Management Implications
Impatiens glandulifera (ornamental jewelweed) is an annual invasive plant commonly growing along waterways where chemical control is prohibited. We evaluated seed survival, hot water weed control at different phenological stages, and cutting at different phenological stages and stem heights. We concluded that the plant’s phenological stage is important for determining the control method. Hot water application was more efficient than cutting in controlling I. glandulifera when performed on plants at an early growth stage, that is, leaf development on the main shoot. This method requires easy access to water. New seedlings emerged after cutting, whereas the hot water largely prevented new seed germination and seedlings to emerge. Furthermore, targeting smaller, rather than larger plants reduced both time and water usage by more than 50%. Cutting plants at the early regenerative stage gave equal control of I. glandulifera compared with the hot water application, regardless of whether the plants were cut below or above the oldest node. No plants produced side shoots after cutting. However, side shoots formed from the oldest node before cutting continued to grow and develop. Hot water application devegetated a larger amount of soil for a longer period of time than cutting. As the vegetation recovered, the grass plant cover increased, while the plant cover of forbs and raspberry (Rubus idaeus L.) plants was unaffected. In cases where establishment of new vegetation shortly after I. glandulifera removal is planned, hot water weed control may be the preferred method, as it prevents germination and seedlings becoming weeds in the reestablished plant cover. This method may also be effective in rough terrain with boulders that hinder access to plants with cutting equipment. Furthermore, hot water application leaves no offcut plant material to collect and transport out of the area, while cutting includes these tasks. In cases where control measures takes place late in the growth season, the use of hot water may not be beneficial due the inefficient use of time and water needed to eradicate larger plants. Nor is hot water weed control a suitable method in areas prone to erosion. Reinvasion or establishment of other unwanted plant species is also a risk in exposed soil. Cutting may be the preferred method in these cases.
Introduction
Ornamental jewelweed (Impatiens glandulifera Royle) is one of the most problematic alien invasive plants in Europe (European Union 2017). The species was introduced to the United Kingdom from the Himalayas in 1839 as an ornamental and nectar-producing plant, and the first naturalizations were reported in 1855 (Beerling and Perrins Reference Beerling and Perrins1993). In most European countries, I. glandulifera has now become naturalized (Pyšek and Prach Reference Pyšek and Prach1995). The process of invasion is still ongoing, and it is expected that considerably more locations will be colonized (Burkhart and Nentwig Reference Burkhart and Nentwig2008; Malíková and Prach Reference Malíková and Prach2010). Impatiens glandulifera was introduced to Norway around 1870 to 1880 and is now widespread as far north as 70°N (Elven et al. Reference Elven, Hegre, Solstad, Pedersen, Pedersen, Åsen and Vandvik2018). Its demonstrated ability to spread and invade new areas has led to a total prohibition against planting and disseminating I. glandulifera in Norway (Forskrift om fremmede organismer 2015).
Impatiens glandulifera is a summer-annual herb that reproduces only by seeds. In Norway it is reported to reach 1.5 m in height (Elven et al. Reference Elven, Hegre, Solstad, Pedersen, Pedersen, Åsen and Vandvik2018), while up to 2.5-m-tall plants are reported farther south in Europe (Burkhart and Nentwig Reference Burkhart and Nentwig2008). The stem is hollow, and the root system is shallow, but adventitious roots provide stability to the tall plants (Ennos et al. Reference Ennos, Crook and Grimshaw1993). The seeds are short-lived and have not been considered to form persistent seedbanks (Beerling and Perrins Reference Beerling and Perrins1993). However, Skálová et al. (Reference Skálová, Moravcová, Čuda and Pyšek2019) reported germinating and dormant seeds following 4 yr of burial. High fecundity and effective seed dispersal mechanisms partly explain why the species easily invades new areas. One individual plant normally produces 700 to 800 seeds at a plant density of 20 m−2. Increased plant density reduces the seed production per plant (Beerling and Perrins Reference Beerling and Perrins1993). When mature, the capsules explode at the slightest touch, and the seeds disperse up to 5 m (Beerling and Perrins Reference Beerling and Perrins1993). Seeds are transported in rivers and drainage ditches.
Several studies have reported that I. glandulifera has a negative influence on ecosystems and ecosystem services, such as plant diversity (Kiełtyk and Delimat Reference Kiełtyk and Delimat2019), soil fungal communities (Gaggini et al. Reference Gaggini, Rusterholz and Baur2019; Pattison et al. Reference Pattison, Rumble, Tanner, Jin and Gange2016; Ruckli et al. Reference Ruckli, Rusterholz and Baur2016), and terrestrial invertebrates (Seeney et al. Reference Seeney, Eastwood, Pattison, Willby and Bull2019). Allelopathic components of I. glandulifera may also negatively affect native vegetation (Bieberich et al. Reference Bieberich, Lauerer, Drachsler, Heinrichs, Müller and Feldhaar2018). In a riparian area in northwest Switzerland, Greenwood and Kuhn (Reference Greenwood and Kuhn2014) found that erosion was greater from sites invaded by I. glandulifera compared with sites with native vegetation. Other studies found no negative ecological effects of I. glandulifera. Bartomeus et al. (Reference Bartomeus, Vila and Steffan-Dewenter2010) found no evidence of I. glandulifera outcompeting native plants for pollinators. The species filled a late-season gap in the flowering season. Furthermore, Ammer et al. (Reference Ammer, Schall, Wördehoff, Lamatsch and Bachmann2011) concluded that I. glandulifera was not a strong competitor against established tree seedlings.
Studies from Canada (Leblanc and Lavoie Reference Leblanc and Lavoie2017) and Austria (Schiffleithner and Essl Reference Schiffleithner and Essl2016) determined the number of work hours spent on controlling I. glandulifera. They concluded that substantial resources are necessary over several years for effective management. Several studies have emphasized the importance of targeting small populations of I. glandulifera before they expand and disperse farther (Jernelöv Reference Jernelöv and Jernelöv2017; Meier et al. Reference Meier, Dullinger, Zimmermann, Baumgartner, Gattringer and Hülber2014). The understanding of how to efficiently control and avoid increased spread of this alien plant species is of great importance (Leblanc and Lavoie Reference Leblanc and Lavoie2017; Schiffleithner and Essl Reference Schiffleithner and Essl2016).
