Introduction
Noonan syndrome is a RASopathy and belongs to the group of Noonan syndrome spectrum disorders caused by pathogenic variants in genes associated with the Ras/MAPK pathway. These disorders include Noonan syndrome (OMIM 163950), Noonan syndrome with multiple lentigines (OMIM 151100), Noonan syndrome-like disorder with or without juvenile myelomonocytic leukaemia (OMIM 613553), Noonan syndrome-like disorder with loose anagen hair (OMIM 607721), cardio-facio-cutaneous syndrome (OMIM 115150), and Costello syndrome (OMIM 218040). Reference Grant, Cushman and Cave1 Noonan syndrome is the most prevalent syndrome of the Noonan syndrome spectrum disorders. Reference Zenker, Edouard, Blair and Cappa2 Autosomal-dominant gain of function pathogenic variants in PTPN11 (40–67%), SOS1 (10–26%), and RAF1 (3–7%) and RIT1 (3–17%) are most common. Reference Lee, Kim and Jin3,Reference Vos, Leenders, Werkman, Udink ten Cate and Draaisma4 Clinical features include distinctive facial features, developmental delay, learning difficulties, short stature, CHD, renal anomalies, lymphatic malformations, and bleeding disorders. Reference Zenker, Edouard, Blair and Cappa2
Approximately 8–20% of patients with a Noonan syndrome spectrum disorder suffer from hypertrophic cardiomyopathy. Reference Colquitt and Noonan5,Reference Norrish, Field and Mcleod6 Noonan syndrome-related hypertrophic cardiomyopathy is often associated with pathogenic variants in the RIT1 and RAF1 genes. Reference Gelb, Roberts and Tartaglia7,Reference Kaltenecker, Schleihauf and Meierhofer8 However, because pathogenic PTPN11 variants are responsible for approximately half of the patients with Noonan syndrome, Noonan syndrome-related hypertrophic cardiomyopathy in patients with pathogenic PTPN11 variant also occurs. Reference Kaltenecker, Schleihauf and Meierhofer8 Sarcomeric hypertrophic cardiomyopathy in children is often due to pathogenic variants in MYH7 and MYBPC genes. Reference Elliott, Anastasakis and Borger9,Reference Olivotto, d’Amati and Basso10 In general, children with Noonan syndrome-related hypertrophic cardiomyopathy present at a younger age and there is significant morbidity and mortality during the first years of life. Reference Kaltenecker, Schleihauf and Meierhofer8,Reference Lioncino, Monda and Verrillo11
Echocardiography is the primary imaging modality to make a diagnosis of hypertrophic cardiomyopathy. Although electrocardiography may also aid in the diagnostic work-up of patients with suspected hypertrophic cardiomyopathy, an electrocardiogram is used less frequently for this purpose due to a greater variety in criteria for diagnosing hypertrophic cardiomyopathy. Reference Rapezzi, Arbustini and Caforio12 However, previous studies have identified specific electrocardiographic abnormalities that may support a diagnosis of Noonan syndrome. Typical Noonan syndrome-associated electrocardiographic features include an abnormal QRS axis (negative aVF), large S-waves in V2, small R-waves in V5 and V6 or abnormal R/S ratio in V5 and V6 and pathologic Q-waves, and are present in up to 50–60% of all children with Noonan syndrome. Reference Vos, Leenders, Werkman, Udink ten Cate and Draaisma4 Importantly, these typical electrocardiographic abnormalities do not relate to the presence or absence of CHD. Reference Vos, Leenders, Werkman, Udink ten Cate and Draaisma4,Reference Croonen, van der Burgt, Kapusta and Draaisma13,Reference Raaijmakers, Noordam and Noonan14 However, a comparison of electrocardiographic features of Noonan syndrome-related hypertrophic cardiomyopathy patients and sarcomeric hypertrophic cardiomyopathy has not been performed yet. Therefore, the purpose of this retrospective multi-centre cohort study is to analyse whether the typical Noonan syndrome-associated electrocardiographic features can be of clinical value to differentiate between Noonan syndrome-related hypertrophic cardiomyopathy and sarcomere hypertrophic cardiomyopathy at first presentation.
