Invasive fungal diseases have become a major threat to haematopoietic stem cell transplantation (HSCT) recipients [Reference Kriengkauykiat, Ito and Dadwal1, Reference Kontoyiannis2]. It is well known that the environment plays an important role in fungal diseases [Reference Bénet3], leading to the recommendation that high-risk patients should be treated in rooms equipped with high-efficiency particulate air (HEPA) filters.
According to recent guidelines for air control in transplantation units [Reference Yokoe4], HSCT allogeneic recipients should be placed in protective rooms, with the following characteristics: ⩾12 air changes per hour, equipped with HEPA filter; directed air flow; positive air pressure with a differential between rooms and hallway ⩾2·5 Pa, with continuous monitoring; and well-sealed rooms with automatic doors. The efficiency of these measures for patients undergoing autologous HSCT has not been established. However, the implementation and maintenance of such measures are expensive and there is controversy around the survival benefit associated with these interventions [Reference Eckmanns, Rüden and Gastmeier5, Reference Humphreys6]. Due to economic restrictions many HSCT patients in developing countries are treated in rooms with no special air filters. This prompted us to investigate the quality of air in two teaching hospitals in which distinct heating, ventilation, air-conditioning (HVAC) systems were in place. The primary purpose of the study was to quantify and monitor the presence of fungal conidia in the air in these distinct HSCT units, and second, to compare the quantity of potentially pathogenic fungi in these areas.
Both hospitals were situated, 1 mile apart, in the city of Porto Alegre, the capital of Rio Grande do Sul state, Southern Brazil. Porto Alegre has a humid subtropical climate with an average annual temperature of 19·5 °C. Hospital 1 is a 1200-bed hospital with a six-bedded HSCT unit in a 10-year-old building with a central HVAC system operative in rooms but in which no HEPA filter is available. Hospital 2 is 40 years old and has 800 beds. Its HSCT ward has 25 beds in which all rooms are equipped with HEPA filters, with positive air pressure in relation to the corridor. Toilets have an exhaust air system creating negative pressure at this location but air in corridors is not pressurized or HEPA filtered. The infection control policies and procedures regarding air control in both institutions are comparable, including the recommendation for patients to wear N-95 masks when leaving rooms, limiting traffic in the units, washing hands, maintaining doors closed, and windows sealed.
Air samples were collected monthly from December 2009 to January 2011 with the six-stage Andersen Sampler (Thermo Scientific, USA) that collects airborne particles on Petri dishes at a constant flow rate of 28·3 l/min, at 1·5 m above the floor. Samples were collected over 20–30 min from corridors, rooms, and toilets (30 min in filtered areas, 20 min elsewhere). Samples were collected monthly in two randomly selected distinct rooms in each of the participating units on plates containing Sabouraud chloramphenicol agar which were incubated at 25 °C and observed daily for fungal growth for 7 days. Fungi were identified to the genus/species level based on their macro and micro morphological features. The amount of culturable fungal conidia in the air was determined in terms of colony-forming units (c.f.u.)/m3. For the purpose of this study, all fungi of the genera Aspergillus, Rhizopus, and Fusarium were considered potentially pathogenic. Cases of invasive mould diseases that occurred during the study period in the HSCT units were documented, and classified according to the revised EORTC/MSG definitions for invasive fungal diseases [Reference De Pauw7].
Descriptive statistics were used to summarize the data. Fungal concentrations in the sites (room, toilet, corridor) were compared between hospitals using the Mann–Whitney (MW) test, and in different sites in the same hospital using the Kruskal–Wallis (KW) test followed by Dunn's multiple comparison (DMC) test. Statistical analyses were performed with GraphPad Prism 5 software (GraphPad Prism Software Inc., USA). Probability (P) values of <0·05 were considered statistically significant.
A total of 117 samples were obtained during the period of study (702 plates). These samples were from corridors (hospital 1: n = 26; hospital 2: n = 13), rooms (n = 26), and toilets (n = 13), in each participating hospital. In both hospitals, dematiaceous fungi particularly Cladosporium spp. were predominant in the corridors, with median (range) concentrations of 53·9 (1·8–390·5) c.f.u./m3 and 91·9 (10·6–242·0) c.f.u./m3 for hospitals 1 and 2, respectively. Overall, corridors from hospitals 1 and 2 (Table 1) revealed similar concentrations of environmental fungi (P = 0·114, MW test), as well as potentially pathogenic moulds (P = 0·622, MW test).
Table 1. Airborne fungal concentration in the rooms of patients admitted in two distinct haematopoietic stem cell transplantation (HSCT) units in Brazil. Only hospital 2 was equipped with HEPA filters. All results are presented in terms of colony-forming units/m3
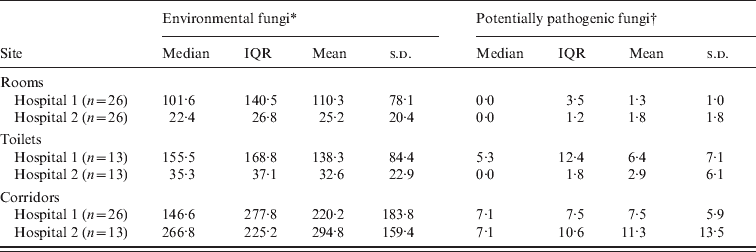
IQR, Interquartile range; n, number of samples; s.d., standard deviation.
* Including fungi belonging to the genus Penicillium, Scytalidium, sterile filamentous fungi, dematiaceous moulds, Rhodotorula, and Candida.
† Including species of Aspergillus, Rhizopus, and Fusarium.