In contrast to the large amount of published work on how I. glandulifera influences ecosystem services, limited research has been published on control measures (Leblanc and Lavoie Reference Leblanc and Lavoie2017). As a summer annual, I. glandulifera might be expected to be easier to control than most perennial weeds. Due to the species’ relatively short-lived seeds, the best method of control is to prevent seed production and dispersal. Methods for control include herbicides and various nonchemical methods (Helmisaari Reference Helmisaari2010). Where I. glandulifera grows along rivers, the use of herbicides is prohibited. Furthermore, the principles of integrated pest management indicate that nonchemical alternatives should be considered before herbicides are applied (Barzman et al. Reference Barzman, Bàrberi, Birch, Boonekamp, Dachbrodt-Saaydeh, Graf, Hommel, Jensen, Kiss, Kudsk, Lamichhane, Messéan, Moonen, Ratnadass, Ricci, Sarah and Sattin2015). Biological control could be an alternative if suitable agents that do not target native species of Impatiens are identified (Burkhart and Nentwig Reference Burkhart and Nentwig2008; Sheppard et al. Reference Sheppard, Shaw and Sforza2006; Tanner et al. Reference Tanner, Pollard, Varia, Evans and Ellison2015). Different methods of thermal weed control are also possible (Rask and Kristoffersen Reference Rask and Kristoffersen2007). Heat applied directly or indirectly to plants causes injury to the plant cells and results in plant desiccation (Ascard et al. Reference Ascard, Hatcher, Melander, Upadhyaya, Upadhyaya and Blackshaw2007). The effect depends on temperature, exposure time, plant development stage, and species (Ascard et al. Reference Ascard, Hatcher, Melander, Upadhyaya, Upadhyaya and Blackshaw2007; Hansson and Ascard Reference Hansson and Ascard2002). Heat treatment may also lead to seed mortality (Dahlquist et al. Reference Dahlquist, Prather and Stapleton2007). Cutting and hand pulling are other possible nonchemical methods (Leblanc and Lavoie Reference Leblanc and Lavoie2017). The early regenerative stage has been recommended as the optimum stage for cutting of I. glandulifera (Helmisaari Reference Helmisaari2010). If cut earlier, plants may start to regrow and produce seeds (Jernelöv Reference Jernelöv and Jernelöv2017), whereas mature seeds may already have developed and dispersed with later cutting. All plant material should be removed after cutting, due to the ability of I. glandulifera to continue to grow from buds on shoot stumps (Helmisaari Reference Helmisaari2010).
The development of an efficient and environmentally friendly method to control I. glandulifera is important. The aim of this study was to evaluate nonchemical methods for control of naturalized populations of I. glandulifera in riparian habitats. In addition, we estimated lethal water temperature for seed and the heat sum (i.e., temperature above the lethal temperature × duration of the hot water exposure) needed to reduce germination by 50%, 90%, and 99%. Flaming is the only thermal weed control technique previously tested on this species (Clements et al. Reference Clements, Feenstra, Jones and Staniforth2008). Because the effect of hot water on I. glandulifera has not been studied before, we conducted experiments with hot water (lab, pot, and field plot experiments) and cutting (field plot experiments) addressing the following hypotheses:
1. Hot water application and cutting will give equal efficient control of I. glandulifera plants.
2. Cutting the stems of I. glandulifera below and above the lowest (i.e., oldest) node will give equal efficient control.
Materials and Methods
Four experiments were conducted in southeast Norway, two experiments targeting seeds of I. glandulifera and two field plot experiments mainly targeting emerged plants of I. glandulifera. The two experiments with seeds of I. glandulifera were conducted as one laboratory experiment and one outdoor pot experiment at the facilities of the Norwegian University of Life Sciences (NMBU), Ås, southeast Norway (59.67°N, 10.77°E, 95 m above sea level [m asl]). Mature I. glandulifera seeds were harvested from untreated plants along Gatebekken stream in October 2015. Seeds were dried for 2 wk at room temperature (about 20 C), followed by stratification at 4 C in darkness for 5 mo in vermiculite moistened with tap water.
Experimental Treatments and Data Sampling in the Laboratory Experiment
In the laboratory experiment (conducted April 1, 2016), we determined lethal water temperature for moist seeds by exposing them to hot water for 10 combinations of water temperature by duration, including an untreated control. For long duration (i.e., 30 min), we used five target temperatures, 45, 50, 55, 60, and 65 C. For short duration, we exposed seeds to water at 80 C for 3, 5, 10, or 30 s. For all treatments, seeds were placed in thin plastic bags and submerged in a hot water bath (Julabo model SW23, Julabo Labortechnik GMBH, Seelbach, Germany). We included 10 seeds in each bag and used 5 replicate bags per treatment, a total of 50 bags, including 5 bags of control seeds not exposed to high temperature. After treatment, all bags were submerged into a cooling water bath (approx. 3 C) before the seeds were placed on four layers of blotting paper watered with distilled water in petri dishes, one dish for each bag. We placed the petri dishes in a climate chamber with a 12-h photoperiod with a light intensity of 15 µmol m−2 s−1 and day/night temperatures of 20/15 C. The number of germinated seeds was counted after 17 d (April 18, 2016).