Materials and methods
Patients
For this two-centre retrospective study, anonymous data collection was done at two tertiary care centres.
Radboud University Medical Center: All Noonan syndrome patients with hypertrophic cardiomyopathy were seen in the outpatient department of the Noonan Syndrome Expertise Center before 2021 with clinically and genetically confirmed Noonan syndrome. The clinical diagnosis of Noonan syndrome was based on the scoring system of van der Burgt et al and confirmed by molecular genetic DNA analysis showing a pathogenic gene variant. Reference Zenker, Edouard, Blair and Cappa2,Reference van der Burgt15 Patients without an available electrocardiogram or echocardiogram before a surgical intervention or balloon valvuloplasty were excluded from the study. All patients with sarcomeric hypertrophic cardiomyopathy were seen in the outpatient department of the Department of Pediatric Cardiology before 2021 with genetically confirmed sarcomeric hypertrophic cardiomyopathy pathogenic gene variants.
Technical University of Munich: All patients with childhood hypertrophic cardiomyopathy presenting at the German Heart Center Munich between January 1978 and July 2018 and meeting a clinical and/or genetic diagnosis of Noonan syndrome or sarcomeric hypertrophic cardiomyopathy. Data from part of this cohort were previously published. Reference Kaltenecker, Schleihauf and Meierhofer8,Reference Schleihauf, Cleuziou and Pabst von Ohain16 Only patients with a genetic diagnosis of Noonan syndrome or genetically confirmed sarcomeric hypertrophic cardiomyopathy were included. Patients without an available electrocardiogram or echocardiogram before a surgical intervention or balloon valvuloplasty were excluded from the study.
Electrocardiography
The first electrocardiogram available was collected and reviewed on specific electrocardiographic features by two investigators independently. Discrepancies between reviewers were resolved by discussion and consensus or involvement of a third reviewer. When consensus was not reached, the item was not scored.
The specific Noonan syndrome-associated electrocardiographic features are abnormal QRS axis, large S-waves in the right pre-cordial leads, small R-waves in the left pre-cordial leads, and abnormal Q-waves. Also, electrocardiographic features associated with hypertrophic cardiomyopathy in general, such as abnormal Q-waves, ST-segment changes, and any T-wave abnormalities, were analysed. Reference Guerrier, Anderson and Pratt17 In detail, ECG features were defined as:
-
QRS axis between 0 and 360 degrees
-
Large S-wave: S-wave is more than the upper limit of normal for the patient’s age in V2 Reference Park18
-
Small R-waves in V5 and/or V6: R deflection over the left pre-cordium with an R/S ratio lower than the lowest limits of normal and R voltage in V5 or V6 is less than 50% of the mean according to Park and Guntheroth Reference Park18
-
Pathologic Q-wave: Q is greater than the upper limit of normal and wider than 0.04 seconds Reference Park18
-
ST-segment abnormalities (elevation or depression)
-
T-wave abnormalities (giant inverted T-waves, giant positive T-waves, or pathological T-wave inversion).
Echocardiography
Diagnosis of hypertrophic cardiomyopathy included in this study was established by the first echocardiogram available with hypertrophic cardiomyopathy. In general, the guidelines and standards for performance of paediatric echocardiograms were used. Reference Lai, Geva and Shirali19 Hypertrophic cardiomyopathy was diagnosed according to the criteria used by Wilkinson et al. and included the following criteria: septal or left ventricle posterior wall thickness exceeding two SDs for body surface area compared with a normal population of children or the presence of localised left ventricle hypertrophy. Reference Wilkinson, Lowe and Salbert20 The location (basal or mid-ventricular) and size of maximum wall thickness were obtained and measured from the parasternal long-axis and four-chamber views. Furthermore, the gradient over the left ventricle outflow tract was measured by continuous wave or pulsed wave Doppler and given in mm Hg. The echocardiographic investigation at presentation was also reviewed for presence of a biventricular hypertrophic cardiomyopathy or additional CHD. The severity of an additional pulmonary stenosis was graded as mild (peak gradient <36 mmHg), moderate (peak gradient 36–64 mmHg), or severe (peak gradient>64 mmHg), depending on the maximal gradient across the valve.
