Meditation is a means of regulating emotion and attention, developed for diverse applications and used for thousands of years (Lutz, Slagter, Dunne, & Davidson, Reference Lutz, Slagter, Dunne and Davidson2008). Numerous studies have investigated the effectiveness of meditation in various domains. Meditation has been suggested to have a potential therapeutic role in treating physical and mental disorders, including chronic pain (Goldberg et al., Reference Goldberg, Tucker, Greene, Davidson, Wampold, Kearney and Simpson2018), anxiety disorders (Hofmann, Sawyer, Witt, & Oh, Reference Hofmann, Sawyer, Witt and Oh2010), depression (Goyal et al., Reference Goyal, Singh, Sibinga, Gould, Rowland-Seymour, Sharma and Haythornthwaite2014; Hofmann et al., Reference Hofmann, Sawyer, Witt and Oh2010) and eating disorders (Kristeller and Hallett, Reference Kristeller and Hallett1999). In addition, positive effects of meditation in reducing smoking (Goldberg et al., Reference Goldberg, Tucker, Greene, Davidson, Wampold, Kearney and Simpson2018) and improving insomnia (Ong, Shapiro, & Manber, Reference Ong, Shapiro and Manber2008) have been reported. Conducting regular meditation practice is regarded as a component of a healthy lifestyle in many cultures.
As meditation has become more popular, interest in understanding the underlying mechanisms has grown. A meta-analysis of brain structure found that meditation is related to structural alterations of brain regions involved in metacognition (frontopolar cortex), exteroceptive and interoceptive awareness (insula), emotion regulation (cingulate cortex) and memory processing (hippocampus; Fox et al., Reference Fox, Nijeboer, Dixon, Floman, Ellamil, Rumak and Christoff2014). Meta-analyses of functional activation/deactivation have also identified activations of these regions along with the recruitment of supplementary motor area (Boccia, Piccardi, & Guariglia, Reference Boccia, Piccardi and Guariglia2015; Fox et al., Reference Fox, Dixon, Nijeboer, Girn, Floman, Lifshitz and Christoff2016; Tomasino, Fregona, Skrap, & Fabbro, Reference Tomasino, Fregona, Skrap and Fabbro2012). Meditation has also been reported to change functional integration among anatomically distinct brain regions (Fox et al., Reference Fox, Dixon, Nijeboer, Girn, Floman, Lifshitz and Christoff2016). However, a systematic meta-analysis on meditation effects on large-scale brain functional networks has yet to be performed.
Summary of types of meditation
Meditation types differ in their underlying principles. For example, mindfulness meditation, one of the most widely practiced types, emphasizes attending to the present moment and nonjudgmental awareness (Brown & Ryan, Reference Brown and Ryan2003; Gu, Strauss, Bond, & Cavanagh, Reference Gu, Strauss, Bond and Cavanagh2015; Kabat-Zinn, Reference Kabat-Zinn2009). It differs from loving-kindness and compassion meditation (LKCM), which focuses on intensifying empathic mental responses, promoting positive emotions and encouraging altruistic thoughts (Lutz et al., Reference Lutz, Slagter, Dunne and Davidson2008; Zeng, Chiu, Wang, Oei, & Leung, Reference Zeng, Chiu, Wang, Oei and Leung2015). Mindfulness meditation can be subcategorized into mindfulness-based stress reduction (MBSR) and mindfulness-based cognitive therapy (MBCT). Both have been validated as therapeutically efficacious in treating physical and psychiatric disorders in experimental and clinical settings (Chiesa & Serretti, Reference Chiesa and Serretti2010; Fjorback, Arendt, Ornbol, Fink, & Walach, Reference Fjorback, Arendt, Ornbol, Fink and Walach2011; Goldberg et al., Reference Goldberg, Tucker, Greene, Davidson, Wampold, Kearney and Simpson2018). Meditation can also be classified in terms of the technique applied, such as focused attention (FA), open monitoring (OM), or LKCM (Fox et al., Reference Fox, Dixon, Nijeboer, Girn, Floman, Lifshitz and Christoff2016; Lippelt, Hommel, & Colzato, Reference Lippelt, Hommel and Colzato2014). Additionally, distinctions among Zen meditation, Tibetan Buddhism meditation and Vipassana meditation are related to cultural or religious considerations.
Although types of meditation can be further subdivided, most share the common goal of involving mental training to induce relaxation, adjust emotions, enhance attentional processes, regulate self-awareness, maintain nonjudgmental awareness, and detach from external stimuli. Also, most meditation practices employ similar approaches (Andresen, Reference Andresen2000) that are intrinsically related (Hofmann, Grossman, & Hinton, Reference Hofmann, Grossman and Hinton2011). For instance, both mindfulness and LKCM incorporate aspects of Buddhist tradition that promote deliberate intention (e.g., breath and mantra recitation) and processing emotional state (e.g., suffering, empathy, and love; Kuhlman, Reference Kuhlman2001). FA and OM practices are key elements of mindfulness meditations, as noted by many scholars and practitioners (Britton et al., Reference Britton, Davis, Loucks, Peterson, Cullen, Reuter and Lindahl2018; Lutz et al., Reference Lutz, Slagter, Dunne and Davidson2008). In a sense, LKCM can be considered a form of FA if the meditative object comes from the targeted person, and it also can be considered a form of OM if no concrete object is needed (Fox et al., Reference Fox, Nijeboer, Dixon, Floman, Ellamil, Rumak and Christoff2014). In the field of neuroimaging, previous meta-analysis studies have combined multiple types of meditations due to their fundamental similarities (Boccia et al., Reference Boccia, Piccardi and Guariglia2015; Fox et al., Reference Fox, Nijeboer, Dixon, Floman, Ellamil, Rumak and Christoff2014; Tomasino et al., Reference Tomasino, Fregona, Skrap and Fabbro2012). Thus, we restricted the types of meditation in this meta-analysis to those that share similar features (mindfulness, loving kindness, breath/attention focused meditation and Zen meditation) to decrease the heterogeneity of studies included.
