To the Editor—We report the case of a 44-year-old man with total parenteral nutrition (TPN) for short-bowel syndrome who was diagnosed with his 17th central-line–associated bloodstream infection (CLABSI). He had primarily been admitted to a single hospital unit during the period of his multiple infections. A timeline of his infections is provided in Figure 1. This case report was reviewed by the IRB of the University of Maryland, Baltimore and determined to be not human-subjects research. The patient provided consent to have his case information published.
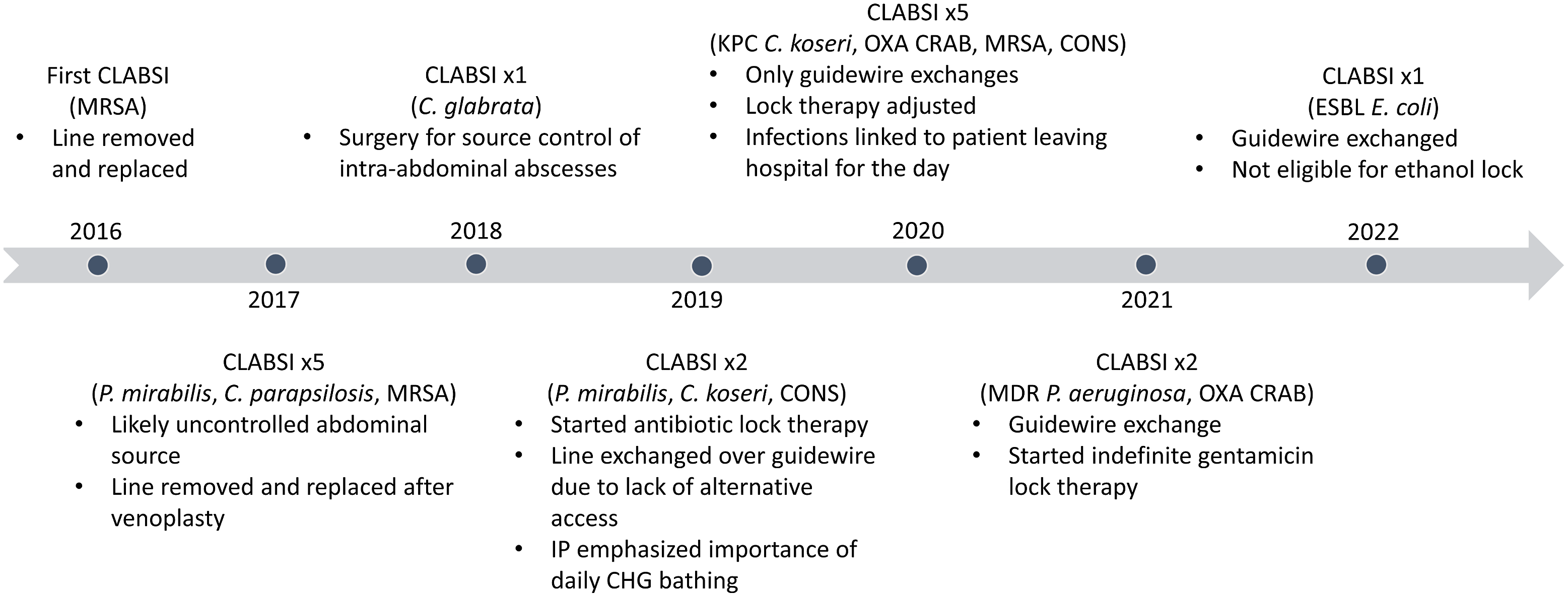
Fig. 1. Timeline of CLABSIs and major interventions. Note. CLABSI, central-line–associated bloodstream infection; MRSA, methicillin-resistant Staphylococcus aureus; C. glabrata, Candida glabrata; KPC, Klebsiella pneumoniae carbapenemase; C. koseri, Citrobacter koseri, OXA, OXA-type beta-lactamase; CRAB, carbapenem-resistant Acinetobacter baumanii; CONS, coagulase-negative staphylococci; ESBL, extended-spectrum β-lactamase; E. coli, Escherichia coli; C. parapsilosis, Candida parapsilosis; P. aeruginosa, Pseudomonas aeruginosa.
Short-bowel syndrome arose from complications of an abdominal gunshot wound. During a difficult and prolonged recovery, he developed extensive bowel necrosis and eventually required total colectomy, partial enterectomy, and placement of a jejunal ostomy. With his entire colon and most of his small bowel removed, he developed severe malnutrition. Bowel transplant was declined due to lack of social support.
