Introduction
Cerambycidae (longhorned beetles) are among the most diverse and economically important families of Coleoptera. In particular, they present significant phytosanitary and biosecurity risks to forest ecosystems. The description of new species has accelerated in recent decades, and currently approximately 36 300 species are described worldwide (Monné et al. Reference Monné, Monné, Wang and Wang2017). Longhorned beetles play an important role in the process of decomposition of wood and recycling of nutrients. They are among the first beetles to colonise dead wood, and larvae initiate the physical process of woody biodeterioration (early successional beetles; Seibold et al. Reference Seibold, Brandl, Buse, Hothorn, Schmidl, Thorn and Müller2015), which facilitates the subsequent entry of other saproxylophagous insects (late-successional beetles; Martikainen et al. Reference Martikainen, Siitonen, Kaila, Punttila and Rauh1999) or infection by wood-rotting fungi (Basham and Belyea Reference Basham and Belyea1960). As a result of their ecological and economic importance and broad distribution and diversity, they also have potential as indicator species for forest ecosystems (Gerlach et al. Reference Gerlach, Samways and Pryke2013).
Longhorned beetles are primarily herbivorous (some are facultative intraguild predators (Schoeller et al. Reference Schoeller, Husseneder and Allison2012)), usually have a long period of larval development (varying from months to years to complete), and some species can develop and survive in wood material a long time after the death of the tree (Brockerhoff et al. Reference Brockerhoff, Jones, Kimberley, Suckling and Donaldson2006; Cocquempot and Lindelöw Reference Cocquempot, Lindelöw, Roques, Kenis, Lees, Lopez-Vaamonde, Rabitsch, Rasplus and Roy2010; Dodds et al. Reference Dodds, Sweeney, Allison, Allison, Paine, Slippers and Wingfield2023). These traits predispose them to be transported in wood products, wood packaging, and dunnage, facilitating the introduction of alien species into new habitats. For example, the most likely invasion pathway of Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae) and Trichoferus campestris (Faldermann) (Coleoptera: Cerambycidae) to North America appears to be in solid wood packing material (Haack et al. Reference Haack, Hérard, Sun and Turgeon2010) and dunnage (Grebennikov et al. Reference Grebennikov, Gill and Vigneault2010), respectively. In this context, several interacting features of invasive species, the pathways by which they travel, and the habitats they invade play an important role to determine their invasion success (Nahrung et al. Reference Nahrung, Liebhold, Brockerhoff and Rassati2023).
In forest ecosystems, some introductions of nonnative species have resulted in complex and long-term impacts (Pyšek et al. Reference Pyšek, Hulme, Simberloff, Bacher, Blackburn and Carlton2020), and most have been accidental. Therefore, forest biosecurity programs focus on prevention and early detection (Allison et al. Reference Allison, Marcotte, Noseworthy and Ramsfield2021). Semiochemical-baited traps are critical tools for early detection programs and can be effective for determining the presence or absence of a species and the pattern of spread of invasive species and for assessing the impact of management interventions. Although horizon scanning can identify potential biosecurity threats, a major challenge to the development of effective monitoring programs is that program targets often are unknown a priori. Consequently, biosecurity programs often aim to sample multiple species simultaneously.