Data handling
Data collected at the Radboud University Medical Center were stored in a dedicated Electronic Data Capture environment database and handled for statistical analysis in an encrypted GDPR-compliant Digital Research Environment (AnDREa consortium). Data from German Heart Center were stored in anonymous form at a local secured database.
Statistical analysis
IBM SPSS Statistics version 25.0 (IBM Corporation) was used for performing the statistical analysis. Due to the few observations for individual cells, non-parametric tests were deployed. Continuous variables are shown as medians (range: minimum–maximum), and categorical data are expressed as the number of cases per group with its associated percentage. Analyses were performed on the agreement between Noonan syndrome-related hypertrophic cardiomyopathy/sarcomeric hypertrophic cardiomyopathy and electrocardiographic features and left ventricle hypertrophic cardiomyopathy/biventricular hypertrophic cardiomyopathy and electrocardiographic features. Categorical data were analysed by means of Fisher exact or Fisher–Freeman–Halton exact test, while the Mann–Whiney U test was used for the analysis of continuous data. Binomial logistic regression was performed to study the influence of Noonan syndrome-related hypertrophic cardiomyopathy/sarcomeric hypertrophic cardiomyopathy, type of hypertrophic cardiomyopathy (biventricular or not) on those electrocardiographic features that were statistically important in both analyses. A (two-sided) p value of <0.05 was considered to be statistically significant.
Ethics
This study was approved by the Radboud University Medical Center Research Ethics Committee (number 2021-8332, 5/10/2021).
This study was approved by the Technical University of Munich Ethics Committee (number 243/17S, 10/16/2017).
Results
Patient characteristics
In total, data from 45 patients were analysed. Of those, 30 patients carried a diagnosis of childhood-onset Noonan syndrome-related hypertrophic cardiomyopathy and 15 of childhood-onset sarcomere hypertrophic cardiomyopathy. Most common pathogenic variants identified were PTPN11 and RAF1 in the Noonan syndrome-related hypertrophic cardiomyopathy group, and MYPBC and MYH7 in the sarcomeric hypertrophic cardiomyopathy group. Detailed clinical and genetic characteristics are depicted in Table 1. In general, there were no statistically significant clinical differences between the two groups, except for the number of additional CHDs, which was statistically more frequent in the Noonan syndrome-related hypertrophic cardiomyopathy group and implantable cardioverter defibrillator insertions, which was statistically more frequent in the sarcomeric hypertrophic cardiomyopathy group. Because of the additional CHD in the group with Noonan syndrome-related hypertrophic cardiomyopathy, analyses were performed with and without additional heart diseases.
Table 1. Clinical and genetic characteristics of the included patients
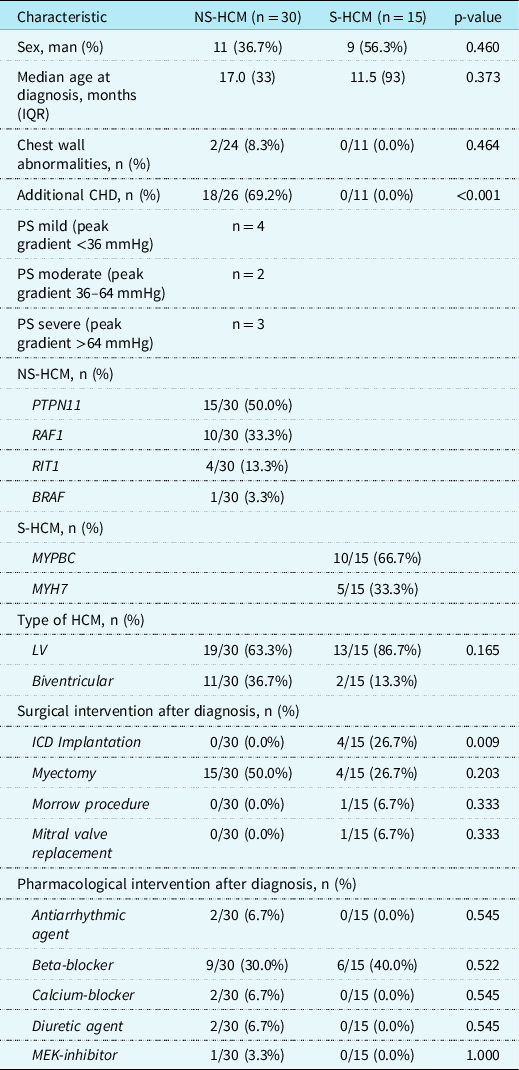
NS-HCM = Noonan syndrome-related hypertrophic cardiomyopathy; S-HCM = sarcomeric hypertrophic cardiomyopathy; IQR = interquartile range; BMI = body mass index; HCM = hypertrophic cardiomyopathy; LV = left ventricle; ICD = implantable cardioverter defibrillator; MEK = mitogen-activated protein kinase.