Current research on meditation
Why and how does meditation affect people’s emotional, cognitive and behavioral performance? Many researchers have tried to explain meditation’s positive effects from a neurobiological perspective. Many studies have shown that the structural architecture and functional organization of the human brain change with experience and in response to systematic learning (Casey, Tottenham, Liston, & Durston, Reference Casey, Tottenham, Liston and Durston2005; Kelly & Garavan, Reference Kelly and Garavan2005), both of which are intensively involved in meditation (Slagter, Davidson, & Lutz, Reference Slagter, Davidson and Lutz2011).
In recent years, magnetic resonance imaging (MRI) studies have suggested that meditation practice can modify brain structures, especially in regions involved in self-referential processing (Boccia et al., Reference Boccia, Piccardi and Guariglia2015). Functional MRI (fMRI) studies have also focused on the effects of meditation on brain function, using approaches inside and outside the scanner. Fox and colleagues (Reference Fox, Dixon, Nijeboer, Girn, Floman, Lifshitz and Christoff2016) explored brain activation and deactivation across multiple meditation categories and found that insula activation was observed in all main types of meditations, suggesting its vital role of promoting interoceptive awareness (Craig, Reference Craig2003, Reference Craig2009; Critchley, Wiens, Rotshtein, Ohman, & Dolan, Reference Critchley, Wiens, Rotshtein, Ohman and Dolan2004; Menon & Uddin, Reference Menon and Uddin2010) and empathy (Lamm, Decety, & Singer, Reference Lamm, Decety and Singer2011; Menon & Uddin, Reference Menon and Uddin2010; Singer, Critchley, & Preuschoff, Reference Singer, Critchley and Preuschoff2009). Activations of the premotor cortex and supplementary motor area were also observed in meditators (Boccia et al., Reference Boccia, Piccardi and Guariglia2015; Fox et al., Reference Fox, Dixon, Nijeboer, Girn, Floman, Lifshitz and Christoff2016; Tomasino et al., Reference Tomasino, Fregona, Skrap and Fabbro2012), explained by their involvement in mental manipulation (Hanakawa et al., Reference Hanakawa, Honda, Sawamoto, Okada, Yonekura, Fukuyama and Shibasaki2002; Nachev, Kennard, & Husain, Reference Nachev, Kennard and Husain2008).
Activated brain regions are increasingly viewed as components of large-scale brain networks. Among these, the default mode network (DMN) has been most frequently examined in meditation (Taylor et al., Reference Taylor, Daneault, Grant, Scavone, Breton, Roffe-Vidal and Beauregard2013). The DMN is a distributed brain network that is frequently deactivated during goal-oriented tasks and is active during wakeful rest (Fox et al., Reference Fox, Snyder, Vincent, Corbetta, Van Essen and Raichle2005). Involvement of the DMN has been revealed in studies of episodic memory, autobiographical process, self-reference, theory of mind, social evaluations and mind wandering (Andrews-Hanna, Reference Andrews-Hanna2012; Buckner, Andrews-Hanna, & Schacter, Reference Buckner, Andrews-Hanna and Schacter2008; Sestieri, Corbetta, Romani, & Shulman, Reference Sestieri, Corbetta, Romani and Shulman2011; Spreng & Grady, Reference Spreng and Grady2009; Svoboda, McKinnon, & Levine, Reference Svoboda, McKinnon and Levine2006). Meditation-associated activations of DMN regions, including the precuneus, ACC and angular gyrus, were shown in Boccia and colleagues’ (Reference Boccia, Piccardi and Guariglia2015) analysis, but the opposite result was found by others (Fox et al., Reference Fox, Dixon, Nijeboer, Girn, Floman, Lifshitz and Christoff2016; Tomasino et al., Reference Tomasino, Fregona, Skrap and Fabbro2012). Deactivation of the DMN is consistent with the meditation practices of bringing the wandering mind back to the present moment (Schacter, Addis, & Buckner, Reference Schacter, Addis and Buckner2007; Tomasino et al., Reference Tomasino, Fregona, Skrap and Fabbro2012) and of weakening self-referential processing (Goldin, Ramel, & Gross, Reference Goldin, Ramel and Gross2009; Manna et al., Reference Manna, Raffone, Perrucci, Nardo, Ferretti, Tartaro and Romani2010).
Patterns of brain activation in response to a specific task inform on the neurobiological correlates of meditation, but their focus on specific loci does not capture the distributed nature of information processing. Functional connectivity (FC) examines the temporal correlations of spontaneous low-frequency blood oxygen level-dependent (BOLD) signal fluctuations between anatomically distinct areas (Biswal, Reference Biswal2012; Friston, Williams, Howard, Frackowiak, & Turner, Reference Friston, Williams, Howard, Frackowiak and Turner1996) to characterize brain neural networks and their interactions with state-dependent activity (Buckner et al., Reference Buckner, Andrews-Hanna and Schacter2008; Gopinath et al., Reference Gopinath, Ringe, Goyal, Carter, Dinse, Haley and Briggs2011; Kilpatrick et al., Reference Kilpatrick, Suyenobu, Smith, Bueller, Goodman, Creswell and Naliboff2011).
