Article contents
Hitachiite, Pb5Bi2Te2S6, a new mineral from the Hitachi mine, Ibaraki Prefecture, Japan
Published online by Cambridge University Press: 15 July 2019
Abstract
Hitachiite, Pb5Bi2Te2S6, is a new mineral discovered in the Hitachi mine, located in the Ibaraki Prefecture of Japan. The mean of 21 electron microprobe analyses gave: Pb 52.01, Bi 23.06, Fe 0.69, Sb 0.17, Te 13.74, S 9.71, Se 0.54, total 100.04 wt.%. The empirical chemical formula based on 15 apfu is (Pb4.75Fe0.23)Σ4.98(Bi2.09Sb0.03)Σ2.12Te2.04(S5.73Se0.13)Σ5.86, ideally Pb5Bi2Te2S6. Synchrotron single-crystal X-ray diffraction experiments indicated that hitachiite has trigonal symmetry, space group P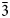 ${\bar 3}$m1, with a = 4.2200(13) Å, c = 27.02(4) Å and Z = 1. The four strongest diffraction peaks shown in the powder X-ray pattern [d, Å (I)(hkl)] are: 3.541(35)(012), 3.391(59)(013), 3.039(100)(015) and 2.114(56)(110). The calculated density (Dcalc) for the empirical chemical formula is 7.54 g/cm3.
${\bar 3}$m1, with a = 4.2200(13) Å, c = 27.02(4) Å and Z = 1. The four strongest diffraction peaks shown in the powder X-ray pattern [d, Å (I)(hkl)] are: 3.541(35)(012), 3.391(59)(013), 3.039(100)(015) and 2.114(56)(110). The calculated density (Dcalc) for the empirical chemical formula is 7.54 g/cm3.
The crystal structure of hitachiite has been refined using synchrotron single-crystal X-ray diffraction data, to R = 7.38% and is based on ABC-type stacking of 15 layers (five Pb, two Bi, two Te, and six S layers) along the [001] direction, and with each layer ideally containing only one kind of atom. The stacking sequence is described as Te–Bi–S–Pb–S–Pb–S–Pb–S–Pb–S–Pb–S–Bi–Te. The discovery of hitachiite implies that the minerals of the Bi2Te2S–PbS join might form a homologous series of Bi2Te2S·nPbS.
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2019
Footnotes
Associate Editor: František Laufek
References
- 4
- Cited by