TPN, which the patient had required for >5 years, was administered through a tunneled catheter in his right external jugular vein. Repeated placement and removal of central lines had rendered other options for venous access unavailable. Bilateral internal jugular veins, brachiocephalic veins, and subclavian veins were either occluded or stenosed. Previous femoral access had been placed and removed in the context of bacteremia and sepsis. He declined placement of permanent transhepatic or translumbar venous access. Consultants from interventional radiology and vascular surgery advised that his current catheter was a “lifeline.” Its removal would likely result in permanent loss of upper-extremity central venous access.
The current line had been placed by exchange over a guidewire 6 months earlier in response to a CLABSI. Gentamicin lock therapy was instilled daily for prophylaxis. Alcohol-impregnated caps were used on all ports. Regular central-line care was provided by attentive staff who reviewed the plan for central-line maintenance with nursing leadership, infection prevention, and the attending physician. Examination of his chlorhexidine-impregnated central-line dressing did not reveal any breaches or areas of concern. He received daily bathing with chlorhexidine gluconate in the preceding week, and he had not recently left the unit. Manipulation of the central line by the patient was not suspected.
Peripheral blood cultures collected after a fever of 39.3°C grew Escherichia coli that was resistant to gentamicin. No localizing symptoms suggested metastatic focus of infection or source besides the catheter. Blood cultures remained positive the following day but subsequently cleared. After initially receiving intravenous meropenem, his line was exchanged over a guidewire, and he was transitioned to ertapenem to complete 14 days of therapy. The antibiotic lock was changed to amikacin. Ethanol lock therapy was considered but was not compatible with the polyurethane catheter.
Discussion
Among healthcare-associated infections, CLABSI has the greatest potential negative impact on patient outcomes, including increased mortality risk and prolonged length of hospital stay. Reference Srinivas, Rivard and Pallotta1–Reference Ziegler, Pellegrini and Safdar3 CLABSI rates are also linked to hospital reimbursement through the Medicare hospital-acquired conditions reduction program and the State of Maryland’s Quality-Based Reimbursement. For these reasons, CLABSI has been declared a “never event,” and significant efforts have been undertaken to prevent its occurrence. Reference Patel, Gupta and Vaughn4
Most US states legally require public reporting of healthcare-associated infections via the National Healthcare Safety Network (NHSN). Reference Herzig, Reagan and Pogorzelska-Maziarz5 According to NHSN definitions, any bloodstream infection that develops in the context of a central line and cannot be attributed to an alternative source is counted as a CLABSI. If the blood culture is positive for a qualifying pathogen, signs or symptoms of infection are not required. Determination of an alternative source of infection depends on the quality of documentation and can be subjective, introducing variability based on who does the chart review. Reference Sexton, Chen and Moehring6–Reference Mayer, Greene and Howell8
On average, the rate of CLABSI in the United States is 1.7 events per 1,000 central-line days. Reference Rock, Thom and Harris9 Based on 17 CLABSIs over a single 2,000-day period, this patient’s individual CLABSI rate was 8.5 events per 1,000 central-line days. These CLABSIs accounted for 27% of all CLABSIs occurring at his admitting hospital. During this period, the NHSN-defined CLABSI rate reflected the largely unmodifiable circumstances of a single patient instead of the quality of care received by most patients at this hospital.
CLABSI prevention is difficult and requires engagement by the entire healthcare team. Efforts to maintain that engagement can be undermined when publicly reported rates are biased to suggest that infection prevention practices do not make a difference. When staff are less motivated, the expectation of recurrent CLABSI can become self-fulfilling. These unintended consequences of public reporting are likely most relevant when options for out-of-hospital care are limited, as is often true for socioeconomically disadvantaged patients.
Although CLABSI is a considered a “never event,” CLABSIs may be inevitable for some patients. For this patient, multiple CLABSIs occurred despite attentive delivery of evidence-based preventive measures. Although this case is an extreme example, recurrent infections are not uncommon in our experience. We have observed similar occurrences for other NHSN-defined infections, including catheter-associated urinary tract infection, Clostridioides difficile infection, and methicillin-resistant Staphylococcus aureus bacteremia.
We suggest that recurrent NHSN-defined infection of the same type in a single patient is more likely to be a consequence of the unmodifiable susceptibility of the patient than the quality of care delivered. To reduce bias, NHSN should consider revising the definition of CLABSI and other healthcare-associated infections to exclude patients who have already developed the same infection during the same hospitalization, regardless of the time elapsed between episodes.
Acknowledgments
Financial support
J.D.B. received support from the University of Maryland Baltimore Institute for Clinical Translational Research (grant no. 1KL2TR003099-03).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.