Studies of FC investigating meditation, either in resting state or during meditation, have been growing. Some studies have compared FC before and after meditation practice and others have contrasted experienced meditators and novices. In between-network FC analyses, meditation has been associated with relatively increased FC between DMN and frontoparietal network (FPN), especially the regions implicated in cognitive regulation and self-monitoring, such as the dorsolateral prefrontal cortex (dlPFC; Brewer et al., Reference Brewer, Worhunsky, Gray, Tang, Weber and Kober2011; Creswell et al., Reference Creswell, Taren, Lindsay, Greco, Gianaros, Fairgrieve and Ferris2016; Hasenkamp & Barsalou, Reference Hasenkamp and Barsalou2012), superior frontal gyrus (SFG; Creswell et al., Reference Creswell, Taren, Lindsay, Greco, Gianaros, Fairgrieve and Ferris2016) and inferior parietal lobule (IPL; Hasenkamp & Barsalou, Reference Hasenkamp and Barsalou2012; Yang et al., Reference Yang, Barros-Loscertales, Pinazo, Ventura-Campos, Borchardt, Bustamante and Walter2016). At the within-network level, meditation training has been linked with increased FC within the dorsal attention network (DAN; Froeliger et al., Reference Froeliger, Garland, Kozink, Modlin, Chen, McClernon and Sobin2012), that is, regions recruited when attention is turned to the external world (Corbetta, Patel, & Shulman, Reference Corbetta, Patel and Shulman2008). Also, decreased FC within the somatomotor network (SMN) and salience network (SN) were found postmeditation training (Cotier, Zhang, & Lee, Reference Cotier, Zhang and Lee2017). Greater FC within auditory and visual networks in experienced meditators than controls was reported in one study (Kilpatrick et al., Reference Kilpatrick, Suyenobu, Smith, Bueller, Goodman, Creswell and Naliboff2011) but not in another (Berkovich-Ohana et al., Reference Berkovich-Ohana, Harel, Hahamy, Arieli and Malach2016). Stronger FC between DMN and medial prefrontal cortex (mPFC), one of the key DMN regions, was found after meditation practice (Jang et al., Reference Jang, Jung, Kang, Byun, Kwon, Choi and Kwon2011), but the opposite result, of decreased FC within the DMN, has also been reported in several studies (Berkovich-Ohana, Harel, Hahamy, Arieli, & Malach, Reference Berkovich-Ohana, Harel, Hahamy, Arieli and Malach2016; Cotier et al., Reference Cotier, Zhang and Lee2017; Hasenkamp & Barsalou, Reference Hasenkamp and Barsalou2012; Yang et al., Reference Yang, Barros-Loscertales, Pinazo, Ventura-Campos, Borchardt, Bustamante and Walter2016).
Length of meditation experience has also been examined. Increased FC within DAN was found in experienced meditators (Froeliger et al., Reference Froeliger, Garland, Kozink, Modlin, Chen, McClernon and Sobin2012; Hasenkamp & Barsalou, Reference Hasenkamp and Barsalou2012). Intra-DMN FC has been negatively correlated with the number of years meditating (Berkovich-Ohana et al., Reference Berkovich-Ohana, Harel, Hahamy, Arieli and Malach2016). Froeliger and colleagues (Reference Froeliger, Garland, Kozink, Modlin, Chen, McClernon and Sobin2012) found that meditation experience predicted increasing FC between DMN and DAN both during resting state and meditation state.
Although meta-analyses of brain structure and functional activations have reached some consensus about the neurobiological processes involved in meditation, no summary on how meditation alters FC across brain networks has been performed. To address the ostensibly simple question “How does meditation affect the interactions of the human brain within and across networks?”, we reviewed all existing FC studies related to meditation and conducted a coordinate-based meta-analysis to lessen bias and mitigate the lack of statistical power entailed by small sample size studies. Considering the specific role of the DMN in meditation effects, this meta-analysis is focused on whether meditation changes functional integration within DMN and between the DMN and other networks. The DMN is usually regarded as being related to self-referential processing, which could be weakened by the meditation practice of focusing on the present moment. To monitor self-referential thinking, regulate attention and control the cognitive process, the involvement of FPN and attention networks (VAN/DAN) may be necessary. The FPN plays an important role in distributing attention (Corbetta & Shulman, Reference Corbetta and Shulman2002), while the DMN is involved in attending internally instead of to the outside (Raichle, Reference Raichle2015). Based on the relationship between the functions of each brain network and the internal mechanism of meditation, we hypothesized that meditation would be associated with significantly increased FC across certain brain networks (DMN-FPN and DMN-VAN/DAN), along with decreased functional connectivity within DMN, as demonstrated in Figure 1.

Figure 1. Schematic of our hypothesis.
Common coordinate-based activation meta-analysis is not typically suitable for functional connectivity measures, as the seeds vary so much across studies (Eickhoff et al., Reference Eickhoff, Laird, Grefkes, Wang, Zilles and Fox2009; Tahmasian et al., Reference Tahmasian, Sepehry, Samea, Khodadadifar, Soltaninejad, Javaheripour and Eickhoff2019; Turkeltaub, Eden, Jones, & Zeffiro, Reference Turkeltaub, Eden, Jones and Zeffiro2002). By selecting studies that used seeds in a common brain network, we assumed that meta-analytical results from these studies could be regarded as the regions co-activated or co-deactivated with these certain brain network areas, which were represented by the corresponding seeds. This assumption had been confirmed by previous studies. For instance, Di, Gohel, Kim, and Biswal (Reference Di, Gohel, Kim and Biswal2013) tested the task-related network through meta-analytic connectivity modeling (MACM) method to find the brain network’s reconfiguration between resting-state and task conditions. This method was also applied to investigate the brain network interactions associated with major depressive disorder (Kaiser, Andrews-Hanna, Wager, & Pizzagalli, Reference Kaiser, Andrews-Hanna, Wager and Pizzagalli2015). In our study, following Kaiser et al. (Reference Kaiser, Andrews-Hanna, Wager and Pizzagalli2015), we exclusively selected studies that placed their seeds within the DMN. This criterion significantly reduced the sample size of the current study, balanced by the benefit of homogeneity of seed placement. In addition, other alternative analytic methods, such as ICA, were not feasible to be analyzed systematically, because relatively few prior studies of meditation effects on FC have implemented these methods. Against this background, we believe there is value in presenting our preliminary results and to withhold definitive interpretation pending future independent replication as the related literature grows over time. Thus, the coordinate-based meta-analysis is utilized to confirm or falsify our hypothesis.
