Giant ragweed is one of the most competitive agricultural weeds plaguing crops in the midwestern United States (Webster et al. Reference Webster, Loux, Regnier and Harrison1994). A single giant ragweed plant m−2 has the potential to reduce soybean yields by 45 to 77%, and 1.4 giant ragweed plants m−2 can reduce corn yields by up to 90% (Harrison et al. Reference Harrison, Regnier, Schmoll and Webb2001; Webster et al. Reference Webster, Loux, Regnier and Harrison1994). As an annual, giant ragweed relies on the seedbank to persist in agricultural fields, and seed production in soybean and field margins is nearly 2,000 seeds plant−1, indicating high potential for seedbank replenishment (Fenner Reference Fenner1995; Goplen et al. Reference Goplen, Sheaffer, Becker, Coulter, Breitenbach, Behnken, Johnson and Gunsolus2016).
Giant ragweed control in corn and soybean rotations has become complicated due to the development of resistance to multiple herbicide mechanisms of action, including both acetolactate synthase (ALS) inhibitors and glyphosate (Heap Reference Heap2015). The availability of multiple glyphosate-resistant crops beginning in 1996 allowed producers to use glyphosate as an effective postemergence, broad-spectrum herbicide with low cost, which led to glyphosate being used as a stand-alone herbicide on millions of hectares of cropland (Duke and Powles Reference Duke and Powles2008). The widespread and repeated use of glyphosate, paired with application to large weeds, caused tremendous selection pressure on weed populations and resulted in glyphosate-resistant weeds. When paired with ALS-inhibitor resistance, which had previously developed in giant ragweed, resistance to multiple herbicide sites of action was stacked within the same plant. As herbicide-resistant giant ragweed becomes more prevalent, there is an increased need for integrative forms of weed control (Shaner and Beckie Reference Shaner and Beckie2014).
Crop rotations have long been recognized as an effective means to control a diversity of weeds (Leighty Reference Leighty1938; Liebman and Dyck Reference Liebman and Dyck1993). Diverse crop rotations provide the opportunity to use different weed control strategies, including a variety of mechanical and chemical methods. Planting crops that permit the use of alternative herbicide sites of action makes control of resistant weeds more manageable and reduces the potential of weeds developing additional resistance (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos, Witt and Barrett2012). More diverse crop rotations also provide a more suitable habitat for seed predators that reduce weed seedbanks (Davis and Liebman Reference Davis and Liebman2003; Westerman et al. Reference Westerman, Liebman, Menalled, Heggenstaller, Hartzler and Dixon2005). Overall, crop sequences that vary in patterns of resource competition, soil disturbance, and mechanical damage create unstable environments hostile to any particular weed species, ultimately decreasing weed populations (Liebman and Dyck Reference Liebman and Dyck1993; Schreiber Reference Schreiber1992). Using crop rotations that diversify herbicide sites of action, in addition to promoting weed seedbank depletion via seed decay and predation, offers potential to effectively manage herbicide-resistant giant ragweed over the long term (Chee-Sanford et al. Reference Chee-Sanford, Davis and Sims2006).
Seed predation by rodents and invertebrates has been shown to remove nearly 90% of giant ragweed seeds within one year in no-tillage corn (Harrison et al. Reference Harrison, Regnier and Schmoll2003). Seed predation increases in wheat and alfalfa compared with annual row crops due to greater crop canopy (Hartzler et al. Reference Hartzler, Liebman and Westerman2007; Westerman et al. Reference Westerman, Liebman, Menalled, Heggenstaller, Hartzler and Dixon2005). In addition, alfalfa is harvested several times throughout the growing season via mowing, eliminating giant ragweed seed production and replenishment of the weed seedbank. Wheat increases early-season competition with emerging giant ragweed by being planted earlier in the growing season than corn or soybean and in narrower rows. Additionally, wheat allows the use of herbicides with alternative mechanisms of action that are more effective against herbicide-resistant populations of giant ragweed. In the event of weed escapes, wheat is harvested prior to giant ragweed seed production (Goplen et al. Reference Goplen, Sheaffer, Becker, Coulter, Breitenbach, Behnken, Johnson and Gunsolus2016), preventing replenishment of the seedbank and allowing time for multiple mechanical and chemical weed control options following wheat harvest.