The literature on cerambycid pheromones suggests that redundancy in pheromone chemistry is common, where related species often use similar or the same chemical structures in their pheromone blends (Hanks and Millar Reference Hanks and Millar2016; Millar and Hanks Reference Millar, Hanks and Wang2017). Surveillance traps can target single or multiple species by manipulating the semiochemicals used to bait traps (Brockerhoff et al. Reference Brockerhoff, Suckling, Roques, Jactel, Branco and Twidle2013, Reference Brockerhoff, Corley, Jactel, Miller, Rabaglia, Sweeney, Allison, Paine, Slippers and Wingfield2023). In support of these programs, known pheromone compounds can be screened for activity in the native fauna of trade partners. These offshore mitigation programs can facilitate the development of inventories of pheromone attractants of potential use for monitoring and surveillance for nonnative species. In Peru, responses by Megacyllene andesiana (Casey) (Coleoptera: Cerambycidae), Oreodera bituberculata Bates (Coleoptera: Cerambycidae), and Discopus eques Bates (Coleoptera: Cerambycidae) to traps baited with anti-2,3-hexanediol, fuscumol, and fuscumol acetate have been reported (Aguirre-Gil et al. Reference Aguirre-Gil, Paredes-Espinosa, Aguilar, Mezones, Guerrero and Monné2021). Responses to traps baited with blends of pheromone attractants were lower than those to individual compounds. Ideally, semiochemical-baited traps in early detection programs would have both high sensitivity and a broad spectrum of activity. Here, we report field trials that characterised the activity of anti-2,3-hexanediol, fuscumol, and fuscumol acetate individually and in binary and ternary blends.
Materials and methods
Field experiment
A field trapping experiment was conducted from 4 September 2021 to 8 January 2022 in a rainforest preserve at Universidad Nacional Agraria de la Selva (UNAS), Tingo Maria, Huánuco, Peru (–9.304 latitude, –75.9991 longitude). The field trial compared the number of adult longhorned beetles (Coleoptera: Cerambycidae) captured by two types of traps baited with all possible combinations of three different pheromone lures. An 8 × 2 factorial experiment with two factors (lure combination (eight levels) × trap type (two levels) for a total of 16 different lure-trap-type combinations) was replicated six times in a completely randomised block design. The bubble cap lures containing the pheromones racemic anti-2,3-hexanediol (mixture of (2R,3S) and (2S,3R)), racemic E,Z-fuscumol, and racemic E,Z-fuscumol acetate were purchased from Synergy Semiochemicals, Delta, British Columbia, Canada. The eight levels of lure combinations were (1) anti-2,3-hexanediol; (2) fuscumol; (3) fuscumol acetate; (4) anti-2,3-hexanediol + fuscumol; (5) anti-2,3-hexanediol + fuscumol acetate; (6) fuscumol + fuscumol acetate; (7) anti-2,3-hexanediol + fuscumol + fuscumol acetate; and (8) a blank control (without pheromone). The two levels of the factor traps were black four-panel (WestGreen Global Technologies, Langley, British Columbia, Canada) and black three-panel intercept traps (Synergy Semiochemicals). The four-panel (n = 48) and three-panel (n = 48) traps used in the field experiment were coated with 12% fluon (EZ fluon kit; Synergy Semiochemicals) to increase trap captures (Allison et al. Reference Allison, Johnson, Meeker, Strom and Butler2011, Reference Allison, Graham, Poland and Strom2016) and were suspended individually from iron frames such that the collection cup was 100 cm above the ground. Traps were distributed randomly within the rainforest preserve, were equipped with wet collection cups with 500 mL of an odourless soap solution, and were spaced 25 m apart. Longhorned beetles were collected from traps weekly, and lures were replaced every four weeks. Samples were returned to the laboratory, and Cerambycidae were identified to species. Voucher specimens of all species were deposited in the Museum of Zoology, UNAS, Tingo Maria, Peru.
Statistics
Trap capture data of all species for which at least 30 individuals were captured were analysed for effects of both factors and the interaction between them. Blocks were defined as replicates, and the number of longhorned beetles captured during the experiment was defined as the dependent variable. The dependent variable was tested by aligned rank transform for nonparametric factorial analyses of variance (α = 0.05). Multiple pairwise comparisons were performed to identify differences between treatments, and Sidak’s method was used to adjust P-values (α = 0.05). Statistical analyses were performed using R software for Windows (R Core Team 2022).
Research permits
Fauna of insects were collected according to Authorisation N° 10-HUA-TM/AUT-IFS-2019-006, and pheromones anti-2,3-hexanediol, fuscumol, and fuscumol acetate were studied according to Research Permissions N° 006-MIDAGRI-SENASA, N° 007-MIDAGRI-SENASA, and N° 008-MIDAGRI-SENASA for the 2021/2022 period, respectively.