To further examine our hypotheses, we also conducted a meta-regression analysis of voxel values across studies in terms of average length of meditation experience of the corresponding samples to capture effects of mean meditation experience duration. We expected our hypothesized FC differences to be related to the duration of meditation experience, with stronger effects in highly experienced meditators than in those with less experience. To the best of our knowledge, this is the first meta-analysis focusing on FC results to explore the underlying neurobiological mechanisms of meditation practice.
Method
Literature search
A literature search was performed using the keywords: “meditation” AND “MRI” AND “functional connectivity” in PubMed, ScienceDirect, ResearchGate, Scopus, Web of Science, NeuroSynth, and Google Scholar databases through September 24, 2019. The flowchart of the search strategy and included studies are shown in Figure 2. The following criteria were used to exclude unrelated studies: (1) only studies that computed whole-brain FC were included; (2) only studies that reported coordinates either in Talairach or Montreal Neurological Institute (MNI) space were included; (3) only studies that focused on state-related (e.g., resting state, meditation state) research were included. Task-related studies were excluded due to their diverse designs; (4) only studies that used participants with real meditation training or experience were included, and studies that explored meditation traits were excluded; (5) only studies that were published in a peer-reviewed journal were included; (6) only noncase studies and those testing nonclinical populations were included. After applying these criteria, 14 studies from 13 articles were chosen. Table 1 presents the details of each study.

Figure 2. Flowchart of the study selection process.
Table 1. Summary of fMRI studies included in meta-analysis (N = 10)

Note: Fourteen studies from 13 publications compared meditators to controls (between-subject), postmeditation training to premeditation training (within-subject); studies from the same article were marked with “*”; Four studies (Chen et al., Reference Chen, Lv, Fang, Yu, Sui, Fan and Jiang2015; Farb et al., Reference Farb, Segal, Mayberg, Bean, McKeon, Fatima and Anderson2007; Liu et al., Reference Liu, Wu, Li and Guo2018; Taren et al., Reference Taren, Gianaros, Greco, Lindsay, Fairgrieve, Brown and Bursley2015) which did not include default mode network seeds were excluded from statistical analysis. FA = focused attention, OM = open monitoring, LK = loving kindness, MBSR = mindfulness-based stress reduction. Revised experience (hours)§: the length of meditation experience in each study was reported in different ways (years, weeks, days, and hours). To make them comparable, all data were converted to hours.
The selected studies used one of two designs. Specifically, some studies focused on the intensive and short-term effects of meditation on brain changes by comparing the difference between pretraining and posttraining results for subjects without previous meditation experience, referred to as the within-subject design. Between-subject studies compared experienced meditators to nonmeditators. These two designs have been applied by researchers to study meditation effects on brain structure and activation/deactivation patterns, as well as FC. We concluded that the two designs could be combined in a meta-analysis since both focus on the same research question, namely, the effects of meditation training of brain functional connectivity. This combination method has been adopted in other neuroimaging meta-analysis of meditation effects (Boccia et al., Reference Boccia, Piccardi and Guariglia2015; Fox et al., Reference Fox, Nijeboer, Dixon, Floman, Ellamil, Rumak and Christoff2014; Tomasino et al., Reference Tomasino, Fregona, Skrap and Fabbro2012).
Data extraction and coding
The current meta-analysis is based on peak coordinates, which represent the brain areas showing significant differences in FC with seed region-of-interests (ROIs) between meditation and control samples or postmeditation versus premeditation states. We first extracted the coordinates of each seed ROI and the peak of each effect region. All peaks in Talairach space were transformed into MNI space. Second, each seed ROI was categorized into a seed network according to Yeo’s 7-network parcellation (Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead and Buckner2011; Table 2; Figure 3). From the 14 studies, we only selected studies that had placed seeds in the DMN, resulting in 10 studies from 9 articles, which were entered into the final statistical analysis: 7 meditator versus nonmeditator (one of them compared highly experienced meditators versus meditators with low experience) and 3 postmeditation versus premeditation. All these studies investigated FC within the DMN and between the DMN and other networks. Third, coordinates were extracted for the following conditions, meditators versus controls, controls versus meditators, postmeditation versus premeditation, premeditation versus postmeditation. We collectively refer to these comparisons as meditation versus control.
Table 2. Summary of seed-networks and anatomical regions of studies included in meta-analysis (n = 14)

Note: Seed regions-of-interest (ROIs) were categorized into seed-networks based on their location within a priori functional networks including frontoparietal network (FPN), somatomotor network (SMN), default mode network (DMN), and limbic system. The other three networks that belongs to Yeo’s 7 network model but not shown above include visual network (VN), ventral attention network (VAN), and dorsal attention network (DAN); anatomical regions in which seed ROIs were located included insula, dorsal medial prefrontal cortex (dmPFC), dorsolateral prefrontal cortex (dlPFC), right posterior parietal lobule (rPPL), dorsal anterior cingulate cortex (dACC), posterior cingulate cortex (PCC), Precuneus (PCu), medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), ventral medial prefrontal cortex (vmPFC), ventral posteromedial cortex (vPMC), pregenual anterior cingulate cortex (pgACC), amygdala. To ensure that a single study could not result in a significant effect, seed-networks were included in meta-analysis if at least three independent studies included seed ROIs within the network.

Figure 3. Locations of Seed ROIs.