Experimental Treatments and Data Sampling in the Outdoor Pot Experiment
The outdoor pot experiment was set up to determine emergence rate of I. glandulifera plants following hot water treatment of seeds in soil. In the spring (March 29, 2016), 100 seeds were sown at a depth of 5 mm in 14-cm-wide bands (1-cm distance from pot edges) in rectangular pots (opening: 16 by 33 cm; volume: 12 L) filled with moist peat soil (P-jord, LOG AS, Oslo, Norway). This sowing density represented 2,165 seeds m−2. The thermal treatments were performed 1 d after sowing, using a hot water weed control machine with a 30-m-long hose and a 16-cm-wide nozzle (Heatweed Mid 22/8, Heatweed Technologies AS, Slitu, Norway). The temperature of the hot water was about 80 C when it reached the soil surface. Soil temperature was 7 to 8 C at the time of treatment. We included three treatments and four replicates: hot water application for either 3.5 or 7 s and an untreated control. The control pots were given the same amount of tap water (about 10 C) as the 3.5-s hot water treatment. The two hot water treatments were equivalent to a water usage of 7.2 and 14.5 L m−2, respectively. Furthermore, these volumes represented work hours of 1.1 min m−2 (54 m−2 h−1) and 2.2 min m−2 (27 m−2 h−1), respectively. Soil temperature was measured at soil depths of 0, 5, and 10 mm in three random pots from each hot water treatment. Temperatures were logged every second using a 0.5-mm-thick thermocouple cable type T connected to a Pico TC-08 (Pico Technology, St Neots, UK) data logger and a laptop computer with PicoLog software. The pots were kept outdoors until the number of emerged plants was recorded after 10 wk (June 6, 2016). For the first 2 wk, we covered the pots with transparent plastic to prevent drought and to protect the seeds from temperatures below zero. The average air temperature during the period was 9.4 C, with minimum and maximum temperatures of −4.8 C and 29.4 C, respectively. The pots were watered with tap water when needed, and no fertilizer was given. The energy inputs in the two hot water applications were calculated from the water volumes and the temperatures of the hot water and tap water (assumed to be 10 C), as described in Hansson and Mattsson (Reference Hansson and Mattsson2002).
Experimental Treatments in Field Experiment 2015 and 2016
The field plot experiments were located in established populations of I. glandulifera along two streams running through flat arable fields in Østfold County, southeast Norway. The locations were Gatebekken stream (59.37°N, 10.75°E, 25 m asl) and Støtvigbekken stream (59.35°N, 10.69°E, 20 m asl). The plots were situated in the relatively steep area between the stream and the arable fields. Field Experiment 2015 included one site, Gatebekken 1, and Field Experiment 2016 included two sites, Gatebekken 2 and Støtvigbekken. The soil type at Gatebekken is Mollic Gleysol (siltic soil), and at Støtvigbekken the soil type is a mix of Mollic Gleysol (siltic soil) and Luvic Stagnosol (siltic soil). In the two field experiments, we evaluated cutting and hot water treatment to control established populations of I. glandulifera. The treatments were applied to plots (1.0 by 1.0 m) arranged in a randomized block design with four or eight replications. All offcuts were removed from the plots after cutting, whereas no plant materials were removed from the hot water–treated plots. For cutting, we used a gasoline brush cutter in 2015 and manual hedge shears in 2016 for more accurate cutting above and below the oldest node on the stems. For hot water weed control, we used the same machine used for the outdoor pot experiment.
Field Experiment 2015
In this field plot experiment, we compared the effect of hot water treatment with cutting the plants as close to the ground as possible. We included three treatments at one site, Gatebekken 1: (1) cutting once, (2) hot water once, and (3) untreated control. The design originally included two more treatments, cutting twice and hot water twice. Due to extremely low regrowth at 4 wk after the first treatment, no second treatments were implemented, and the data for these plots were included in the data for the two other treatments. Hence, the cutting and hot water treatment had eight replicates, while the untreated control had four replicates. The two control measures were applied on the same day (June 22) when the I. glandulifera plants had started leaf development of main shoot (BBCH 1; Hess et al. Reference Hess, Barralis, Bleiholder, Buhr, Eggers, Hack and Stauss1997). On the day of treatment, the average height of the tallest plant across all plots was 57 cm and the average plant cover of I. glandulifera was 47%.
Field Experiment 2016
We included four treatments at two sites, Gatebekken 2 and Støtvigbekken, in this field plot experiment: (1) low cutting once, (2) high cutting once, (3) hot water once, and (4) untreated control. The low-cut plants were cut below the first (oldest) node without roots connected to the ground. The high-cut plants were cut above the first (oldest) node without roots connected to the ground. Unlike in Field Experiment 2015, the treatments were performed at what was presumed to be ideal time for this type of control measure.
The hot water treatment was applied on June 6, at an earlier plant development stage than in Experiment 2015, under the assumption that the treatment would be less time-consuming (Hansson and Ascard Reference Hansson and Ascard2002). The I. glandulifera plants had 2 to 3 whorls of unfolded leaves (BBCH 12–13; Hess et al. Reference Hess, Barralis, Bleiholder, Buhr, Eggers, Hack and Stauss1997) at both sites, but the population was taller and more dense at Støtvigbekken than at Gatebekken 2. The average height of the tallest plant across all plots was 39 cm at Støtvigbekken and 28 cm at Gatebekken 2, and the ground cover was 48% and 21%, respectively.
The cutting was done 5 wk later than the hot water application (July 13), which was at a later growth stage of I. glandulifera than in 2015. At both sites in 2016, plants were in their early regenerative phase, growth stage 5 (inflorescence emergence of the main shoot) according to the BBCH scale. The population at Støtvigbekken was at a later phenological stage than at Gatebekken 2: BBCH 55–59 (visible flowers and petals [still closed]) and BBCH 51–55 (visible flower buds and flowers), respectively. The average height of the tallest plants across all plots was 115 cm at Støtvigbekken and 92 cm at Gatebekken 2, and the ground cover was 73% and 44%, respectively. After high cutting, the average stump heights at Støtvigbekken and Gatebekken 2 were 21 and 12 cm, respectively. After low cutting, the average stump heights at Støtvigbekken and Gatebekken 2 were 5 and 4 cm, respectively.
Data Sampling in Field Experiments 2015 and 2016
Before treatments, the height of the tallest I. glandulifera plant in each plot was measured and visual assessments of the portion of living vegetation and bare ground, including dead vegetation, were taken. The living vegetation (% cover) was categorized as (1) I. glandulifera, (2) grass, or (3) forbs and woody species. These assessments were done June 22, 2015, and June 3 and July 13, 2016. In 2015, the posttreatment assessments of the same categories were done 4 wk (July 21) and 10 wk (August 28) after the treatments. In 2016, the posttreatment assessments were done August 24, that is, 11 wk after the hot water treatment and 5 wk after the cutting. The time required to apply the hot water treatment was recorded (in seconds) per plot in both experiments, while the time for cutting and clearing was only recorded in 2015. In Field Experiment 2016, the cutting was done more precisely (above and below the oldest node) than in Field Experiment 2015, and hence was slower and not representative for practical management.