Although crop rotation is known to be an effective form of weed control, the effects of crop-rotation systems on giant ragweed seedbank dynamics have not been thoroughly evaluated. The objectives of this research were to determine how cropping systems common to the midwestern United States affect giant ragweed’s (1) timing of emergence, (2) total emergence, and (3) seedbank depletion after two growing seasons. This research will help define which crop-rotation systems promote soil conditions most conducive to minimizing giant ragweed emergence and maximizing seedbank depletion, allowing crop producers to determine the most effective ways to proactively manage herbicide-resistant giant ragweed infestations.
Materials and Methods
Site Details
Two replicated experiments were initiated in 2012 and 2013 at different sites near Rochester, MN (43.91°N, 92.56°W). Both sites were on a Port Byron silt loam (fine-silty, mixed, superactive, mesic Typic Hapludolls) with a pH of 7.0 and 4.0% organic matter with a history of a 2-yr corn–soybean rotation, with soybean immediately preceding both experiments. The research sites had known populations of giant ragweed resistant to glyphosate and ALS-inhibitor herbicides.
Crop Management
Each experiment had six crop rotations arranged in a randomized complete block design with four replications. Crop-rotation treatments consisted of: continuous corn (CCC); soybean–corn–corn (SCC); corn–soybean–corn (CSC); soybean–wheat–corn (SWC); soybean–alfalfa–corn (SAC); and alfalfa–alfalfa–corn (AAC). Plots were 10 by 15 m. Corn and alfalfa cultivars had resistance to glyphosate and corn and soybean cultivars were glufosinate-resistant. DEKALB® DKC53-78RIB corn was planted at 86,500 seeds ha−1 in 76 cm rows. Soybean plots were planted with Stine® 19LD08 at 345,900 seeds ha−1 in 76 cm rows. Inoculated alfalfa (DEKALB® DKA41-18RR) was direct seeded with a no-till drill at 16.8 kg ha−1 in 19 cm rows. MN RB07 wheat was direct seeded with a no-till drill at 135 kg ha−1 in 19 cm rows. Fertilizer was applied according to University of Minnesota guidelines (Kaiser et al. Reference Kaiser, Lamb and Eliason2011). Phosphorus, potassium, and sulfur were broadcast using mono-ammonium phosphate, potash, and ammonium sulfate, respectively, across the entire study area in the fall of each year to maintain adequate levels of these nutrients for all crops grown. At planting, ammonium nitrate was applied at 191, 135, 0, and 129 kg N ha−1 for corn following corn or wheat, corn following 1 yr of alfalfa, corn following 2 yr of alfalfa, and wheat following soybean, respectively.
Corn plots were chisel plowed to a depth of 20 cm in the fall after corn harvest and stover chopping, and were field cultivated twice in the spring just prior to planting. Soybean plots were field cultivated twice in the spring just prior to planting. Soybean stubble following harvest was chisel plowed to a depth of 20 cm in the fall when corn was to be planted the following year and was left fallow when wheat or alfalfa was to be seeded the following year. Wheat was no-tilled into standing soybean stubble, and wheat stubble was chisel plowed to a depth of 20 cm in the fall after harvest at the same time as chisel plowing occurred in the other crop-rotation systems. Alfalfa plots that were seeded in the first year of the rotation received a single pass with a field cultivator prior to planting, while alfalfa plots seeded in the second year of the rotation were no-till seeded into soybean stubble.
Fields were scouted for insect pests and diseases, and none reached levels warranting treatment according to University of Minnesota guidelines (University of Minnesota Extension 2015). Wheat plots were sprayed prophylactically with tebuconazole (alpha-[2-(4-chlorophenyl)ethyl]-alpha-(1,1-dimethylethyl)-1H-1,2,4-triazole-1-ethanol) (Bayer CropScience® Folicur 3.6F) at 126 g ai ha−1 at flower initiation (Zadoks 61) to prevent the development of Fusarium head blight (Fusarium graminearum L.) (Simmons et al. Reference Simmons, Oelke and Anderson1995).