Results
Field experiment
In total, 490 longhorned beetles were captured by traps baited with cerambycid pheromones in the Peruvian rainforest. Longhorned beetles captured in high enough numbers (> 30) for analysis were M. andesiana from the subfamily Cerambycinae (tribe Clytini), with 268 individuals, and O. bituberculata, D. eques, and Aegomorphus longitarsis (Bates) (Coleoptera: Cermbycidae) (synonymised with Psapharochrus longitarsis by Santos-Silva et al. Reference Santos-Silva, Botero and Wappes2020) from the subfamily Lamiinae (tribe Acanthoderini) with 59, 37, and 31 individuals, respectively (Table 1).
Table 1. Total number of cerambycid beetles captured by pheromone baited traps in Tingo Maria, Peru. Abbreviations: C6, anti-2,3-hexanediol; Fu, fuscumol; FA, fuscumol acetate; Control, blank control (unbaited trap)
Effect of traps and pheromones on the capture of longhorned beetles
There was no effect of the factor trap on the total number of longhorned beetles captured (F 1,80 < 0.001, P = 0.997). Similarly, the effect of traps on the capture of M. andesiana (F 1,80 = 0.348, P = 0.557), O. bituberculata (F 1,80 = 2.145, P = 0.147), D. eques (F 1,80 = 2.365, P = 0.130), and A. longitarsis (F 1,80 = 0.288, P = 0.593) was not significant.
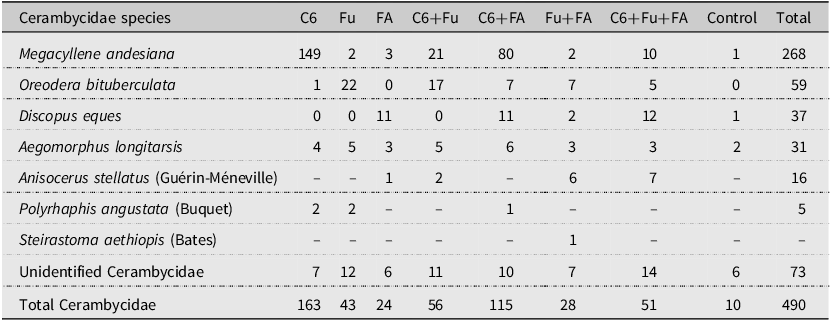
There was an effect of the factor pheromone on the total number of longhorned beetles captured (F 7,80 = 12.922, P < 0.001). In general, significantly more longhorned beetles were captured by traps baited with anti-2,3-hexanediol alone, in binary blends with fuscumol or fuscumol acetate, or in the tertiary blend of all three compounds than by unbaited traps (Fig. 1). There was a significant effect of the factor pheromones on the total number of M. andesiana (F 7,80 = 11.709, P < 0.001; Fig. 2A), as well as of males (F 7,80 = 13.974, P < 0.001; Fig. 2B) and of females (F 7,80 = 6.933, P < 0.001; Fig. 2C). In general, both male and female M. andesiana were attracted to traps baited with anti-2,3-hexanediol, and the effect of anti-2,3-hexanediol was reduced by the addition of either or both fuscumol and fuscumol acetate.

Figure 1. Boxplots of the total number of longhorned beetles collected by traps baited with pheromones, Abbreviations: C6, anti-2,3-hexanediol; Fu, fuscumol; FA, fuscumol acetate; Control, blank control (unbaited trap). Boxplots with different letters are statistically different (aligned rank transform for nonparametric factorial analysis of variance, P < 0.05). Dots above barplots represent a singular data point on dataset.