Statistical analysis
We used Signed Differential Mapping (SDM) software (Radua, Mataix-Cols et al., Reference Radua, Mataix-Cols, Phillips, El-Hage, Kronhaus, Cardoner and Surguladze2012) to meta-analyze brain areas with statistically significant FC with DMN seeds. SDM has become widely used in neuropsychiatric research (Bora et al., Reference Bora, Fornito, Radua, Walterfang, Seal, Wood and Pantelis2011; Radua, Borgwardt et al., Reference Radua, Borgwardt, Crescini, Mataix-Cols, Meyer-Lindenberg, McGuire and Fusar-Poli2012); detailed description can be found elsewhere (Radua, Borgwardt et al., Reference Radua, Borgwardt, Crescini, Mataix-Cols, Meyer-Lindenberg, McGuire and Fusar-Poli2012; Radua & Mataix-Cols, Reference Radua and Mataix-Cols2009, Reference Radua and Mataix-Cols2012). Briefly, only peak coordinates attaining statistical significance at the whole-brain level were included in the first phase of the meta-analysis. Then, an effect-size map of the meditation versus control FC was computed by transforming the maximum t values of the peak coordinates into Hedges’ effect sizes. Afterwards, an anisotropic Gaussian kernel was used to allot larger effect sizes to voxels that are more strongly associated with the peaks (Radua et al., Reference Radua, Rubia, Canales-Rodriguez, Pomarol-Clotet, Fusar-Poli and Mataix-Cols2014). Finally, a random-effects model was used to integrate those studies included in this meta-analysis. SDM is based on the null hypothesis that effect sizes rather than peaks alone are randomly distributed across the whole brain (Radua & Mataix-Cols, Reference Radua and Mataix-Cols2012). The threshold was set as p = .005 (uncorrected) combined with z > 1. Radua, Mataix-Cols et al. (Reference Radua, Mataix-Cols, Phillips, El-Hage, Kronhaus, Cardoner and Surguladze2012) do not recommend relying solely on uncorrected or corrected p values, but rather they strongly suggest using the combination of two thresholds and complementing the analysis with several tests to assess the robustness and heterogeneity of the findings. Based on the empirical validation in their study, Radua, Mataix-Cols et al. proposed using an uncorrected p = .005 as the main threshold, as this was found to optimally balance sensitivity and specificity and to be an approximate equivalent to a corrected p value = .05 (indeed .025) in SDM. Still, this equivalence is only approximate, so that some thresholding error is expected when using this approach, and researchers might prefer other thresholds. To reduce the possibility of false positive results, Radua, Mataix-Cols et al. suggested an additional z-based threshold (e.g., z > 1). We note that z > 1 would be associated with a clearly nonsignificant p-value under the standard normal distribution, but this is not the case under the empirical distributions produced by permutation tests, as the use of null effect sizes when preprocessing voxels far from any peak coordinate makes z > 1 much more unlikely (i.e., associated with much lower p values).
Results
Brain areas with statistically significant effect sizes in the meditation versus control meta-analysis were categorized according to Yeo’s 7-network parcellation (Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead and Buckner2011). This network parcellation was selected because: (1) it was defined in a large sample; (2) it was replicated in an independent sample (Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead and Buckner2011); (3) it closely corresponded with networks derived from alternative FC analytic strategies and task-based patterns of co-activation.
Results showed that meditation was related to increased FC between DMN seeds and regions of the left inferior frontal gyrus that fall within the FPN. Increased FC was also found between DMN seeds and VAN regions, such as left insula and right anterior supramarginal gyrus. In addition, increased FC between DMN seeds and left parahippocampal gyrus, a component of the limbic network, was also observed. Functional connectivity between the right cerebellum and DMN seeds increased as well (Table 3; Figure 4).
Table 3. Results of meta-analysis of state functional connectivity associated with meditation, based on DMN seeds (n = 10)

Note: Results of comparison in meditation vs. control (M > C; M < C) functional connectivity with default model network (DMN). The setting threshold was p < .005 (uncorrected) combined with z > 1; networks examined included frontoparietal network (FPN), limbic network, ventral attention network (VAN), somatomotor network (SMN), and default mode network (DMN). Voxels = number of 2 × 2 × 2 mm voxels; P = statistical significance level.

Figure 4. Results of meta-analysis of state functional connectivity associated with meditation, based on DMN seeds (n = 10).
Decreased connectivity within the DMN was characterized by decreased FC between DMN seeds and precuneus extending to posterior cingulate gyrus. Decreased FC between DMN seeds and somatomotor areas within the right paracentral lobule was also found (Table 3; Figure 4).
Meditation experience (indexed as number of hours) was entered into a meta-regression analysis to explore differences between highly experienced meditators and less experienced meditators. Results showed decreased FC between DMN seeds and areas of the medial superior frontal cortex and right posterior cingulate gyrus – both located in the DMN – in individuals with extensive meditation experience compared to those with briefer experience. Increased FC between DMN seeds and the left inferior frontal gyrus (FPN), left fusiform (DAN), and left inferior temporal gyrus (limbic network) were found in highly experienced practitioners in contrast to those with less experience (Table 4; Figure 5).
Table 4. Results of meta-regression of state functional connectivity associated with meditation experience (hours), based on DMN seeds (N = 10)

Note: Results of meta-regression of functional connectivity with default mode network (DMN). The setting threshold was p < .005 (uncorrected) combined with z > 1; networks examined included frontoparietal network (FPN), dorsal attention network (DAN), limbic network, and default mode network (DMN). Voxels = number of 2 × 2 × 2 mm voxels; max P = maximum proportion of studies exhibiting the effect at the peak density, weighted by sample size. L = long term; S = short term.

Figure 5. Results of meta-regression of state functional connectivity associated with meditation experience (hours), based on DMN seeds (n = 10).