A zero-weed threshold was maintained throughout the study to determine giant ragweed seedbank depletion. Due to the presence of glyphosate-resistant and ALS inhibitor–resistant giant ragweed populations, herbicides specifically targeting resistant giant ragweed were used. When herbicides with residual activity on giant ragweed were used, quadrats where emergence was monitored were covered at time of application to prevent herbicide coverage. Quadrats were not covered for POST applications of herbicides without residual activity, as seedlings were counted and removed prior to herbicide application. Corn and soybean plots received a single PRE application of S-metolachlor at 2.14 kg ai ha−1 on the date of planting each year. Corn and soybean plots received two POST applications of glufosinate-ammonium at 450 g ai ha−1 at approximately 3 and 6 wk post-planting. Alfalfa plots received a single application of 2,4-DB, dimethylamine salt at 1.12 kg ae ha−1 2 wk following planting in the seeding year, while second-year alfalfa received no herbicide application. Wheat plots received a tank-mixed POST application of a prepackaged mixture of clopyralid monoethanolamine salt and fluroxypyr, 1-methylheptyl ester at 105 g ae ha−1 each (Widematch) and MCPA isooctyl (2-ethylhexyl) ester at 389 gaeha−1 approximately 2 wk post-planting, when wheat was beyond the 3-leaf stage (Zadoks 13) (Simmons et al. Reference Simmons, Oelke and Anderson1995). Weeds escaping herbicide control were hand weeded to ensure there were no inputs into the seedbank.
Seedbank Monitoring
Giant ragweed seedbank densities were determined after emergence terminated in mid-July in the initial and final year of each crop-rotation system. Seedbank samples in the first year at the first experimental location were taken from three fixed quadrat locations in each plot by sampling a single 25 by 40 cm area to a depth of 15 cm. Due to the amount of time necessary to extract each sample, an alternative sampling method was used to determine seedbank densities in the final year at the first experimental location and in both the initial and final years at the second experimental location. The alternative sampling procedure, adapted from Forcella (Reference Forcella1992), used a 10-cm-diameter golf-hole cutter to obtain a composite of 10 samples to a 15 cm depth collected from the same three quadrat locations in each plot. Weed seeds were separated from combined samples using a modified version of a physical extraction procedure adapted from Ball and Miller (Reference Ball and Miller1989), Cardina and Sparrow (Reference Cardina and Sparrow1996), and Standifer (Reference Standifer1980), in which compiled samples were wet sieved to separate seeds. Samples were soaked with water and mixed several times over 20 min using a paint-stirring attachment on an electric drill. Once the soil was in suspension, samples were poured through a 0.16 cm sieve to extract seeds. Remaining soil was soaked again and mixed until the entire sample passed through the sieve. A low-pressure shower of water was sprayed on the sample to speed the passing of soil through the sieve. Once organic material >0.16 cm was separated from soil, the seedbank extract was placed in a 60 C forced-air oven for 2 d to dry the sample before seeds and seed fragments were handpicked from the samples. Seeds were then determined to be potentially viable or nonviable by dissecting the seeds to determine the presence of an intact embryo and counted, similar to methods defined previously (Ball and Miller Reference Ball and Miller1989; Cardina and Sparrow Reference Cardina and Sparrow1996; Standifer Reference Standifer1980).
Emergence Monitoring
Giant ragweed emergence counts were made on a weekly basis starting at the onset of emergence and continued for at least 10 wk or until emergence ceased each year. Giant ragweed emergence was monitored in six permanent 30 by 76 cm quadrats within each plot. Three quadrats were placed between rows, and three quadrats were placed over the crop row. Each week, seedlings were counted and removed from the quadrat by clipping seedlings at the soil surface without disturbing the soil.
