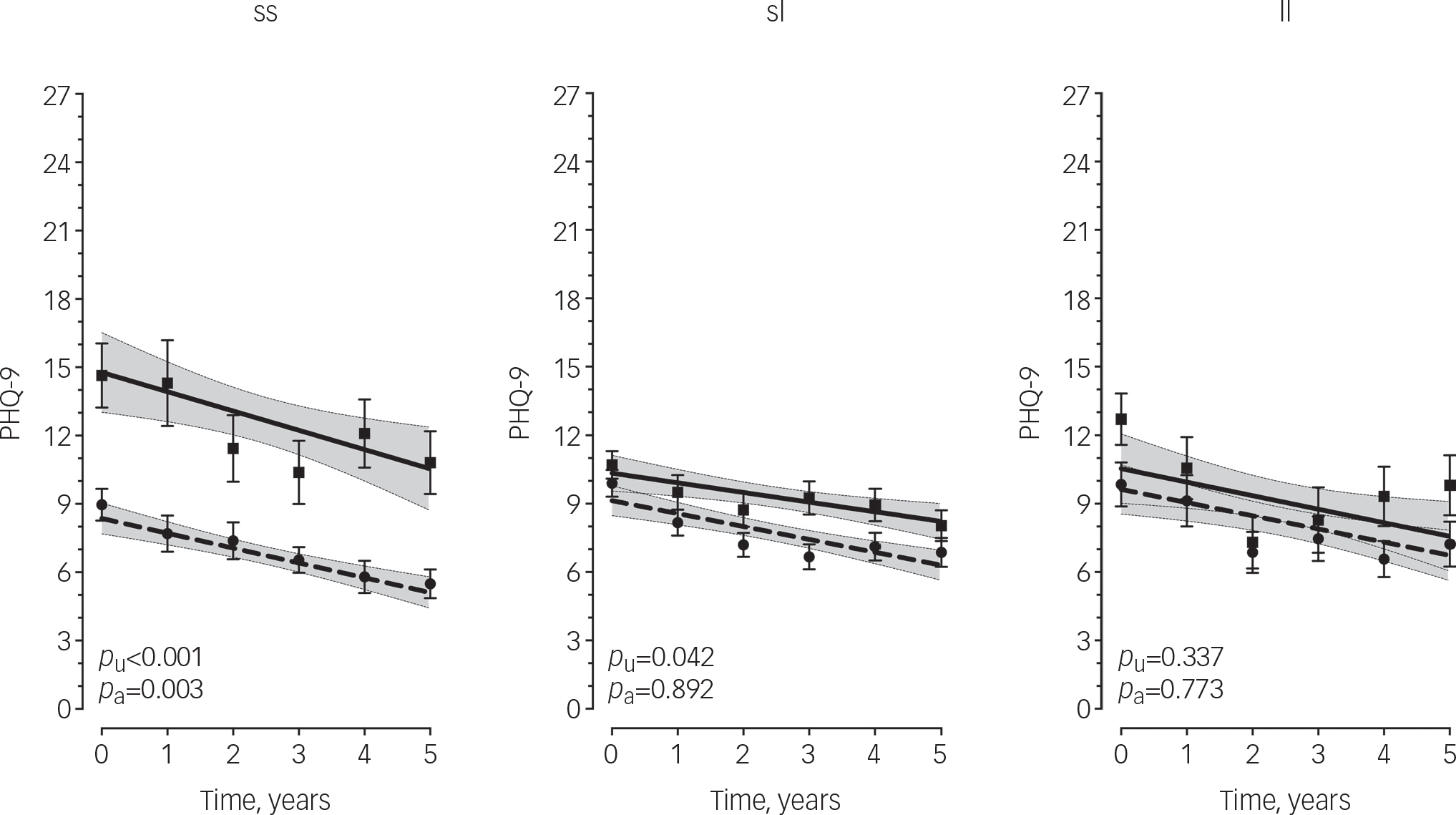
Fig. 1 Longitudinal measurements of depressive symptoms over 5 years by 5HTTLPR and history of severe child abuse.
Points are observed mean PHQ-9 scores with standard error bars. Lines are predicted values with 95% confidence intervals shaded based on linear mixed model analysis. Dashed line/circles, no history of severe child abuse; solid line/squares, history of severe child abuse; PHQ-9, Primary Care Evaluation of Mental Disorders Patient Health Questionnaire-9; p U, covariate-unadjusted P-value; p A, covariate-adjusted P-value. P-values based on estimated marginal means.