We extracted the z values of voxels located in the seven-network model – visual network, SMN, DAN, VAN, FPN and DMN – to summarize the distribution of the z values of voxels in each network for both the meta-analysis of FC and meta-regression. The violin plot shows meditation-associated decreased FC within DMN and DMN-SMN, along with significant DMN-FPN and DMN-VAN increased FC (Figure 6). Overall, meta-analysis and meta-regression results agreed except for increased FC between DMN and VAN and decreased FC between DMN and SMN, both of which were absent in the meta-regression (Figure 7). Thus, our hypothesis that meditation is associated with significantly increased FC between DMN and FPN, and between DMN and VAN, and significantly decreased FC within DMN was supported. Other than that, the unexpected finding of decreased FC between DMN and SMN was additional to our final result.

Figure 6. Meta-analysis: violin plot of the Z values of each of the voxels in the seven-networks for both the increased (left) and decreased (right) functional connectivity related to meditation.

Figure 7. Meta-regression considering the factor of meditation experience (practice hours): violin plot of the Z values of each of the voxels in the seven-network parcellation for both increased (left) and decreased (right) functional connectivity related to meditation.
Discussion
The present study provides the first, albeit preliminary, meta-analytic evidence that meditation practices alter the interactions within and between specific brain networks, that is, DMN, SMN, FPN and VAN (Figure 8). These findings are beneficial for building a neurocognitive model to characterize meditation’s underlying neural mechanisms. If confirmed as the number of studies continues to accumulate, our findings could also facilitate the application of meditation in clinical settings. Based on our results, we propose that reduced FC within DMN and between DMN and SMN, as well as increased FC between DMN and both FPN and VAN, can be tested as biomarkers of meditation training effects. The meta-regression of meditation experience supports this pattern of both decreases and increases in FC.
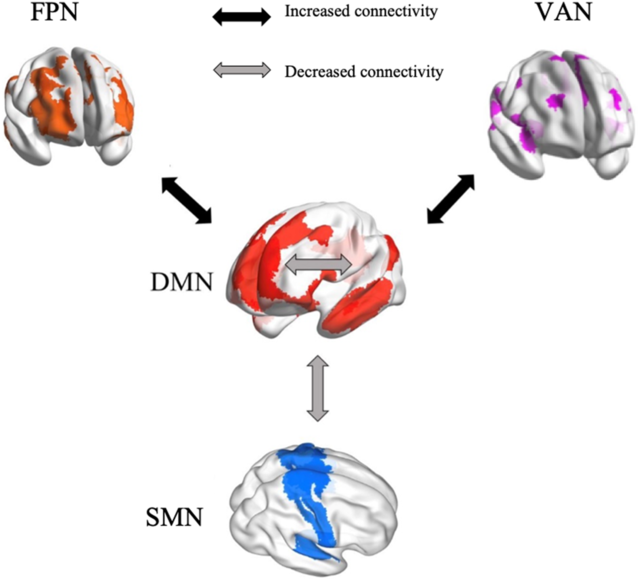
Figure 8. A neurocognitive network model of brain network interactions associated with meditation.
Increased functional connectivity between DMN and FPN
From a theoretical perspective, meditators are trained to monitor their cognition and decrease self-referential thinking to maintain present-moment attention. This implies enhancement of communication between “task-positive” brain regions involved in executive control and the “task-negative” DMN (Brewer et al., Reference Brewer, Worhunsky, Gray, Tang, Weber and Kober2011; Spreng, Reference Spreng2012). This implication was confirmed by our findings of increased FC between brain areas of DMN involved in internal awareness and FPN regions related to executive control and cognitive monitoring. Objectives of meditation include improving cognitive control (Moore & Malinowski, Reference Moore and Malinowski2009), presumably through frontoparietal systems and reducing mind wandering and self-referential thinking, which are associated with the DMN (Brewer et al., Reference Brewer, Worhunsky, Gray, Tang, Weber and Kober2011; Lutz et al., Reference Lutz, Slagter, Dunne and Davidson2008). The left inferior frontal gyrus, a major component of the FPN, is involved in conscious inhibition (Miyake et al., Reference Miyake, Friedman, Emerson, Witzki, Howerter and Wager2000; Swick, Ashley, & Turken, Reference Swick, Ashley and Turken2008). The interaction between left inferior frontal gyrus and DMN areas might underlie the reduction of mind wandering. Thus, the meditation-related interaction of FPN with DMN regions is reflected by enhanced FC. This result has also been observed consistently (Brewer et al., Reference Brewer, Worhunsky, Gray, Tang, Weber and Kober2011; Creswell et al., Reference Creswell, Taren, Lindsay, Greco, Gianaros, Fairgrieve and Ferris2016; Gerlach, Spreng, Madore, & Schacter, Reference Gerlach, Spreng, Madore and Schacter2014; Spreng, Reference Spreng2012). The pattern was found to be more significant in highly experienced meditators compared to individuals with less experience, which further supports the conclusion that meditation changes interactions between the DMN and the FPN.
Increased connectivity between DMN and VAN
We observed increased FC associated with meditation between DMN seeds and the VAN, regions involved in detecting unexpected stimuli and reorienting attention (Corbetta & Shulman, Reference Corbetta and Shulman2002). Fox and colleagues (Reference Fox, Corbetta, Snyder, Vincent and Raichle2006) linked the VAN to awareness and attentional control related to stimuli generated spontaneously in addition to external stimuli. Attentional control is a core part of meditation practice, especially regarding internal awareness. During meditation, practitioners are guided to attend internally, which might account for increased communication between the DMN involved in self-awareness and the VAN related to attention control. Insular cortex, as a major part of the ventral attention system, participates in many interoception activities (Craig, Reference Craig2003, Reference Craig2009; Critchley et al., Reference Critchley, Wiens, Rotshtein, Ohman and Dolan2004; Farb, Segal, & Anderson, Reference Farb, Segal and Anderson2013) and meta-awareness processing (Fleming & Dolan, Reference Fleming and Dolan2012). Meditation training involves monitoring respiration, heart rate and other internal body states, sustaining focused attention and being aware of mind wandering. Therefore, significant increase in FC between insula and DMN regions is consistent with meditation being intimately associated with attentional functions. The lack of a difference in FC between the DMN and the VAN related to meditation experience may reflect the initial focus of most meditation training on attention control, which presumably remains stable in long-term meditators.