Environmental Effects
Daily precipitation, soil temperature, and growing degree days (GDDs) were monitored to determine their effects on giant ragweed emergence. Daily precipitation and minimum and maximum air temperatures were obtained from the National Weather Service station nearest the study locations. Soil temperature was monitored at a 5 cm depth using temperature sensors (Hobo Water Temp Pro v. 2), logging temperature at hourly intervals (Figure 1). GDDs were calculated using Equation 1,
where T max is the maximum daily air temperature, T min is the minimum daily air temperature, b 0 is the base temperature (2 C), and S 1 and S 2 are April 1 and July 31, respectively (Table 1) (Abul-Fatih and Bazzaz Reference Abul-Fatih and Bazzaz1979).

Figure 1 Mean daily soil temperature throughout the giant ragweed emergence period at 5 cm depth in the second year of each crop-rotation system in 2013 and 2014. aC, S, W, and A represent the sequence of corn, soybean, wheat, and alfalfa in each 3 yr crop-rotation system.
Table 1 Mean air temperature, total growing degree days (GDDs), and total precipitation by month and across the growing season (April to October) during the study period from the nearest National Weather Service station.Footnote a

a Weather data obtained from site identification: KRST (43.9041°N, −92.4916°W).
b GDDs calculated using Equation 1 (2 C base temperature).
Statistical Analysis
Seedbank depletion, total giant ragweed emergence each year, and the proportion of the seedbank depleted due to emergence were each analyzed using the MIXED procedure of SAS, with crop-rotation system considered a fixed effect and experimental location, block (nested within location), interactions, and subsampling considered random effects (SAS Institute 2012). Data from both experimental locations were combined following analysis of interactions. Mean comparisons were made for each set of analyses using Fisher’s protected LSD test (α=0.05) and transformed means (described below) were back transformed for presentation.
Seedbank depletion was calculated as a percentage of the initial giant ragweed seedbank density. Since seedbank depletion between the first and third years of each crop-rotation system was a percentage and exhibited a skewed distribution, data were arcsine transformed prior to analysis. The total giant ragweed emergence in each year exhibited a nonnormal distribution and was transformed with the natural log transformation prior to analysis. Additionally, the initial seedbank density was included as a random effect covariate, to control for spatial variation in the analysis of total giant ragweed emergence each year. Since the initial seedbank samples were taken after giant ragweed emergence, the proportion of the seedbank depleted by emergence was calculated as the total giant ragweed emergence from the second and third year of the crop-rotation systems, therefore representing the emergence that occurred between the initial and final seedbank samples. The proportion of seedbank depletion due to emergence exhibited a skewed distribution and was transformed with the natural log transformation prior to analysis.
To evaluate emergence timing of giant ragweed, weekly emergence counts were converted to a cumulative percentage of the total seedlings that emerged each year. Cumulative percent emergence of giant ragweed in each crop-rotation system was modeled using a logistic regression equation as a function of day of year (doy) (Equation 2). The emergence data in both experimental locations were similar and were combined for analysis. The emergence rate (ER) and inflection point (IP) of Equation 2
were estimated for the second year of each crop-rotation system and were used to predict the dates when 50 and 90% emergence occurred in each crop-rotation system. Only emergence in the second year of each crop-rotation system was modeled, because all four crops were present in the second year, illustrating the greatest cropping diversity effects on giant ragweed emergence.
Results and Discussion
Total Emergence
Across both experimental locations, 125, 37, and 6 seedlings m−2 yr−1 emerged in years 1, 2, and 3 of the crop-rotation systems, respectively, representing a major threat for crop yield and control costs. Initial seedbank densities over the study locations ranged from 0 to 317 seeds m−2. Due to spatial variation of the initial weed seedbank at both locations, estimates of the starting seedbank density were included as a covariate in analysis of total emergence in each year of each cropping system (R2=0.31, P<0.001). In the first year, there were no differences in giant ragweed emergence among crop-rotation systems (P=0.73; Table 2), which was expected, since all crops were planted into a site with the same management history. Additionally, crop planting was delayed in the first year of the cropping systems at the second experimental location in 2013 due to large amounts of precipitation (Table 1), resulting in a large percentage of giant ragweed emergence prior to crop planting, thus minimizing any cropping effects.