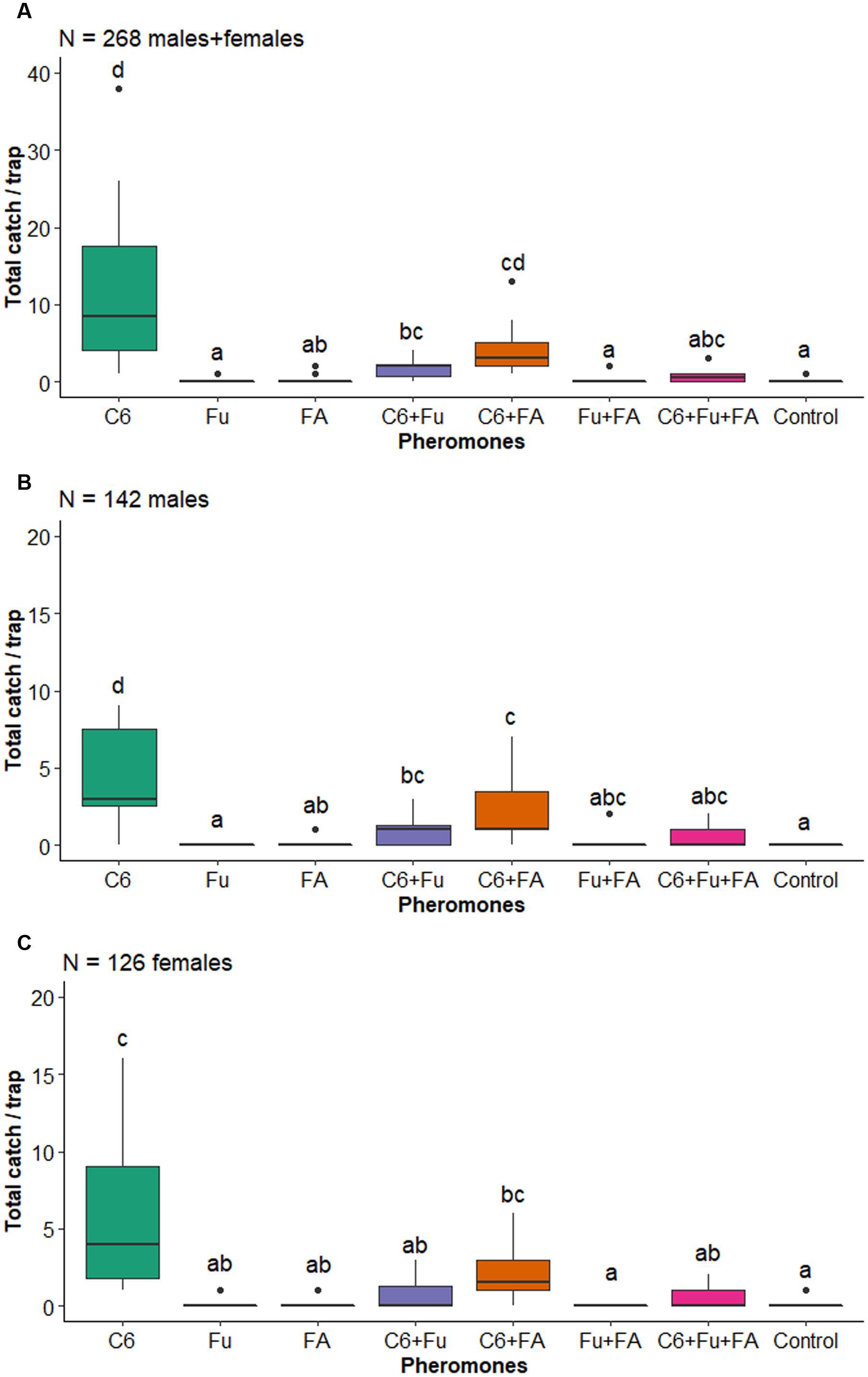
Figure 2. Boxplots of the number of Megacyllene andesiana, A, total catch, B, males, and C, females collected by traps baited with pheromones, Abbreviations: C6, anti-2,3-hexanediol; Fu, fuscumol; FA, fuscumol acetate; Control, blank control (unbaited trap). Boxplots with different letters are statistically different (aligned rank transform for nonparametric factorial analysis of variance, P < 0.05). Dots above barplots represent a singular data point on dataset.