Data Analysis
Data from the field plot experiments (2015 and 2016) were analyzed using Minitab 18 Statistical software. For the data from the laboratory and pot experiments, we also used SAS v. 9.4 statistical software. In general, all multiple comparisons were done using Tukey’s test and a significance level of P = 0.05. Details on the statistical analyses used for each experiment are given here.
In the laboratory experiment, the germination data were fit to a three-parameter log-logistic regression model (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2016):
 $$Y = {D \over {1 + \exp \{ b \cdot [\log (x) - \log ({\rm{L}}{{\rm{D}}_{50}})]\} }}$$
$$Y = {D \over {1 + \exp \{ b \cdot [\log (x) - \log ({\rm{L}}{{\rm{D}}_{50}})]\} }}$$
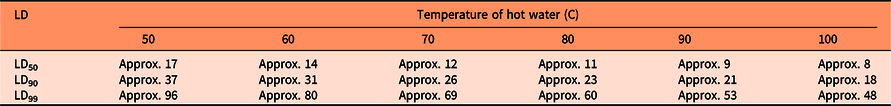
where Y is the percentage of germination, D is the parameter for maximum germination, LD50 is the parameter denoting the heat sum (x) required to reduce the maximum germination by 50%, and b is the relative slope parameter around LD50. Heat sum (x) was calculated as log10 (“degree seconds” = temperature in degrees Celsius × duration in seconds). Because no seeds germinated after exposure to water at 50 C and higher (for 30 min), whereas exposure to 45 C gave a germination rate of 20%, we used a base temperature of 50 C to calculate the heat sum. Model parameters, as well as the parameters LD90 and LD99 were estimated with nonlinear regression (PROC nlmixed in SAS).
In the pot experiment, we used a generalized linear mixed model (PROC glimmix in SAS) and the estimation algorithm Laplace with treatment as a fixed factor (three levels: untreated control and 3.5- and 7.0-s hot water applications), pot within treatment as a random factor, and emergence rate of I. glandulifera as response. The Wald test was used to test whether the variance of the random factor was significantly higher than zero.
In the field plot experiments, we analyzed the posttreatment plant cover of I. glandulifera as a response variable. The pretreatment plant cover of I. impatiens was used as a covariate. In 2015 the data were analyzed using a linear mixed model with treatment, assessment time, and their interaction as fixed factors, and block and plot within block as random factors. In 2016 the data were analyzed using a linear mixed model with treatment as a fixed factor, and site, block within site, and plot within block and site as random factors. At Gatebekken 2 (2016), one replicate block was unintentionally damaged after the control measures were applied, and we were left with three blocks at this site in August 2016. No transformation of data was necessary for Field Experiment 2015, while we transformed the response variable and the covariate by the natural logarithm of the variable + 1 to achieve approximately normal distributed residuals with homogenous variance for Field Experiment 2016.
Results and Discussion
Effect of Hot Water on Germination of Impatiens glandulifera Seeds (Laboratory and Outdoor Pot Experiments)
Laboratory Experiment
No seeds germinated after long-duration (i.e., 30-min) exposure to a hot water bath at 50 C and higher. The average germination rate was 20% for the seeds exposed to 45 C and 32% for the untreated control (Figure 1). Hence, the lethal temperature found in our experiment was between 45 and 50 C. This was in general accordance with others. Dahlquist et al. (Reference Dahlquist, Prather and Stapleton2007) found temperatures of 50 C and above to be lethal for seeds of six weed species when simulating soil solarization. When exposing seeds in wet soil to oven heat, Thompson et al. (Reference Thompson, Jones and Blair1997) found temperatures between 50 and 80 C to be critical to prevent seed germination. Critical temperature varied depending on species. When the seeds were exposed to 80 C for a short duration (i.e., 3 to 30 s), the germination rate decreased with increased exposure time, as expected. Hence, the germination rate depended on the duration above the lethal temperature. Exposure for 3, 5, 10, and 30 s resulted in average germination rates of 32%, 28%, 16%, and 0%, respectively (Figure 1). Compared with the untreated control, these germination rates corresponded to control rates of 0%, 13%, 50%, and 100%, respectively. These results were in general agreement with the findings of De Wilde et al. (Reference De Wilde, Buisson, Yavercovski, Willm, Bieder and Mesléard2017), who discovered that when the power for microwave soil heating was reduced, the duration had to increase to achieve the same effect on germination in three invasive species (Bohemian knotweed [Polygonum × bohemicum (J. Chrtek & Chrtková) Zika & Jacobson [cuspidatum × sachalinense]], giant goldenrod [Solidago gigantea Aiton], and jimsonweed [Datura stramonium L.]). Thompson et al. (Reference Thompson, Jones and Blair1997) found the maximum temperature to be of greater importance than the duration in preventing seed germination in 10 common arable weed seeds exposed to oven heat in moist soil.