Discussion
In accordance with our hypothesis and previous cross-sectional studies, Reference Karg, Burmeister, Shedden and Sen6 we observed greater depressive symptom severity among individuals with the s/s genotype who reported a history of severe child abuse compared with those without a history. We also provide novel evidence which suggests that the s/s genotype confers a persistent moderating effect on depressive symptom severity without alteration to the rate of change over time. There was no significant difference in depressive symptoms between those with or without a history of child abuse in the s/l or l/l genotype groups. These findings support the notion that long-lasting consequences of adverse childhood experiences may be dependent on an individual's genomic context. Reference Labonte, Suderman, Maussion, Navaro, Yerko and Mahar28 More specifically, these findings suggest that the s/s genotype may be associated with susceptibility to more severe depressive symptom trajectories in adulthood following exposure to severe abuse in childhood.
Among the s/s group, those with a history of severe, child abuse had a mean PHQ-9 score greater than 10 (i.e. moderate depression) at each time point over the 5-year period, whereas those without a history of child abuse consistently scored under 10 (i.e. mild depression), a differential effect not observed among s/l and l/l genotype carriers. However, among individuals without a history of severe child abuse, we detected a trend in which s/s genotype carriers had more favourable depressive symptom trajectories compared with s/l and l/l genotype carriers. This pattern of results suggests that the 5HTTLPR polymorphism may be a marker of ‘phenotypic plasticity’ rather than ‘vulnerability’ in that s/s carriers appear to be the most susceptible to the negative effects of severe child abuse (i.e. increased depressive symptom severity) but also marginally more likely to benefit from the absence of a severe child abuse experience, albeit only a trend difference (P=0.068) was seen between s/s carriers and their l/l or l/s carrying counterparts in the absences of severe child abuse. Nonetheless, this so-called ‘differential susceptibility’ Reference Belsky, Jonassaint, Pluess, Brummett and Williams29 has been observed previously. Most notably, in the landmark paper by Caspi et al Reference Caspi, Sugden, Moffitt, Taylor, Craig and Harrington5 in that s/s genotype carriers with stressful life events reported the most depressive symptoms but conversely those without such events reported the fewest depressive symptoms. Similar results for the s/s genotype have been observed more recently. Eley et al Reference Eley, Sugden, Corsico, Gregory, Sham and McGuffin30 showed the s/s genotype conferred more susceptibility to depression among female adolescents in high-stress environments but s/s genotype carriers in low-stress environments were less likely to be depressed. Furthermore, Wilhelm et al Reference Wilhelm, Mitchell, Niven, Finch, Wedgwood and Scimone31 showed that the probability of lifetime major depression was highest among s/s carriers with more than one adverse life event in the past 5 years but lowest for s/s carriers with no such events.
If the 5HTTLPR polymorphism is a marker of ‘phenotypic plasticity’, it may have clinical utility in guiding psychosocial intervention in the future. In fact, recent work showed that children with anxiety with the s/s genotype were more likely to respond to cognitive–behavioural therapy compared with s/l and l/l genotypes, Reference Eley, Hudson, Creswell, Tropeano, Lester and Cooper32 albeit this was not observed in a randomised control trial of cognitive-behavioural therapy among adults with recurrent depression. Reference Bockting, Mocking, Lok, Koeter and Schene33 Furthermore, the antidepressant pharmacogenomics literature suggests that, among White but not Asian people, the l/l rather than the s/s genotype infers ‘phenotypic plasticity’ in that s allele carriers are less likely to respond to selective serotonin reuptake inhibitors compared with individuals with the l/l genotype. Reference Porcelli, Fabbri and Serretti34 Likewise, there is evidence, primarily in adolescent studies, suggesting the s/s genotype effect is stronger among females and in some cases in the opposite direction in males. Reference Uher and McGuffin35 In fact, in our fully adjusted model both gender and gender × genotype showed larger effects on depressive symptom severity than we detected for child abuse × genotype. Thus, further clinical trials, with particular attention to ancestry and gender, are required to determine the utility of 5HTTLPR genotype-guided psychosocial intervention in clinical practice.