When rooms, toilets and corridors were compared regarding overall fungal concentration, a significant difference was found by KW test (P < 0·05 and P < 0·001 for hospitals 1 and 2, respectively). Rooms in both hospitals showed lower fungal concentrations compared to their respective corridors (P < 0·05 and P < 0·0001, DMC test, for hospitals 1 and 2, respectively). Comparing toilets and rooms in each of the hospitals, a trend towards higher fungal counts in toilets was observed (hospital 1: median 102 and 161 c.f.u./m3 for rooms and toilets, respectively; hospital 2: median 24 and 39 c.f.u./m3 for rooms and toilets, respectively). These results were not statistically different (P > 0·05, DMC test in both hospitals).
In hospital 1, there was a significantly lower amount of potentially pathogenic fungi in rooms compared to corridors (P < 0·0001, KW test compared to P < 0·05, DMC test). Albeit not statistically significant, the concentration of environmental fungi (median and mean) was also lower in rooms in hospital 1 compared to corridors (P = 0·059, KW test). For hospital 2, the number of potentially pathogenic moulds and environmental fungi was reduced in rooms compared to corridors (P < 0·0001, KW test compared to P < 0·0001, DMC test). There were lower concentrations of airborne fungi in rooms of hospital 2 than in hospital 1 (P < 0·0001, MW test) but when only potentially pathogenic moulds were considered, similar results were found in both hospitals (P = 0·714, MW test).
During the period of investigation, 47 patients were hospitalized in the HSCT unit in hospital 1, and 144 in hospital 2. In hospital 1 patients were mostly autologous HSCT recipients (83·0%, n = 39) followed by allogeneic HSCT recipients (17·0%, n = 8). Most patients admitted to hospital 1 were male (57·4%, n = 27), with mean age 46 years (range 19–64 years), and mean length of hospital stay 28·7 days (range 14–68 days). Most patients admitted to the HSCT ward in hospital 2 had acute leukaemia (39·6%, n = 57), followed by allogeneic (27·8%, n = 40) and autologous (24·3%, n = 35) HSCT recipients, and other haematological conditions (8·3%, n = 12). These patients were mostly male (54·9%, n = 79), with mean age 37·2 years (range 1–71 years) and mean length of hospital stay 56·7 days (range 7–132 days).
The incidence (proven/probable cases) of invasive mould disease in hospitals 1 and 2 was 2·1% (n = 1) and 7·6% (n = 11), respectively. Despite the increased incidence of invasive mould diseases in hospital 2, results were not statistically different (P = 0·300, Fisher's exact test). These infections affected the respiratory tract (n = 10) and the skin (n = 2). Fungi recovered from these patients included Aspergillus section Fumigati (n = 3), Fusarium spp. (n = 2, including one case of skin infection) and Curvularia spp. (n = 1, skin infection).
Several studies [Reference Falvey and Streifel8–Reference Hospenthal, Know-Chung and Bennet13] have attempted to correlate the air fungal burden with the incidence of invasive fungal infections. Here we compared two hospitals that were geographically very close but had very distinct features, both in terms of hospital architecture and patient population. Rooms in hospital 2 were equipped with HEPA filters and the amount of fungal conidia in these areas was markedly reduced compared to corridors. Surprisingly, air concentration of potentially pathogenic fungi was similar in the rooms of both hospitals, despite the use of different ventilation systems. This could possibly be the result of several measures that together reduced the amount of aerial fungal spores, including sealed windows, closed doors, using appropriate ceiling tiles, reducing pedestrian traffic, as well as implementing an effective hand disinfection policy [Reference Yokoe4, Reference Fournel14]. Fungal burden in hospital 1 may also had been influenced by the location of its HSCT unit in a recently constructed building.
During the study period, construction works occurred in both hospitals. These were mostly classified as activities that generated a moderate to high amount of dust and may explain the elevated concentration of environmental fungi in the HSCT units, particularly for hospital 1. However, protective measures to control dispersion of dust were in place at all times, in both hospitals, which may have contributed to limit the amount of potentially pathogenic fungi in patients' rooms. Previous studies in HSCT units equipped with HEPA filters revealed counts of <2 c.f.u./m3 for Aspergillus spp. [Reference Falvey and Streifel8–Reference Curtis11] and a total fungal count of ∼10 c.f.u./m3 [Reference Falvey and Streifel8].
The concentration of airborne fungi may vary with different seasons of the year. Some authors have reported that fungi are less pronounced in the environment during autumn and winter seasons, with an increase during summer months [Reference Leenders15–Reference Panackal17]. Panackal et al. [Reference Panackal17] compared two HSCT centres and showed a higher incidence of invasive aspergillosis and fungal spore counts during summer months, which was also associated with low precipitation, facilitating the dispersion of hydrophobic conidia. Even though our sample size was relatively small we did not find any association between fungal concentration and season.
However, this study was limited by a few factors, including the number of samples analysed. In addition, since a single incubation temperature was used (25 °C), growth of thermotolerant fungi such as Aspergillus section Fumigati might have been underestimated. Since the incubation period of Aspergillus infections is unknown it was not possible to determine the precise source of infection (i.e. community-acquired vs. hospital-acquired), mainly because molecular typing was not performed to compare environmental and clinical samples. However, since most patients were in the hospital for more than 14 days at the time of air sampling, it is quite possible that invasive aspergillosis cases documented during the survey represented nosocomial infections.
In conclusion, we observed that a low fungal burden may be present in the air of rooms of HSCT recipients despite the absence of HEPA filtration. As discussed, additional protective measures may also play an important role in environmental control, therefore HEPA filters might not be essential for all HSCT units. However, in hospitals in which severely immunocompromised patients are admitted, a reduction in fungal spores by means of HEPA filters is highly desirable.
ACKNOWLEDGEMENTS
Professor Pasqualotto receives a research grant from CNPq (Brazilian National Research Counci).
DECLARATION OF INTEREST
None.