Table 2 Total giant ragweed emergence in each year, percentage of seedbank depletion, and the percentage of depletion accounted for by emergence in each crop-rotation system, across both experimental locations in 2012 to 2015.Footnote a
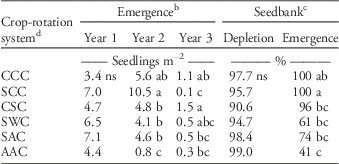
a Means with different letters indicate a significant difference at the 0.05 probability level using Fisher’s protected LSD.
b Total seedling emergence in each year is the corrected mean from the seedbank density covariate.
c Seedbank depletion represents the percentage of seeds depleted between the first and third year of the crop-rotation systems, while emergence represents the percentage of the seedbank depletion that was accounted for by emergence during the same time period.
d C, S, W, and A represent the sequence of corn, soybean, wheat, and alfalfa in each 3 yr crop-rotation system.
There were differences in total giant ragweed emergence in the second year of the crop-rotation systems, in that second-year alfalfa had the least emergence of all crop-rotation systems. Although not significantly different from the continuous corn treatment, corn following soybean had the greatest amount of giant ragweed emergence in the second year of the crop-rotation systems (Table 2). The other crops planted following soybean, including wheat and alfalfa in the SWC and SAC treatments, had less emergence of giant ragweed than SCC in the second year of the rotations, despite similar overwinter and early-spring conditions. However, the pre-planting tillage and planting date differed among the SWC, SAC, and SCC systems in the second year of the rotations, with alfalfa and wheat planted an average of 22 d prior to corn and soybean. There were differences in total emergence in the third year of the crop-rotation systems, despite having consistent tillage type and timing the preceding fall and spring prior to planting (Table 2). Overall, differences in emergence could have been due to variations in soil temperature (Figure 1) and crop residues, since even slight changes in planting date, cultivation timing, and residue management can influence vertical seed movement, seed dormancy dynamics, and overall emergence of giant ragweed (Buhler Reference Buhler1995; Buhler et al. Reference Buhler, Hartzler and Forcella1997; Cousens and Moss Reference Cousens and Moss1990; Dyer Reference Dyer1995; Staricka et al. Reference Staricka, Burford, Allmaras and Nelson1990).
The second year of the AAC system had the least giant ragweed emergence among crop-rotation systems, which was expected, since giant ragweed is least adapted to the perennial environment of alfalfa. In the fall of the first year of the AAC system, there was significant alfalfa canopy coverage, likely buffering the soil environment from extreme temperature changes throughout the winter. In the spring of the second year, this canopy coverage likely contributed to reduced fluctuations in soil temperature and cooler soil temperature in the established alfalfa than in the exposed soil of the other treatments, which was most extreme from May 20 to June 12 in 2014 (Figure 1). These differences in soil temperature were minimized after June 12 in 2014, as alfalfa was harvested on this date. Additionally, less tillage occurred in the AAC system. Previous work has shown that weed emergence depth is shallower with no-tillage than chisel and moldboard plowing (Buhler and Mester Reference Buhler and Mester1991). Seeds of large-seeded weeds like giant ragweed tend to remain near the soil surface with less intensive tillage, which has been shown to inhibit establishment of these species (Lueschen and Anderson Reference Lueschen and Andersen1980). Therefore, lower soil temperatures along with less intensive tillage likely provided a soil environment less conducive to giant ragweed emergence. This environment may also have been more conducive to seedbank degradation through seedling mortality, seed degradation, and seed predation, resulting in less emergence with similar levels of seedbank depletion (Table 2).