Decreased functional connectivity within DMN
We found decreased FC in meditators relative to controls and in postmeditation training relative to pretraining within the DMN. Meta-regression confirmed significantly decreased FC in highly experienced meditators in contrast to individuals with less experience. The cognitive roles of the DMN include self-referential thinking, social evaluations and mind wandering (Andrews-Hanna, Reference Andrews-Hanna2012; Buckner et al., Reference Buckner, Andrews-Hanna and Schacter2008; Davey, Pujol, & Harrison, Reference Davey, Pujol and Harrison2016; Svoboda et al., Reference Svoboda, McKinnon and Levine2006). One of the core nodes of the DMN is the posterior cingulate cortex (PCC; Leech & Sharp, Reference Leech and Sharp2014). Relative deactivation of the PCC during various types of external attention demanding tasks has been found in many fMRI studies (Singh & Fawcett, Reference Singh and Fawcett2008). Increased activation of the PCC in retrieving autobiographical memories, making personal decisions and self-evaluations, and planning for the future has been repeatedly observed (Addis, Wong, & Schacter, Reference Addis, Wong and Schacter2007; Mason et al., Reference Mason, Norton, Van Horn, Wegner, Grafton and Macrae2007; Shannon & Buckner, Reference Shannon and Buckner2004; Szpunar, Watson, & McDermott, Reference Szpunar, Watson and McDermott2007). Both phenomena suggest that the PCC has an important role in maintaining internally directed cognitive activities (Buckner et al., Reference Buckner, Andrews-Hanna and Schacter2008). The finding of decreased FC between DMN seeds and the PCC fits with decreased internal cognition after meditation training, which focuses on regulating self-awareness, detaching from self-referential thinking, and maintaining a nonjudgmental stance (Sperduti, Martinelli, & Piolino, Reference Sperduti, Martinelli and Piolino2012). Through regular practice of self-awareness control activities, the internal FC of the brain network associated with self-referentiality (the DMN) appears to be reduced. In addition, the DMN is involved in rumination, a mental state characterized by repetitive thinking about self-related negative experiences (Berman et al., Reference Berman, Peltier, Nee, Kross, Deldin and Jonides2011). Decreased coupling within the DMN has been related to a reduction of ruminative thinking (Jacobs et al., Reference Jacobs, Watkins, Peters, Feldhaus, Barba, Carbray and Langenecker2016). Therefore, meditation may represent an approach to cope with rumination, with its effectiveness supported behaviorally and in an EEG study (Keune, Bostanov, Hautzinger, & Kotchoubey, Reference Keune, Bostanov, Hautzinger and Kotchoubey2011; Teismann et al., Reference Teismann, von Brachel, Hanning, Grillenberger, Hebermehl, Hornstein and Willutzki2014). The meditation-associated change in decreasing within-DMN FC appeared to be more pronounced as the number of meditation practice hours increased. This further supports that long-term meditation practice helps people to control self-awareness, reduce the occurrence of negative ruminative thinking, and thus avoid the risks of unhealthy mentation to some extent.
Decreased functional connectivity between the DMN and the SMN
Reduced FC was also observed in meditators compared to controls and in postmeditation training compared to pretraining between the DMN and the SMN. The SMN is composed of multiple somatosensory and motor regions that are traditionally regarded as contributing to individuals’ control and detection of movement (Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead and Buckner2011). Few studies have discussed the interaction of the SMN with the DMN in cognitive processing since their functional divisions are relatively clear (Mesulam, Reference Mesulam1998). The regions in the SMN are involved in the detection and processing of motor and somatosensory stimuli (Downar, Crawley, Mikulis, & Davis, Reference Downar, Crawley, Mikulis and Davis2000), whereas the DMN area is related to individual’s self-referential thinking (Buckner et al., Reference Buckner, Andrews-Hanna and Schacter2008; Northoff et al., Reference Northoff, Heinzel, de Greck, Bermpohl, Dobrowolny and Panksepp2006). This results in a low coupling with each other in general. The revealed decreased connectivity between the SMN and the DMN in our meta-analysis might indicate that meditation practice further strengthens the differentiation of these two networks. Through meditation training, individuals are more able to separate external stimuli from internalized attention when dealing with the perception and processing of these external stimuli (Rubia, Reference Rubia2009). In other words, meditation practice can enhance the ability to resist interference by perceived external stimuli with internal consciousness.
Implications for clinical settings
Meditation has been widely recognized as an effective therapeutic intervention in the treatment of many physical and mental disorders, such as depression, stress-related disturbance (e.g., posttraumatic stress disorder, anxiety disorder), and chronic pain. The findings of the present meta-analysis may explain meditation’s clinical effectiveness from a neuroscience perspective.