Electrographic abnormalities in patients with Noonan syndrome-related hypertrophic cardiomyopathy versus sarcomere hypertrophic cardiomyopathy
A negative aVF was seen in 25/30 (83%) patients with Noonan syndrome-related hypertrophic cardiomyopathy, in contrast to 4/15 (27%) patients with sarcomeric hypertrophic cardiomyopathy (p < 0.001). Of the patients with Noonan syndrome-related hypertrophic cardiomyopathy, 15/30 (50%) had an extreme QRS axis in the north-west corner (Fig 1). Large right pre-cordial S-waves were seen in 13/30 (43%) of patients with Noonan syndrome-related hypertrophic cardiomyopathy and in 4/15 (27%) of patients with sarcomeric hypertrophic cardiomyopathy (p = 0.225). Small R-waves in the left pre-cordial leads V5 and V6 were present equally in both groups (p = 0.132 for V5 and p = 0.119 for V6). However, an abnormal R/S ratio was more seen in patients with Noonan syndrome-related hypertrophic cardiomyopathy than in patients with sarcomeric hypertrophic cardiomyopathy (p < 0.001). Pathologic Q-waves were seen statistically more frequently in patients with sarcomeric hypertrophic cardiomyopathy (p = 0.009). The occurrence of ST-segment and T-wave pathology did not statistically differ between the two groups (Table 2). These results generally hold when patients with additional CHD were excluded (Table 2).
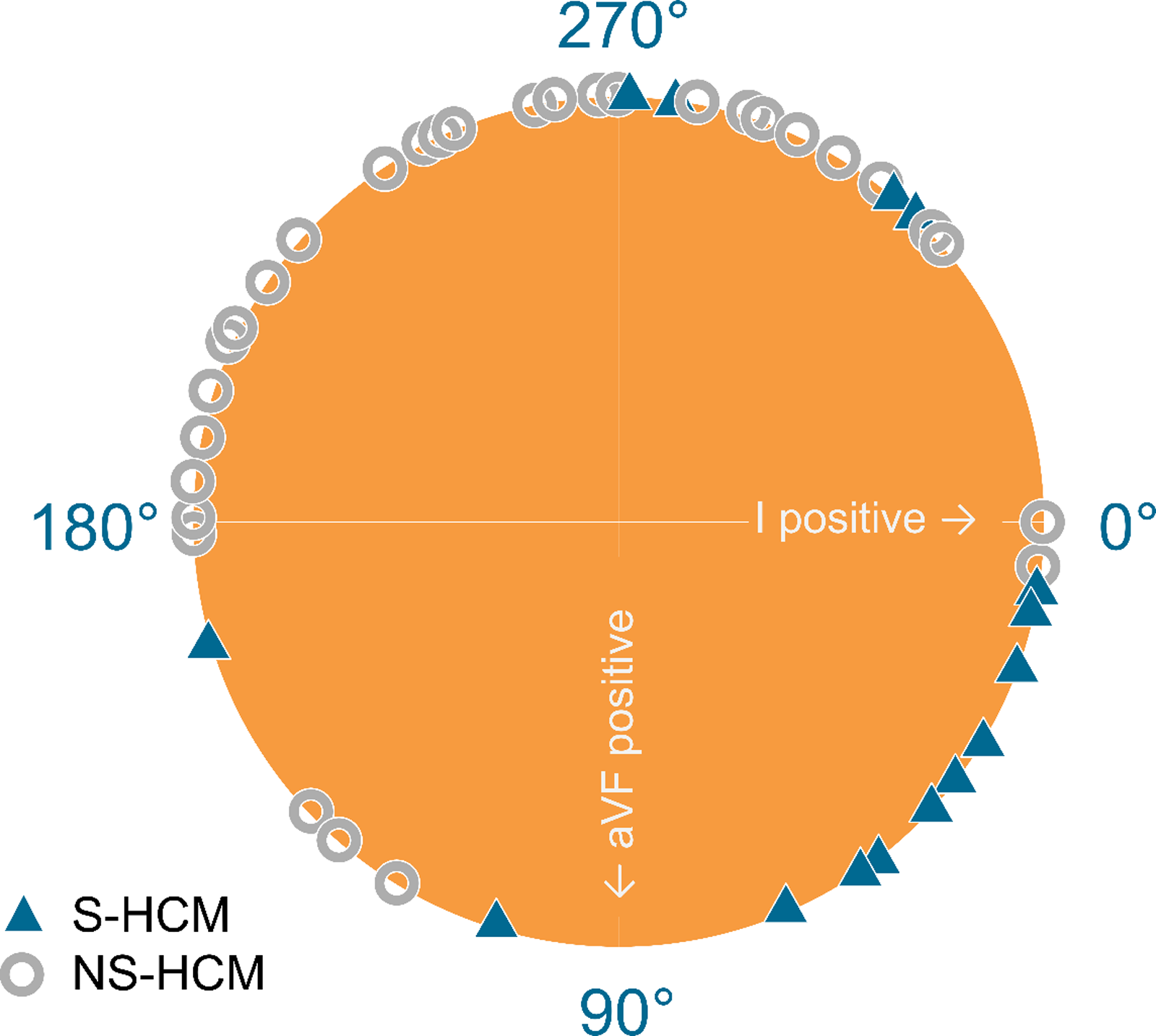
Figure 1. Heart axis in patients with Noonan syndrome-related hypertrophic cardiomyopathy (NS-HCM) and sarcomeric hypertrophic cardiomyopathy (S-HCM).
Table 2. Specific electrocardiographic characteristics in patients with Noonan syndrome-related hypertrophic cardiomyopathy and sarcomeric hypertrophic cardiomyopathy with additional CHD included, and without additional CHD
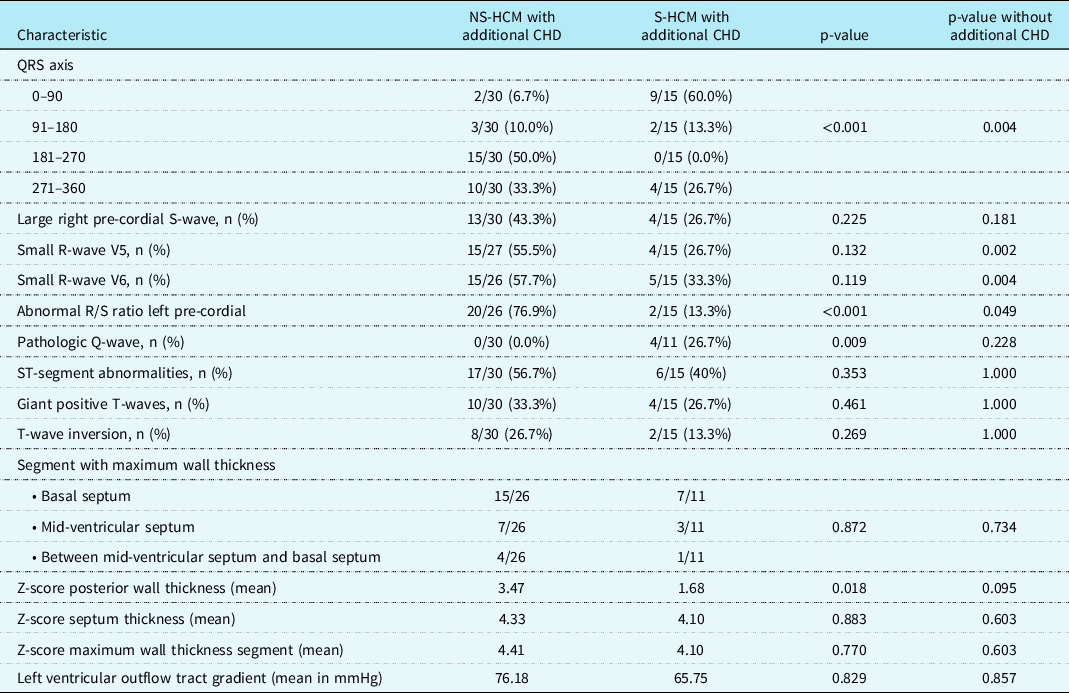