Emergence Timing
Giant ragweed exhibited similar emergence patterns in each year of the crop-rotation system treatments, following a logistic growth curve relative to date (Figure 2; Table 3). Only emergence from the second year of the crop-rotation system is presented here, because corn, soybean, wheat, and alfalfa were all planted in the second year and represent the greatest cropping diversity in a single year. Giant ragweed began emerging slowly in the early weeks of each growing season before having a period of rapid emergence throughout May. Emergence tapered off and nearly terminated mid-June. Giant ragweed emergence initiated at a similar date in 2013 and 2014, but emergence occurred slightly more rapidly in 2013, which was likely caused by greater precipitation from April to June in 2013 (Table 1). Despite slight yearly differences, across all treatments, 50 and 90% of giant ragweed emergence in all years occurred on May 21 and June 4, respectively (Table 3), indicative of the early emergence pattern of giant ragweed in much of the midwestern United States (Werle et al. Reference Werle, Sandell, Buhler, Hartzler and Lindquist2014). Giant ragweed in this study did not exhibit a biphasic emergence pattern, as has been identified in Ohio (Schutte et al. Reference Schutte, Regnier and Harrison2012). All crop-rotation systems exhibited a similar pattern of cumulative emergence, except for the second year alfalfa in the AAC system. The second year of the AAC system achieved 50% emergence at the same time as the other crop-rotation systems, indicated by the similarity in inflection points of the crop-rotation systems (Table 3). However, the AAC system had a longer duration of emergence, reflected by the slower emergence rate, and did not reach 90% emergence until June 18, several weeks after the other crop-rotation systems (Table 3; Figure 2). Giant ragweed emergence has been shown to be associated with the accumulation of thermal time (Archer et al. Reference Archer, Forcella, Korth, Kuhn, Eklund and Spokas2006; Davis et al. Reference Davis, Clay, Cardina, Dille, Forcella, Lindquist and Sprague2013; Schutte et al. Reference Schutte, Regnier, Harrison, Schmoll, Spokas and Forcella2008; Werle et al. Reference Werle, Sandell, Buhler, Hartzler and Lindquist2014). The prolonged pattern of emergence of giant ragweed in the second year of the AAC system may be related to the greater early-season crop canopy of alfalfa, which reduced the amount of solar radiation reaching the soil surface, thereby decreasing soil temperature and slowing the accumulation of thermal time at the soil level (Figure 1).

Figure 2 Cumulative percentage of giant ragweed emergence in the second year of each crop-rotation system at both experimental locations based on the best-fit logistic regression equations (Table 3). aC, S, W, and A represent the sequence of corn, soybean, wheat, and alfalfa in each 3 yr crop-rotation system.
Table 3 Parameter estimates and predicted dates of 50 and 90% cumulative giant ragweed emergence in the second year of each crop-rotation system, across both experimental locations.
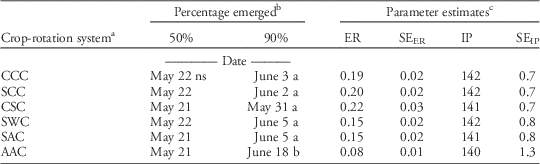
a C, S, W, and A represent the sequence of corn, soybean, wheat, and alfalfa in each 3 year crop-rotation system.
b Dates for percentage emerged are calculated from the logistic regression parameter estimates. Dates with different letters indicate significant difference.
c Parameter estimates are from the best-fit logistic regressions (Equation 2) from each crop-rotation system based on cumulative giant ragweed emergence. Abbreviations: ER, emergence rate; IP, inflection point; SEER, standard error for ER parameter estimate; SEIP, standard error for IP parameter estimate.
Seedbank Depletion
There were no differences in giant ragweed seedbank depletion among crop-rotation systems when a zero-weed threshold was maintained (P=0.57). On average, 96% of the giant ragweed seedbank was depleted in 2 yr in any crop-rotation system (Table 2), indicating that the giant ragweed seedbank is short-lived regardless of the cropping system. Harrison et al. (Reference Harrison, Regnier, Schmoll and Harrison2007) found that depletion of the giant ragweed seedbank was dependent on burial depth and that seeds closer to the soil surface were degraded more quickly than seeds >10 cm deep. A small percentage of giant ragweed seed remaining in the seedbank has been shown to persist for up to 15 yr, which exemplifies the importance of long-term weed management (Hartnett et al. Reference Hartnett, Hartnett and Bazzaz1987; Loux and Berry Reference Loux and Berry1991).