In the case of O. bituberculara, there was a significant effect of the factor pheromones on the capture of the total number of O. bituberculata (F 7,80 = 6.593, P < 0.001; Fig. 3A) and males (F 7,80 = 12.922, P < 0.001; Fig. 3B) of this longhorned beetle. Both males and the total number of O. bituberculata were attracted to traps baited with fuscumol, and the addition of fuscumol acetate reduced the number of males captured (Fig. 3B). In contrast, there was an effect of the factor pheromones on the capture of D. eques (F 7,80 = 4.621, P < 0.001), but these effects did not differ from the blank control, and there was no effect on the capture of A. longitarsis (F 7,80 = 0.252, P = 0.970). However, when data for D. eques catch were analysed using a simple Chi-square goodness-of-fit test with the null hypothesis that D. eques trap catch is independent of the presence of fuscumol acetate where catch in traps with and without fuscumol acetate does not differ from an expected 1:1 ratio, it was observed that catch of D. eques is not independent of fuscumol acetate (X 2 = 33.108, df = 1, P < 0.001).
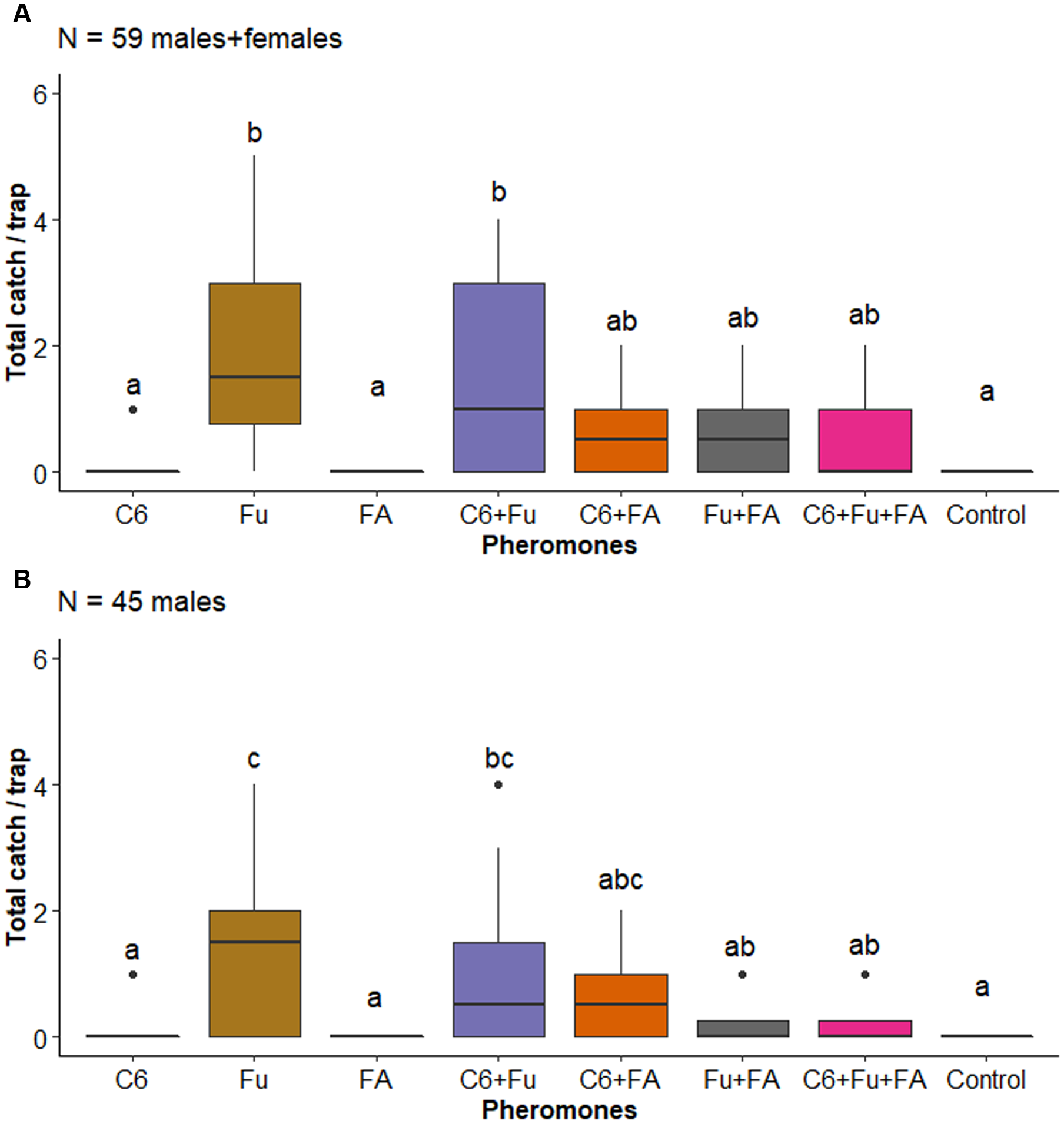
Figure 3. Boxplots of the number of Oreodera bituberculata, A, total catch, and B, males collected by traps baited with pheromones, Abbreviations: C6, anti-2,3-hexanediol; Fu, fuscumol; FA, fuscumol acetate; Control, blank control (unbaited trap). Boxplots with different letters are statistically different (aligned rank transform for nonparametric factorial analysis of variance, P < 0.05). Dots above barplots represent a singular data point on dataset.
Discussion
It has been predicted that North and South America could be invaded by up to 1500 alien arthropod species by 2050 (Seebens et al. Reference Seebens, Bacher, Blackburn, Capinha, Dawson and Dullinger2020). The biology of many longhorned beetles makes them likely to be transported in wood products. In support of surveillance programs for nonnative longhorned beetles, trials often screen known pheromones to develop inventories of attraction and inhibition on different fauna. Previously, we demonstrated that several species of longhorned beetles in Peru were attracted to individual compounds but that responses were low to traps baited with blends of compounds (Aguirre-Gil et al. Reference Aguirre-Gil, Paredes-Espinosa, Aguilar, Mezones, Guerrero and Monné2021). Here, we demonstrate that for M. andesiana, attraction