NS-HCM = Noonan syndrome-related hypertrophic cardiomyopathy; S-HCM = sarcomeric hypertrophic cardiomyopathy.
Electrographic abnormalities in biventricular hypertrophic cardiomyopathy versus (only) left ventricle hypertrophic cardiomyopathy
A negative aVF was seen in 8/13 (61.5%) patients with biventricular hypertrophic cardiomyopathy and 21/32 (65.6%) patients with left ventricle hypertrophic cardiomyopathy (p = 1.0). However, of the patients with biventricular hypertrophic cardiomyopathy, 7/13 (53.8%) had an extreme QRS axis in the north-west corner, contrasting with 8/32 (25.0%) of patients with left ventricle hypertrophic cardiomyopathy (p = 0.07). For the QRS axis as a group, the difference between patients with biventricular hypertrophic cardiomyopathy and left ventricle hypertrophic cardiomyopathy was statistically significant (p < 0.001).
There were no other statistically significant clinical differences between the two groups, except for giant positive T-waves that occurred statistically more frequent in patients with left ventricle hypertrophic cardiomyopathy (p = 0.038). This difference disappeared when patients with additional CHD were excluded (Table 3).
As the heart axis was significantly different between patients with Noonan syndrome-related hypertrophic cardiomyopathy and sarcomeric hypertrophic cardiomyopathy, and between patients with biventricular hypertrophic cardiomyopathy and left ventricle hypertrophic cardiomyopathy, binomial logistic regression was used to analyse the relationship between the presence of Noonan syndrome-related hypertrophic cardiomyopathy and heart axis deviation and compared this with the relationship between the type of hypertrophic and heart axis deviation. The model for Noonan syndrome-related hypertrophic cardiomyopathy was statistically significant with Chi-square = 9.85 and p = 0.002. This is in comparison with the statistically insignificant model for the type of hypertrophic cardiomyopathy (Chi-square = 1.90 and p = 0.168). This model shows that the heart axis deviation is determined by the presence of Noonan syndrome-related hypertrophic cardiomyopathy.