Depression has been characterized by hyper-connectivity within the DMN system compared to healthy controls (Alexopoulos et al., Reference Alexopoulos, Hoptman, Kanellopoulos, Murphy, Lim and Gunning2012; Berman et al., Reference Berman, Peltier, Nee, Kross, Deldin and Jonides2011; Kaiser et al., Reference Kaiser, Andrews-Hanna, Wager and Pizzagalli2015). Hypo-connectivity between pregenual anterior cingulate cortex (DMN) and insula (VAN; Horn et al., Reference Horn, Yu, Steiner, Buchmann, Kaufmann, Osoba and Walter2010) and between the DMN and cerebellar regions (Guo et al., Reference Guo, Liu, Xue, Gao, Liu, Xiao and Zhao2013) have been observed in patients with depressive disorder. The effect of meditation practice on changing the abnormal interactions of these brain networks (through reducing within-DMN FC and increasing the FC between DMN and both VAN and cerebellum) may partially explain improvements in depression symptoms. Of note, a multisite large sample (N > 1000) study was recently conducted on FC in the DMN in patients with major depressive disorder (MDD; Yan et al., Reference Yan, Chen, Li, Castellanos, Bai, Bo and Zang2019). Although the results did not reveal enhanced within-DMN FC in MDD patients, reduced DMN FC was observed in patients treated with antidepressants, which is consistent with the notion that decreasing FC within the DMN is associated with improving depression. In social anxiety disorder, stronger within-DMN FC (Liao et al., Reference Liao, Xu, Mantini, Ding, Machado-de-Sousa, Hallak and Chen2011) and decreased FC between brain regions related to cognitive control and bilateral angular gyri, important DMN components, have been found (Qiu et al., Reference Qiu, Liao, Ding, Feng, Zhu, Nie and Gong2011). A recent meta-analysis focusing on resting-state FC studies found hypo-connectivity and decoupling of the FPN and DMN in patients with anxiety disorder (Xu et al., Reference Xu, Van Dam, Feng, Luo, Ai, Gu and Xu2019). Thus, the effect of meditation in enhancing FPN-DMN FC and reducing within-DMN FC may be relevant to the treatment of anxiety disorder. Enhanced within-DMN FC has also been correlated with posttraumatic stress disorder symptoms (Bluhm et al., Reference Bluhm, Williamson, Osuch, Frewen, Stevens, Boksman and Lanius2009; Lanius et al., Reference Lanius, Bluhm, Coupland, Hegadoren, Rowe, Theberge and Brimson2010; Qin et al., Reference Qin, Wang, Sun, Wan, Su, Zhou and Xu2012). Positive effects of meditation in treating anxiety and stress-related disorders may be related to regulation of within-DMN FC and its interaction with brain areas in the VAN system (Li et al., Reference Li, Li, Dong, Luo, Han, Xiong, Guo and Li2012). In the context of chronic pain, abnormal inter-network and between-network dysfunctions of the DMN have been found (Baliki, Baria, & Apkarian, Reference Baliki, Baria and Apkarian2011; Napadow et al., Reference Napadow, LaCount, Park, As-Sanie, Clauw and Harris2010), and meditation may play a neuromodulation role in pain relief interventions. Thus, the effects of meditation on changing brain network interactions may underlie its effectiveness in treating mental disorders.
Limitations
Several limitations in the present meta-analysis warrant further research. First, there have been relatively few brain imaging studies of meditation effects with even fewer seed-based FC studies. Among these studies, variation in the seeds selected made it difficult to conduct large sample size based meta-analyses. Therefore, the number of studies included in statistical analyses was limited and our results should be considered preliminary. Second, the present meta-analysis was limited to FC changes with ROIs within the DMN only. Interactions within and between non-DMN networks should be better examined to provide a more comprehensive model of brain functional integration. Third, standards for data acquisition and preprocessing differed across studies (e.g., difference in MRI facilities, scanning time, motion correction methods, and instructions regarding eye status). The effects of these variables should be examined in future work. Fourth, we estimated the durations of meditation practice time based on the limited information provided in each paper. Fifth, we used seed ROIs to represent networks. Although functions within each brain network tend to be consistent, local differences are not negligible. For example, in the DMN, Andrews-Hanna and colleagues (Reference Andrews-Hanna, Reidler, Sepulcre, Poulin and Buckner2010) were able to distinguish three subnetworks with different cognitive functions. Further parcellation of networks in the future should provide results that are more accurate. Finally, types of meditation were combined. Although the same approach has been adopted by prior brain imaging reviews and meta-analyses (Boccia et al., Reference Boccia, Piccardi and Guariglia2015; Fox et al., Reference Fox, Nijeboer, Dixon, Floman, Ellamil, Rumak and Christoff2014; Sperduti et al., Reference Sperduti, Martinelli and Piolino2012; Tomasino et al., Reference Tomasino, Fregona, Skrap and Fabbro2012), some distinctions between types of meditation may be substantive. Analysis of types of meditations and their brain network interactions may lead to greater understanding of meditation mechanisms and could help identify which type of meditation is best for a specific condition. Thus, we encourage the conduct of comparative studies of meditation to facilitate such efforts in the future.
Conclusion
In this meta-analysis, we found that meditation effects are associated with both decreased FC (DMN-DMN and DMN-SMN) and increased FC (DMN-FPN and DMN-VAN). Stronger DMN-FPN FC and weaker DMN-DMN FC was more prominent in highly experienced meditators in contrast to those with less experience, which further supports the inference that meditation alters brain network interactions. These findings provide insights into the mechanisms underlying meditation and have implications for guiding clinical assessment of meditation interventions in treating certain physical and mental disorders.
Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFC1309902), the National Natural Science Foundation of China (81671774, 81630031 and 31900777), the Hundred Talents Program of the Chinese Academy of Sciences, and Beijing Municipal Science & Technology Commission (Z161100000216152).
Financial Support
None.
Conflict of Interest
The authors declare no competing financial interests.
Data Availability Statement
The meta-data is available at Github: https://github.com/Chaogan-Yan/PaperScripts/blob/master/Shen_2020_JPRP/Shen_Coordinates.xlsx
