to anti-2,3-hexanediol is reduced by the addition of fuscumol and fuscumol acetate alone or in combination. For O. bituberculata, we demonstrate that attraction to fuscumol is reduced by the addition of fuscumol acetate. These results suggest that surveillance traps co-baited with these pheromones may sacrifice sensitivity for increased diversity of Cerambycidae surveyed.
Antagonism in behavioural responses to pheromones in members of the subfamilies Cerambycinae and Lamiinae have been reported in South America. In Brazil, Hylettus seniculus (Germar) (Coleoptera: Cerambycidae: Lamiinae, Acanthocinini) was attracted to (R)-fuscumol acetate and antagonised by (S)-fuscumol acetate (Silva et al. Reference Silva, Zou, Hanks, Bento and Millar2019), and the congener Megacyllene acuta (Germar) (Coleoptera: Cerambycidae: Cerambycinae, Clytini) was attracted to racemic 3-hydroxyhexan-2-one + racemic 2-methylbutan-1-ol and antagonised by syn-2,3-hexanediol (Silva et al. Reference Silva, Millar, Hanks, Costa, Leite, Tonelli and Bento2018). In addition, Cotyclytus curvatus (Germar) (Coleoptera: Cerambycidae: Cerambycinae, Clytini) was attracted to racemic 3-hydroxyhexan-2-one and antagonised by racemic 2-methylbutan-1-ol and syn-2,3-hexanediol (Silva et al. Reference Silva, Millar, Hanks, Costa, Leite, Tonelli and Bento2018). Antagonism in other tribes of Cerambycinae has also been reported in Brazil. Chrysoprasis linearis (Bates) (Coleoptera: Cerambycidae) (tribe Heteropsini) was attracted to racemic 3-hydroxyhexan-2-one and antagonised by racemic 2-methylbutan-1-ol (Silva et al. Reference Silva, Millar, Hanks, Costa, Leite, Tonelli and Bento2018), and Ambonus electus (Gahan) (Coleoptera: Cerambycidae) (tribe Elaphidiini) was attracted to racemic 3-hydroxyhexan-2-one: 1-(1H-pyrrol-2-yl)-1,2-propanedione and antagonised by 3-methylthiopropan-1-ol (Silva et al. Reference Silva, Zou, Bento, Hanks and Millar2017). Some species can even recognise differences in the configuration of the compounds (i.e., (R)- versus (S)-fuscumol acetate in Silva et al. (Reference Silva, Zou, Hanks, Bento and Millar2019)). This study reports that Peruvian fauna (M. andesiana and O. bituberculata) are antagonised by fuscumol and fuscumol acetate. Our results suggest that M. andesiana and O. bituberculata likely recognise specific compounds to avoid cross attraction and prevent competition for food resources and space.