Discussion
Infants and children with Noonan syndrome may present with hypertrophic cardiomyopathy as the first clinical presentation. In 2013, the European Society of Cardiology working group on myocardial and pericardial diseases published a diagnostic work-up in cardiomyopathies. Reference Rapezzi, Arbustini and Caforio12 Additional hints given by the standard electrocardiogram that aid in diagnosis of familial/genetic and non-familial/non-genetic causes of hypertrophic cardiomyopathy were given. As diagnostic hint for Noonan syndrome an extreme superior (north-west QRS axis deviation) was suggested. This may be more important because of specific causative treatment for severe progressive Noonan syndrome-related hypertrophic cardiomyopathy. Fast diagnosis in severe progressive Noonan syndrome-related hypertrophic cardiomyopathy is critical to allow initiation of causative treatment with inhibition of the Ras/MAPK pathway and/or the mTOR pathway. Reference Kaltenecker, Schleihauf and Meierhofer8,Reference Lioncino, Monda and Verrillo11 Identifying Noonan syndrome-related hypertrophic cardiomyopathy-specific electrocardiographic features might help in diagnosis, in addition to the gold standard of molecular genetic testing, which is more time-consuming. Reference Schleihauf, Cleuziou and Pabst von Ohain16 However, only 81% of paediatric cardiologists initiate genetic testing, of which 53% do this themselves and 45 refer to a clinical geneticist. Reference Wolf, Zenker and Burkitt-Wright21 The suspicion on Noonan syndrome-related hypertrophic cardiomyopathy may accelerate and optimise this process.