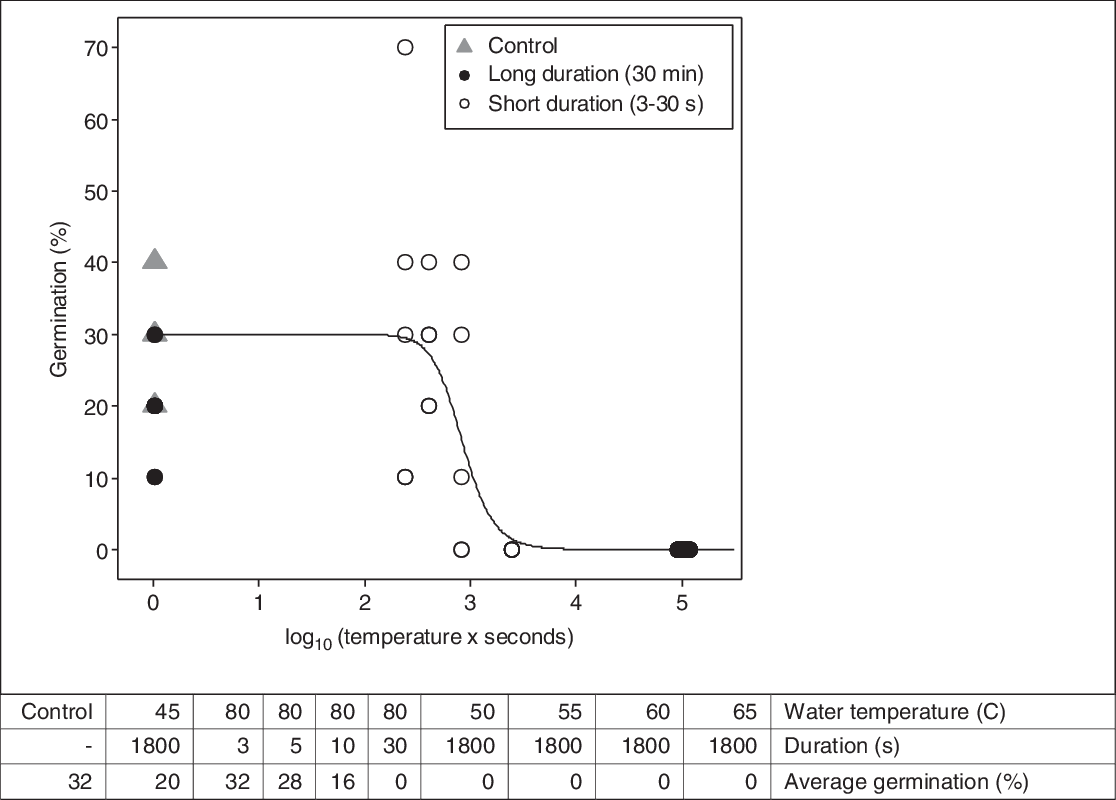
Figure 1. Dose–response curve fit by Equation 1 to the germination data from the laboratory experiment with seeds in hot water baths. Germination of Impatiens glandulifera seeds (y-axis) was modeled as a function of the heat sum experienced by the seeds (x-axis). The threshold (base) temperature of the hot water used to calculate the heat sum was 50 C. Each data point is the germination percentage based on 10 seeds.
Seed germination was modeled as a function of heat sum (water temperature × duration) with a base temperature of 50 C (Figure 1). The approximate parameter estimates were LD50= 842, LD90 = 1,840, and LD99 = 4,795 “degree seconds” (Table 1). These parameter values were used to calculate the number of seconds necessary to reduce seed germination by 50%, 90%, and 99% for various temperatures of hot water (Table 2). LD99 can be considered a threshold for complete seed mortality (Vidotto et al. Reference Vidotto, De Palo and Ferrero2013). For 70 and 80 C water, for example, the estimated LD99 value corresponded with exposure periods of approximately 69 and 60 s, respectively (Table 2). These durations were well above the findings of Vidotto et al. (Reference Vidotto, De Palo and Ferrero2013), who estimated the LD99 for six Italian weed species to vary between 66 and 81 C when heating soil and seeds in hot water for only 2 to 5 s.
Table 1. Parameter estimates of the dose–response regression model for the germination data of Impatiens glandulifera seeds in the laboratory experiment based on Equation 1, their SEs, lower and upper 95% confidence limits, and P-values of t-tests.a
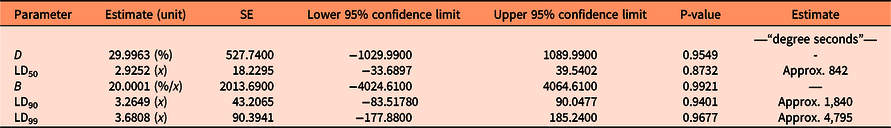
a df = 50. Unit of x is log10 of the heat sum calculated in “degree seconds” (temperature of hot water in degrees Celsius × duration in seconds). Threshold (base) temperature used to calculate heat sum was 50 C.
Pot Experiment
In response to hot water applied to moist seeds in soil, the plant emergence percentage, was significantly reduced compared with the untreated control (Figure 2). The emergence percentages of the three treatments differed significantly. The untreated control had 44.0% emergence, in contrast to 9.7% and 3.2% emergence following 3.5- and 7-s treatments with hot water (80 C), respectively. Compared with the control, the 3.5- and 7-s treatments reduced the emergence by 78% and 93%, respectively.
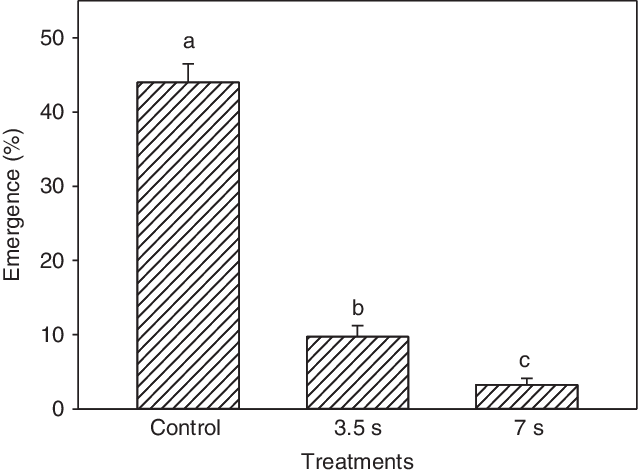
Figure 2. Emergence rate of Impatiens glandulifera plants at 10 wk after hot water (80 C) treatment of seeds in soil (seeded at 5-mm depth) for either 3.5 or 7 s, corresponding to 7.2 and 14.5 L m−2 in the pot experiment. These volumes represented work hours (capacity) of 1.1 min m−2 (54 m−2 h−1) and 2.2 min m−2 (27 m−2 h−1), respectively. Least-squares means with the same letter are not significantly different.