In parallel with identification of pheromones and attractants for Cerambycidae fauna globally, several studies have assessed operational parameters of traps (i.e., design, placement) to increase trapping efficiency (Allison and Redak Reference Allison and Redak2017; Allison et al. Reference Allison, Strom, Sweeney and Mayo2019; Staton et al. Reference Staton, Girling, Redak, Smith and Allison2023). Significant differences in trapping efficiency between trap designs have been reported for Monochamus scutellatus (Say) (Coleoptera: Cerambycidae) and Monochamus notatus (Drury) (Coleoptera: Cerambycidae) (Lamiinae, Monochamini), and differences in plume structure downwind of these designs may be involved (Bouwer et al. Reference Bouwer, MacQuarrie, Aguirre-Gil, Slippers and Allison2020). In the current study, it was expected that the four-panel trap would capture more longhorned beetles than the three-panel trap would because the four-panel trap has greater interception surface area than the three-panel trap does (panel surface area of 1.069 and 0.386 m2, respectively). Hypothetically, more surface area could increase the proportion of attracted longhorned beetles that are captured. Similar studies suggest that the presence of a dark silhouette is an important cue to host finding for M. scutellatus but not for Monochamus mutator LeConte (Haldeman) (Coleoptera: Cerambycidae) (De Groot and Nott Reference De Groot and Nott2001). Our results indicate that the four- and three-panel traps did not differ in their performance for Lamiinae (O. bituberculata, D. eques, and A. longitarsis) and Cerambycinae (M. andesiana) in a rainforest preserve in Peru.
One potential limitation of this study could be the absence of host plant volatiles: several studies have demonstrated that volatiles such as ethanol and alpha-pinene (Brockerhoff et al. Reference Brockerhoff, Jones, Kimberley, Suckling and Donaldson2006; Allison et al. Reference Allison, McKenney, Millar, McElfresh, Mitchell and Hanks2012; Hanks et al. Reference Hanks, Millar, Mongold-Diers, Wong, Meier, Reagel and Mitchell2012) can synergise the capture of longhorned beetles by pheromone-baited traps. Furthermore, the present study was limited to a single longhorned beetle flight activity period, the field site had few downed trees and logs, and the flora was dominated by Cedrelinga catenaeformis (Ducke) (Fabaceae) and Tapirira guianensis (Aublet) (Anacardiaceae). Despite these limitations, this study demonstrated that the capture of adult M. andesiana (Cerambycinae, Clytini) is antagonised by fuscumol and fuscumol acetate alone or in combination and that the capture of adult O. bituberculata (Lamiinae, Acanthoderini) is antagonised by fuscumol acetate. We suggest that future studies attempt to identify host plant volatiles that would improve the capture of these Cerambycidae beetles.
Acknowledgements
The authors thank the Canadian Forest Service, Universidad Nacional Agraria de la Selva (Peru), and Synergy Semiochemicals Corp. for supporting this research.
Competing interests
The authors declare they have no competing interests.