In this cohort, the distribution of the causative pathogenic gene variants of Noonan syndrome-related hypertrophic cardiomyopathy is the same as earlier described; however, the distribution of the causative pathogenic gene variants of sarcomeric hypertrophic cardiomyopathy is more in favour of MYBPC. Reference Kaltenecker, Schleihauf and Meierhofer8 The mean age at diagnosis is earlier in sarcomeric hypertrophic cardiomyopathy than in Noonan syndrome-related hypertrophic cardiomyopathy, in contrast to published studies. Reference Norrish, Field and Mcleod6,Reference Kaltenecker, Schleihauf and Meierhofer8 Part of the explanation might be that in the Netherlands only Noonan syndrome-related hypertrophic cardiomyopathy seen in the outpatient department of the Noonan Syndrome Expertise Center, was included initially. Although biventricular hypertrophic cardiomyopathy appeared frequently in our cohort in Noonan syndrome-related hypertrophic cardiomyopathy and has been suggested to be a clinical clue for the diagnosis of Noonan syndrome-related hypertrophic cardiomyopathy, there was no statistical difference between the prevalence of biventricular hypertrophic cardiomyopathy in Noonan syndrome-related hypertrophic cardiomyopathy and sarcomeric hypertrophic cardiomyopathy in our series. Reference Lioncino, Monda and Verrillo11 In our series, no patient with Noonan syndrome-related hypertrophic cardiomyopathy and implantable cardioverter defibrillator insertion was included, in contrast to 26.7% of patients with sarcomeric hypertrophic cardiomyopathy (p = 0.009). This is in agreement with earlier publications. Reference Colquitt and Noonan5,Reference Kaltenecker, Schleihauf and Meierhofer8,23 Myectomy was performed equally in both groups, this is contrary to literature. Reference Kaltenecker, Schleihauf and Meierhofer8 The reason might be a selection bias in that patients presenting at a tertiary care centre usually are more severely affected and therefore more often have an indication for invasive outflow tract resection.
There was no difference in severity of hypertrophy and specific segments of hypertrophy of the left ventricle, except for the posterior wall thickness. The severity was more in patients with Noonan syndrome-related hypertrophic cardiomyopathy. This is in agreement with an earlier publication. Reference Kaltenecker, Schleihauf and Meierhofer8
Of the typical electrographic features for Noonan syndrome (abnormal QRS axis (negative aVF), large S-waves in V2, small R-waves in V5 and V6 or abnormal R/S ratio in V5 and V6 and pathologic Q-waves) only an abnormal QRS axis and abnormal R/S ratio in V5 and V6 were seen more often in patients with Noonan syndrome-related hypertrophic cardiomyopathy. However, when the group with additional CHD was excluded, also small R-waves in V5 and V6 occurred more in the group with Noonan syndrome-related hypertrophic cardiomyopathy. An extremely superior QRS axis (181–270; north-west axis) was seen exclusively in patients with Noonan syndrome-related hypertrophic cardiomyopathy. However, pathologic Q-waves were exclusively seen in patients with sarcomeric hypertrophic cardiomyopathy. The fact that pathologic Q-waves were not seen in patients with Noonan syndrome-related hypertrophic cardiomyopathy is in agreement with an earlier study. Reference Vos, Leenders, Werkman, Udink ten Cate and Draaisma4 Abnormal repolarization was seen in both groups. Recently, Lioncino et al stated that an extreme (north-west) QRS axis reflects biventricular involvement. Reference Lioncino, Monda and Verrillo11 For this reason, we also analysed the electrocardiographic characteristics in patients with biventricular hypertrophic cardiomyopathy and (only) left ventricle hypertrophic cardiomyopathy. The heart axis was significantly different in patients with biventricular hypertrophic cardiomyopathy; however, it could be explained by the fact that most of them had Noonan syndrome. A giant positive T-wave occurred more often in left ventricle hypertrophic cardiomyopathy. There are no standard criteria for the electrocardiographic diagnosis of biventricular hypertrophic cardiomyopathy in children. In 1999, some electrocardiographic patterns in adults with biventricular hypertrophic cardiomyopathy were described. Reference Jain, Chandna, Silber, Clark and Denes22 These criteria included: S/R in V5, V6 greater than 1, and S-wave in V5 and V6 greater than 7 mm. Although we did not analyse this in depth, we included an abnormally low R/S ratio left pre-cordial, which can be seen as an equivalent measure. We did not find a difference between biventricular en left ventricle hypertrophic cardiomyopathy with regard to these electrocardiographic patterns.