The 3.5- and 7-s treatments used 7.2 and 14.5 L m−2 of water, respectively. We achieved higher and longer duration of the high soil temperature with 14.5 L m−2 of hot water compared with half the water use (7.2 L m−2) (Figure 3). The soil temperature fell rapidly the first 5 s. Following the 7-s (14.5 L m−2) treatment, the temperature stayed above 50 C for almost 2 min (116 s) at all the soil depths measured. Following the 3.50-s (7.2 L m−2) treatment, the soil temperature stayed above 50 C for only 82 s at the soil surface and 5-mm soil depth. However, at the 10-mm soil depth, soil temperature never reached above 50 C. At the sowing depth (5 mm), the seeds experienced heat sums of 5,239 and 7,114 “degree seconds” (using threshold [base] temperature of 50 C) with the 3.5- and 7-s treatments, respectively. Under other more field-like circumstances, where seeds may be buried at 10 mm or deeper, volumes of 7.2 L m−2 (3.5 s) and 14.5 L m−2 (7 s) would probably have resulted in higher emergence, and hence a lower control rate, than our 78% and 93%, respectively.
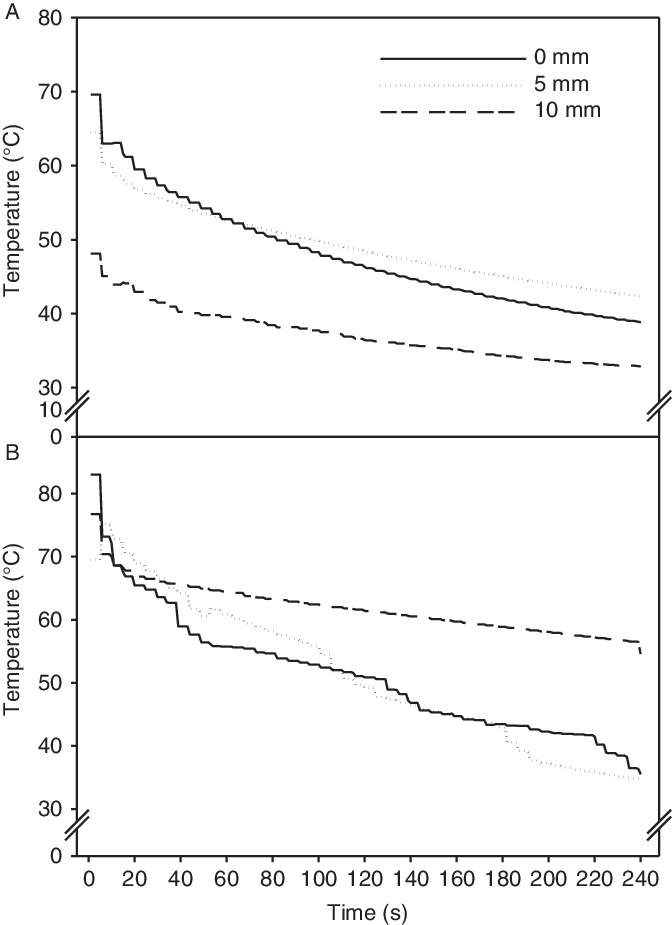
Figure 3. Soil temperature at the soil surface and at soil depths of 5 and 10 mm following treatment with hot water (80 C) for 3.5 s (7.2 L m−2) (A) and 7 s (14.5 L m−2) (B). The Impatiens glandulifera seeds were seeded at 5 mm.
This pot experiment was done in open soil with no plants emerged. Under field conditions, other living and/or dead vegetation, including plants of I. glandulifera, may have caused an insulating effect and protected the seedbank from the heat. Furthermore, soil humidity affects the time needed for seeds to reach lethal temperature (De Wilde et al. Reference De Wilde, Buisson, Yavercovski, Willm, Bieder and Mesléard2017). Latsch et al. (Reference Latsch, Anken, Herzog and Sauter2017) found soil humidity to play a less important role than the water temperature and the amount of hot water. However, they found increased soil moisture to require a larger amount of hot water to reach the target control rate of bitter dock (Rumex obtusifolius L.). Therefore, if the hot water machine had been used in dry summer conditions, we could have expected better control rates than in the current experiment, which was performed in late March with moist cold soils. Furthermore, soil texture can influence the level of control for the same amount of energy applied (Latsch et al. Reference Latsch, Anken, Herzog and Sauter2017).
Hansson and Ascard (Reference Hansson and Ascard2002) reported a 90% reduction in the number of annual white mustard (Sinapis alba L.) plants (at their 2-leaf stage) in a field experiment with hot water (116 C just before nozzle outlet) as foliar spray at a rate of 10,000 L ha−1 (i.e., 3,970 MJ ha−1). The volumes of hot water applied in our pot experiment corresponded to 72,350 and 144,700 L ha−1, and the control rates achieved were 78% and 93%, respectively. These volumes represented very high direct-energy inputs (i.e., 21,170 and 42,339 MJ ha−1, respectively).
Effect of Hot Water Treatment and Cutting in Established Impatiens glandulifera Populations
Field Experiment 2015
Hot water treatment and stem cutting of a field population in late June (June 22), effectively reduced plant cover (Figure 4A). The cover of I. glandulifera was significantly lower following hot water treatment than following cutting at 4 and 10 wk after treatments. Compared with the nontreated control, the I. glandulifera cover at 10 wk after treatment (late August) was reduced by 97% and 79% following hot water treatment and cutting, respectively. After cutting, the cover significantly increased to double the amount from mid-July (week 4) to late August (week 10) due natural regrowth (Figure 4A). At 10 wk after cutting, new I. glandulifera plants had established in all eight cut plots. They were blooming in four plots and had started to develop fruit in one plot. Of the eight hot water–treated plots, three plots had I. glandulifera plants. They were blooming, but had not developed fruit. In all the untreated control plots, the I. glandulifera plants had developed fruit.