There are multiple ways weed seeds can be depleted from the seedbank. Weed seeds can germinate and emerge or die, fungi and other soil microorganisms can decay the seeds, and predators such as birds and rodents can consume seeds (Buhler et al. Reference Buhler, Hartzler and Forcella1997; Chee-Sanford et al. Reference Chee-Sanford, Davis and Sims2006; Kremer Reference Kremer1993). Each of these mechanisms of seedbank degradation has potential to cause significant seedbank losses. Harrison et al. (Reference Harrison, Regnier and Schmoll2003) found that up to 90% of giant ragweed seeds deposited on the soil surface of a no-tillage cornfield can be eliminated by predation in a single year. Additionally, the rate of seed predation increases as the crop canopy develops within a field, with wheat and alfalfa typically having greater seed predation than corn (Hartzler et al. Reference Hartzler, Liebman and Westerman2007; Westerman et al. Reference Westerman, Liebman, Menalled, Heggenstaller, Hartzler and Dixon2005).
A large portion of giant ragweed seedbank depletion was due to emergence in all crop-rotation systems, ranging from 41 to 100% among crop-rotation systems (Table 2). In the CCC, SCC, and CSC systems, nearly 100% of seedbank depletion was due to emergence, although CCC and CSC did not have significantly greater depletion due to emergence than SWC and SAC, which accounted for 61 and 74%, respectively. Less emergence in the second year of the AAC treatment likely resulted in the AAC treatment having less seedbank depletion due to emergence than the CCC and SCC crop-rotation systems (Table 2). These results indicate that there was slightly more depletion of the giant ragweed seedbank due to factors other than emergence in the AAC system, which is supported by Brust and House (Reference Brust and House1988), who found that depletion of weed seedbanks due to seed predators and soil microorganisms is greater in more diverse cropping systems with more habitat for seed predators.
Conclusions
These results align with previous research indicating that giant ragweed is a relatively early-emerging weed with a short duration of emergence compared with other common weeds in the midwestern United States (Werle et al. Reference Werle, Sandell, Buhler, Hartzler and Lindquist2014). This early-season emergence pattern indicates that there is potential to enhance giant ragweed control through the timing of field operations. Although delayed planting may reduce crop yield potential, it allows a greater percentage of seedlings to emerge and be destroyed prior to planting when tillage and/or herbicides can be used to control early-emerging weeds just prior to planting (Gill Reference Gill1996; Walsh and Powles Reference Walsh and Powles2007). Crops also have a faster rate of growth when planted later in the growing season, providing an additional competitive advantage over weeds (Tsimba et al. Reference Tsimba, Edmeades, Millner and Kemp2013). It is also expected that delayed planting will improve control of giant ragweed populations that have a biphasic emergence pattern like those found in Ohio, since these populations still have an early flush of emergence that can be affected by planting date (Schutte et al. Reference Schutte, Regnier and Harrison2012). Additionally, delayed planting provides the opportunity for PRE herbicide residual activity to extend weed control later into the growing season.
This study indicates that the giant ragweed seedbank is short-lived and that crop-rotation systems do not differ in the amount of seedbank depletion when a zero-weed threshold is implemented. More specifically, these results indicate that weed seed inputs in the cropping systems studied only need to be prevented for 2 yr to reduce the giant ragweed seedbank by 96%. Implementing a zero-weed threshold may be easier if total annual emergence is reduced. Corn following soybean had the greatest total emergence of giant ragweed. Conversely, emergence in the AAC system was less than that of the other cropping systems, indicating that the inclusion of alfalfa in the cropping system has the greatest potential to improve giant ragweed control. Although the emergence period was longer in the AAC system, the harvesting schedule of established alfalfa prevents seedbank inputs without reliance on herbicides. Overall, these results indicate that there is potential to manage fields infested with giant ragweed by eliminating seedbank inputs and depleting the weed seedbank to ultimately improve herbicide-resistant giant ragweed control.
Acknowledgments
This research was funded by the Monsanto Graduate Fellowship, the Rapid Agricultural Response Fund of the Minnesota Agricultural Experiment Station, and the Torske Klubben Graduate Fellowship. The authors would also like to express appreciation to staff and students for their assistance, in particular Brad Kincaid and Doug Miller.