A limitation of this retrospective cohort study is that the electrocardiograms (and echocardiograms) were made in a clinical setting, and it was not possible to interpret all the characteristic features, although at least two reviewers evaluated each electrocardiogram. Moreover, the groups are relatively small. However, in an interim analysis of the patients of only one of the two centres, the same conclusions could be drawn.
In conclusion, this study confirms that the earlier established specific electrocardiographic Noonan syndrome features, especially a negative aVF (north-west axis pathognomonic) and abnormal low R/S ratio in the left pre-cordial leads, can differentiate between Noonan syndrome-related hypertrophic cardiomyopathy and sarcomere hypertrophic cardiomyopathy. On the other hand, a pathologic Q-wave (earlier established as a specific Noonan syndrome electrocardiographic feature) did not appear in Noonan syndrome-related hypertrophic cardiomyopathy but did appear in sarcomeric hypertrophic cardiomyopathy.
Table 3. Specific electrocardiographic characteristics in patients with biventricular hypertrophic cardiomyopathy and left ventricle hypertrophic cardiomyopathy with additional CHD included, and without additional CHD

HCM = hypertrophic cardiomyopathy; LV HCM = left ventricle hypertrophic cardiomyopathy; CHD = congenital heart disease.
Acknowledgements
Several authors of this publication are members of the European Reference Network for Developmental Anomalies and Intellectual Disability (ERN-ITHACA) and of the European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (GUARD-Heart).
Author contribution
Robert W. L. Hauptmeijer: conceptualisation and design of the study, data analysis and interpretation, drafting of the manuscript, and approval of the final manuscript. Lea Lippert: collection of data, data analysis, conceptualisation and design of the study, critical review of the manuscript, and approval of the final manuscript. Floris E. A. Udink ten Cate: conceptualisation and design of the study, drafting of the manuscript, and approval of the final manuscript. Zina Feijzic: conceptualisation and design of the study, drafting of the manuscript, and approval of the final manuscript. Erika Leenders: drafting of the manuscript and approval of the final manuscript. Cordula M. Wolf: collection of data, data analysis, conceptualisation and design of the study, drafting of the manuscript, critical review of the manuscript, and approval of the final manuscript. Jos M.T. Draaisma: conceptualisation and design of the study, data analysis and interpretation, critical review of the manuscript, and approval of the final manuscript.
Financial support
N/A.
Competing interests
CMW is a consultant to Biomarin, Day One Biopharmaceuticals, Adrenomed, and Pliant and has ownership interest. All other authors declare that they have no conflict of interest.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the Medical Ethics Committee (CMO) of the district Arnhem/Nijmegen (file number 2021-8332) and the Technical University of Munich Ethics Committee (number 243/17S, 10/16/2017).
Consent to participate
Informed consent to participate was obtained from the parents of the patients included in the study.
Consent for publication
N/A.
Data availability
The data of this study are available on request from the corresponding author.
Code availability
N/A.