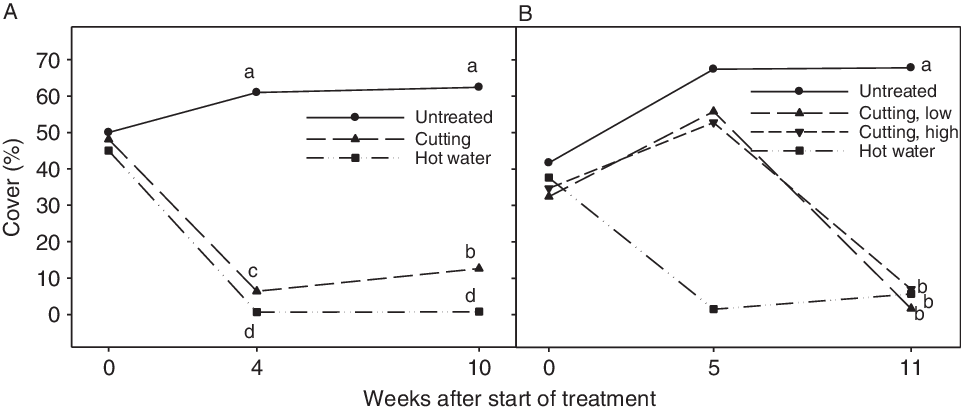
Figure 4. Cover of Impatiens glandulifera (%) in plots before and after treatments in 2015 (A) and 2016 (B). Least-squares means that do not share a letter are significantly different (Tukey’s test). (A) In 2015, all treatments were done on June 22 (week 0) at one site (Gatebekken 1). The assessments were done after 4 wk (July 21) and 10 wk (August 28). (B) In 2016, plots were treated with hot water at the start of experiment (June 6, i.e., week 0) and low and high cutting were done on July 13 (week 5). Low and high cutting are cutting below and above the first (oldest) stem node, respectively. All treatments in 2016 were done at two sites (Gatebekken 2 and Støtvigbekken). The assessments were done 5 wk (July 13) and 11 wk (August 24) after the start of the experiment.
Field Experiment 2016
The three different control measures resulted in significantly reduced cover of I. glandulifera compared with the untreated control (assessed in late August) (Figure 4B). The hot water treatment had been conducted in early June and cutting in mid-July. Compared with the nontreated control, the I. glandulifera cover in late August was reduced 99% for low cutting and 91% for both the high cutting and hot water treatments. The I. glandulifera cover after these three treatments was not significantly different at the assessment time in late August. In plots with high cutting, new seedlings and old side shoots were present at both sites in late August. No plants had formed new side shoots after high cutting, but side shoots formed before the cutting had continued to grow and develop (Figure 5). In plots with low cutting, one single plant was present in three of four plots at Støtvigbekken. At Gatebekken 2, low cutting and hot water gave 100% control in late August (data not shown). On the other hand, after the hot water treatment at Støtvigbekken, there were a few plants present in three out of eight plots, and the plant cover slightly increased from mid-July to late August as the plants grew. These plants had either escaped the hot water treatment or had emerged after the treatment. These survivors not only lacked competition and grew larger than they would in a denser population, they also completed their life cycle and were at the same developmental stage as the untreated control plants (BBCH 81–89, ripening or maturity of fruit and seed) in late August. In comparison, the most developed plants in the cut plots had just started to flower (BBCH 61). These results demonstrate the importance of revisiting any treated population later in the season to handle potential survivors and prevent seed dispersal. Ineffective control of I. glandulifera may lead to larger spread distance than no control, as simulated by Meier et al. (Reference Meier, Dullinger, Zimmermann, Baumgartner, Gattringer and Hülber2014). They simulated a better total effect at a lower overall cost for control strategies with high initial spending compared with strategies with low initial spending.

Figure 5. Example of an Impatiens glandulifera plant at 4 wk after high cutting (i.e., above the oldest node). The side shoot formed before cutting was blooming at 4 wk (Støtvigbekken 2016).
Seed Viability after Hot Water Application
Loss of seed viability is beneficial in the long term, as I. glandulifera seeds may still be dormant or germinate after 4 yr (Skálová et al. Reference Skálová, Moravcová, Čuda and Pyšek2019). Hot water weed control may be efficient over a period of more than 1 yr due to the effect on seed viability, as our laboratory and pot experiments showed. The hot water prevented emergence of I. glandulifera seedlings in 8 of 22 field plots (Figure 6). However, we cannot exclude that seeds may have spread from the untreated neighboring plants after the hot water application.

Figure 6. Effect of hot water treatment at Støtvigbekken in 2016. (A) Before the hot water treatment (June 6), the plot was almost totally covered with Impatiens glandulifera plants. (B) Impatiens glandulifera plants during the hot water treatment. (C) At 5 wk after treatment (July 11), all I. glandulifera plants were eradicated, and only native grass species were present. (D) At 13 wk after treatment (i.e., 2 wk after the last assessment, September 8), no I. glandulifera plants had emerged. The average volume of hot water (80 C) applied in 2016 was 13.7 L m−2 (see Table 3). (A, C, D) Plot no. 103; (B) plot no. 301.
Time of Management
Hot water application reduced the I. glandulifera plant cover significantly when applied to relatively small plants in early June (2016) and to larger plants in late June (2015) (Figure 4). The outcome for cutting, however, depended more on the plant’s phenological stage and the time of year. Targeting plants in their early regenerative stage in mid-July gave (2016) gave better control compared with cutting juvenile plants (2015), due to emergence of new seedlings with potential to develop mature seeds. The early regenerative stage has been recommended as an optimum time for cutting I. glandulifera (Helmisaari Reference Helmisaari2010). If cut as juveniles, they may start to regrow and produce seeds after cutting (Jernelöv Reference Jernelöv and Jernelöv2017). On the other hand, if they are cut too late, mature seeds may develop and disperse before the treatment. In a Canadian study, Leblanc and Lavoie (Reference Leblanc and Lavoie2017) found that one hand pulling during summer was not enough when done at a plant height of 60 cm. At the second hand pulling performed in mid-August, 14% of the stems had developed flowers and could potentially have produced seeds if not removed. To ignore a second control treatment may give an overall higher cost, as new seeds will spread and the problem remains.