The mechanism(s) by which the 5HTTLPR polymorphism confers differential susceptibility to the negative effects of child abuse is not clear. However, previous work has shown a link between child abuse and methylation in a CpG residue immediately upstream of the 5HTTLPR Reference Vijayendran, Beach, Plume, Brody and Philibert36 and the s allele appears to be more susceptible to methylation. Reference Philibert, Madan, Andersen, Cadoret, Packer and Sandhu37 Supporting this observation, a more recent study showed a decrease in SLC6A4 transcription in peripheral blood cells among s allele carriers who reported a history of early trauma compared with s allele carriers without early trauma and l allele carriers with or without early trauma. Reference Wankerl, Miller, Kirschbaum, Hennig, Stalder and Alexander38 Thus, the differential susceptibility conferred by the s/s genotype that we and others have observed may in part be explained by DNA methylation mediated SLC6A4 transcription and possibly translation, although the data linking SLC6A4 methylation to its protein expression is limited. Further clinical as well as human post-mortem brain studies with accompanying ante-mortem data on child abuse/adverse life events are needed before firm conclusions on the mechanisms can be made.
The current study has several notable strengths absent in previous studies. We included comprehensive covariate adjustment, modelled both fixed and random effects, and used a longitudinal design. However, six key caveats should be acknowledged. First, depressive symptom severity was the primary outcome measure and as such results cannot be extrapolated to the categorical diagnosis of major depressive disorder. However, the use of quantitative traits and a dimensional perspective affords greater statistical power and the PHQ-9 has previously been validated as a replicable measure of symptom severity that correlates with decreasing physical and social functioning, increased disability days, and increased healthcare utilisation and thus is likely to be more meaningful compared with a dichotomous clinical diagnostic classification. Reference Kroenke, Spitzer and Williams14 Second, although all participants were of Northern/Western European ancestry, a strength in genetic association studies, we are not able to generalise these results to other ancestry groups, particularly given that frequencies of the s and l alleles are known to vary by ancestry. Reference Gelernter, Kranzler and Cubells39 Third, detailed measures of depression treatment history were not available and adjustment of possible effects of antidepressant or psychotherapy treatment beyond the scope of self-reported use at baseline was not possible. Although we did not observe a difference in baseline antidepressant use by genotype, future follow-up studies should include detailed measurements of antidepressant use to evaluate whether the effect reported here is confounded by pharmacotherapy. Fourth, our linear mixed model approach required a large number of covariates and interactions to be examined in order to properly control for potential confounds. Reference Keller26 As a result, statistical power was likely suboptimal and our post hoc stratified analyses by genotype may have overestimated the true effects. Fifth, a priori we selected an additive genetic model but post hoc evaluation of our results suggests a recessive model (s/s v. l carriers) may also fit our data. Although previous studies in the literature have almost exclusively assumed an additive model, future studies may benefit from examination of a recessive model. Last, this study did not examine all types of potential childhood adversity and relied on self-report. Childhood emotional abuse or neglect have also been associated with depressive symptoms in the primary care setting Reference Spertus, Yehuda, Wong, Halligan and Seremetis40 but were not queried and may have important effects not accounted for in this study. Furthermore, some have criticised the use of self-reported childhood abuse due to a number of biases (e.g. underreporting). Reference McKinney, Harris and Caetano41,Reference Hardt and Rutter42 However, it is likely that these biases would obscure the moderation effect observed, resulting in more conservative parameter estimates.
In summary, our results provide evidence that the 5HTTLPR s/s genotype moderates the effects of severe childhood abuse on depressive symptom severity among adult primary care attendees. This effect was detected at baseline, persisted over the 5-year observation period and withstood robust adjustment of key confounders. If replicated, the 5HTTLPR polymorphism may serve as one of many candidate markers that could aid in identifying susceptibility to persistent depressive symptoms among individuals with a history of severe childhood abuse.
Funding
The diamond study is funded by the National Health and Medical Research Council (IDs 299869, 454463, 566511 and 1002908) and the Victorian Centre for Excellence in Depression and Related Disorders, an initiative between beyondblue and the Victorian Government. The collection of DNA and genotyping was funded by the LEW Carty Chartable Fund (ID 7284). No funding body had a role in the study design; the collection, analysis and interpretation of data; or the writing of the manuscript for publication. We acknowledge the 30 dedicated general practitioners their patients and practice staff for making this research possible.
Acknowledgements
We thank the diamond project team, including associate investigators and researchers involved in the diamond study: Ms Aves Middleton, Ms KonstancjaDensley, Professor Helen Herrman, Professor Christopher Dowrick, Dr Gursharan Chana and casual research staff.






eLetters
No eLetters have been published for this article.