Workload
Application of hot water was more time-consuming (on average 4.8 min m−2) than cutting and clearing (on average 2.4 min m−2) when we targeted larger plants in late June in 2015 (Table 3). Time and water consumption were reduced by 56% for smaller plants treated with hot water in early June (2.1 min m−2, 2016) compared with targeting larger plants in late June (4.8 min m−2, 2015). For efficient hot water weed control, the treated plants should be as young as possible. De Cauwer et al. (Reference De Cauwer, Bogaert, Claerhout, Bulcke and Reheul2015) tested hot water treatment (89 C) against annual and perennial weeds. They concluded that best effects were achieved when applications were done on young plants. This was also found by Hansson and Ascard (Reference Hansson and Ascard2002), who reported a two-thirds reduction in energy requirements when targeting S. alba at the 2-leaf stage compared with the 6-leaf stage when using hot water. Leblanc and Lavoie (Reference Leblanc and Lavoie2017) found that the time required to control I. glandulifera with hand pulling was almost 1,400 hours ha−1 over 2 yr, which gives an average of 4.2 min m−2 year−1. This is close to the average time, 4.8 min m−2, we spent on hot water application targeting larger plants in late June (Field Experiment 2015) (Table 3). In our pot experiment targeting seeds, the 3.5- and the 7-s hot water treatments (Figure 2) represented 54 m2 h−1 and 27 m2 h−1, respectively. The latter value, which resulted in a 93% reduction in germination, was similar to the average capacity achieved when targeting smaller plants in Field Experiment 2016 (28.5 m2 h−1; see Table 3).
Table 3. Work hours and water consumption for control on established naturalized stands of Impatiens glandulifera in the field plot experiments.
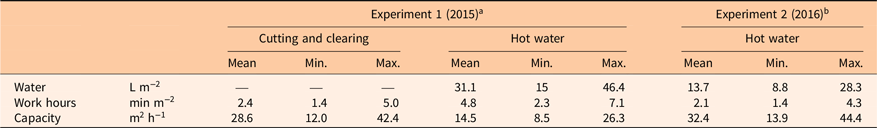
a Average maximum plant height was 57 cm when treatments were performed on June 22, 2015.
b Average maximum plant height was 34 cm when hot water treatment was performed on June 6, 2016.
Effect on Other Vegetation of Removal of Impatiens glandulifera
Grass was dominant in all treated plots at the last visual assessment in August in both field plot experiments (Figure 7). In general, grasses are tolerant to mowing. The dominant grass species in both experiments was quackgrass [Elymus repens (L.) Gould], a creeping perennial with the ability to regrow from belowground rhizomes. The ability to regrow from belowground meristems gives the plant a high resistance to heat applied at the soil surface (Ascard et al. Reference Ascard, Hatcher, Melander, Upadhyaya, Upadhyaya and Blackshaw2007). Grasses also have narrow erect leaves, resulting in low water retention and high heat loss (De Cauwer et al. Reference De Cauwer, Bogaert, Claerhout, Bulcke and Reheul2015). This finding may explain the success of the grasses in our study. Leblanc and Lavoie (Reference Leblanc and Lavoie2017) also found an increase in grass species with vegetative spread (rhizomes) after I. glandulifera removal. However, they also observed a decrease in forbs, while we found little effect on forbs (Figure 7).

Figure 7. Cover (%) of grasses, forbs and woody species (raspberry), Impatiens glandulifera, and open soil in plots before and after treatments in 2015 (A) and 2016 (B). (A) In 2015, all treatments were done on June 22 (start of treatment) at one site (Gatebekken 1). The data collection was done after 4 and 10 wk. (B) In 2016, plots were treated with hot water at the start of experiment (June 6, week 0) and low and high cutting were done on July 13 (week 5). All treatments were done at two sites (Gatebekken 2 and Støtvigbekken). The data collection was done 5 and 11 wk after the start of experiment. Arrows indicate timing of treatments.
After treatment of I. glandulifera, the proportion of open soil increased temporarily. This was more evident after the hot water treatment than after cutting (Figure 7). If new vegetation is not established immediately after removal of I. glandulifera along rivers, erosion might be a problem, and measures for revegetation should be considered. Furthermore, removal of I. glandulifera may increase richness and diversity of other nonnative species (Hulme and Bremner Reference Hulme and Bremner2006). On the other hand, Hejda and Pyšek (Reference Hejda and Pyšek2006) found that removal of I. glandulifera did not have any consequences for species diversity.
Cutting and hot water treatment gave high control rates, 79% to 99% compared with the untreated control, in our two field plot experiments. We conclude that the growth stage at application influenced the effect of the two control methods. Water use and time consumption was reduced by more than 50% when using the hot water treatment on smaller plants (average of tallest plants was 34 cm, Field Experiment 2016) compared with larger plants (average of tallest plants was 57 cm, Field Experiment 2015). Cutting was more time efficient than use of hot water when targeting larger plants. Therefore, our first hypothesis that hot water application and cutting provide equally efficient control of I. glandulifera plants was rejected. The lethal temperature for moist I. glandulifera seeds was found to be between 45 and 50 C. For hot water treatment of emerged plants to also be effective for the seeds, the dose (i.e., water temperature above the lethal temperature × duration of period above lethal temperature) needs to be sufficiently high. The best control rate for seeds buried in soil was 93% using 14.5 L m−2 of hot water, which corresponded to a direct energy input of 42,339 MJ ha−1, which was about the same amount as in Experiment 2016 (40 086 MJ ha−1) and more than 50% less energy than in Experiment 2015 (90,999 MJ ha−1).
Our second hypothesis, that cutting the stems of I. glandulifera below and above the lowest (oldest) node on the stem provides equal control, was therefore confirmed. Compared with the control, cutting below and above the oldest node gave control rates of 99% and 91%, respectively, not significantly different. Cutting below the first (oldest) node will prevent later side shoot development, but can be hard to conduct consistently in practice, especially in rugged landscapes. When cutting is chosen as the control method, the later in the season it is conducted, the less time will be available for remaining side shoots or new seedlings to develop ripe seeds.
Acknowledgments
This work was funded by Regionale Forskningsfond Oslofjordfondet project no. 245824, NMBU and the Norwegian Public Roads Administration. The cooperation with Heatweed Technologies AS and Landbrukskontoret Moss Rygge Råde are highly appreciated. We thank Marit Helgheim, Sophie van Meteren, and Samuel Habte for excellent technical assistance. No conflicts of interest have been declared.